Use of Mechanical Enhanced Colonoscopy to Improve Polyp Detection During Colorectal Cancer Screening: A Real-World Healthcare Database Analysis
Abstract
1. Introduction
2. Methods
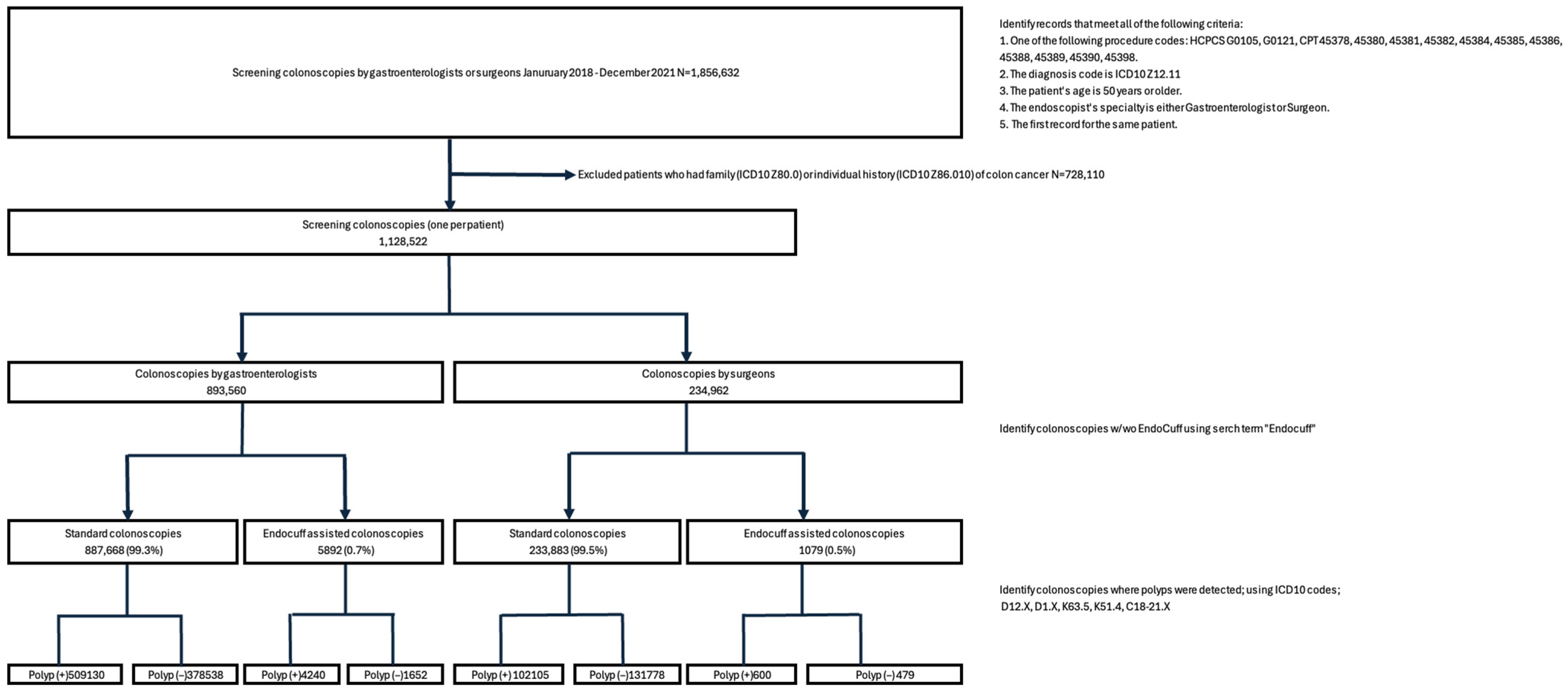
2.1. Study Design and Inclusion Criteria
2.2. Data Sources
2.3. Outcomes
2.4. Statistical Analysis
2.5. Role of Funding Source
3. Results
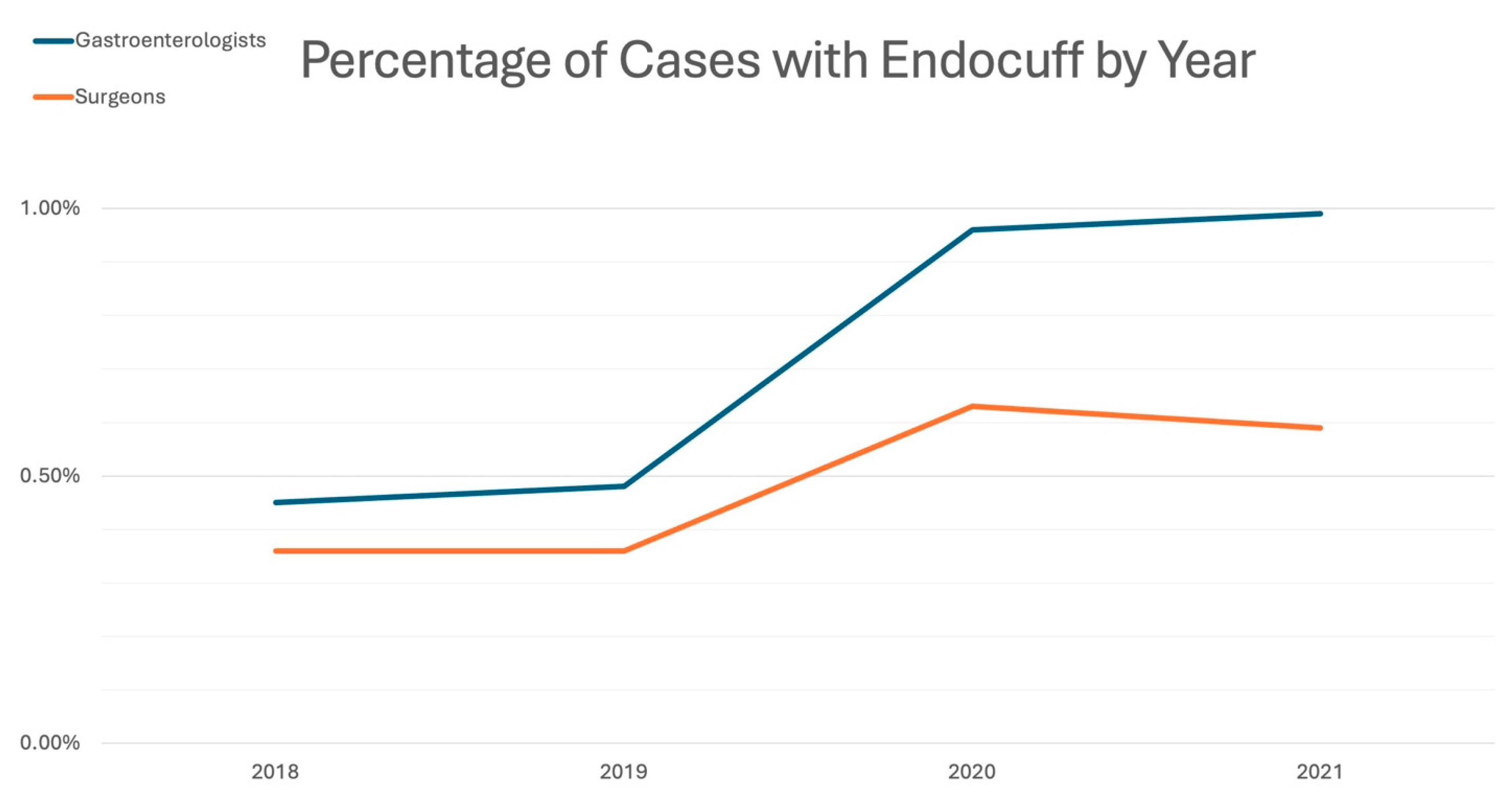
3.1. Colonoscopy Characteristics and EAC Utilization
3.2. Outcomes
3.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.C.; Sandler, R.S.; Sanoff, H.K.; Yang, Y.C.; Lund, J.L.; Baron, J.A. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clin. Gastroenterol. Hepatol. 2017, 15, 903–909.e6. [Google Scholar] [CrossRef]
- Shaukat, A.; Kahi, C.J.; Burke, C.A.; Rabeneck, L.; Sauer, B.G.; Rex, D.K. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am. J. Gastroenterol. 2021, 116, 458–479. [Google Scholar] [CrossRef]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017, 112, 1016–1030. [Google Scholar] [CrossRef]
- Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; Kubik, M.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar]
- Kahi, C.J.; Imperiale, T.F.; Juliar, B.E.; Rex, D.K. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin. Gastroenterol. Hepatol. 2009, 7, 770–775. [Google Scholar] [CrossRef]
- Zauber, A.G.; Winawer, S.J.; O’Brien, M.J.; Lansdorp-Vogelaar, I.; van Ballegooijen, M.; Hankey, B.F.; Shi, W.; Bond, J.H.; Schapiro, M.; Panish, J.F.; et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012, 366, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Chang-Claude, J.; Jansen, L.; Knebel, P.; Stock, C.; Hoffmeister, M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology 2014, 146, 709–717. [Google Scholar] [CrossRef]
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Schapiro, M.; Bond, J.H.; Panish, J.F.; et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N. Engl. J. Med. 1993, 329, 1977–1981. [Google Scholar] [CrossRef]
- Brenner, H.; Chang-Claude, J.; Seiler, C.M.; Rickert, A.; Hoffmeister, M. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann. Intern. Med. 2011, 154, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.; Murray, A.; Heitman, S.J.; Ruan, Y.; Antoniou, S.A.; Boyne, D.; Murthy, S.; Baxter, N.N.; Datta, I.; Shorr, R.; et al. Association Between Endoscopist Specialty and Colonoscopy Quality: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1931–1946. [Google Scholar] [CrossRef]
- Rex, D.K.; Anderson, J.C.; Butterly, L.F. Quality indicators for colonoscopy. Gastrointest. Endosc. 2024, 100, 352–381. [Google Scholar] [CrossRef]
- Millien, V.O.; Mansour, N.M. Bowel Preparation for Colonoscopy in 2020: A Look at the Past, Present, and Future. Curr. Gastroenterol. Rep. 2020, 22, 28. [Google Scholar] [CrossRef]
- Causada-Calo, N.S.; Gonzalez-Moreno, E.I.; Bishay, K.; Shorr, R.; Dube, C.; Heitman, S.J.; Hilsden, R.J.; Rostom, A.; Walsh, C.; Anderson, J.T.; et al. Educational interventions are associated with improvements in colonoscopy quality indicators: A systematic review and meta-analysis. Endosc. Int. Open 2020, 8, E1321–E1331. [Google Scholar] [CrossRef]
- Bishay, K.; Causada-Calo, N.; Scaffidi, M.A.; Walsh, C.M.; Anderson, J.T.; Rostom, A.; Dube, C.; Keswani, R.N.; Heitman, S.J.; Hilsden, R.J.; et al. Associations between endoscopist feedback and improvements in colonoscopy quality indicators: A systematic review and meta-analysis. Gastrointest. Endosc. 2020, 92, 1030–1040.e1039. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Lee, J.K.; Doubeni, C.A.; Zauber, A.G.; de Boer, J.; Fireman, B.H.; Schottinger, J.E.; et al. Adenoma detection rate and risk of colorectal cancer and death. N. Engl. J. Med. 2014, 370, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Tuskey, A.; Rao, V.L.; Dominitz, J.A.; Murad, M.H.; Keswani, R.N.; Bazerbachi, F.; Day, L.W. Interventions to improve adenoma detection rates for colonoscopy. Gastrointest. Endoscopy 2022, 96, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Ruan, Y.; Yuan, Y.; Khalaf, K.; Sabrie, N.S.; Gimpaya, N.; Scaffidi, M.A.; Bansal, R.; Vaska, M.; Brenner, D.R.; et al. Relative Efficacies of Interventions to Improve the Quality of Screening-Related Colonoscopy: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Gastroenterology 2024, 167, 560–590. [Google Scholar] [CrossRef]
- Aziz, M.; Haghbin, H.; Sayeh, W.; Alfatlawi, H.; Sharma, S.; Weissman, S.; Kamal, F.; Lee-Smith, W.M.; Nawras, A.; Sharma, P.; et al. Comparison of Artificial Intelligence with Other Interventions to Improve Adenoma Detection Rate for Colonoscopy: A Network Meta-analysis. J. Clin. Gastroenterol. 2024, 58, 143–155. [Google Scholar] [CrossRef]
- Rex, D.K.; Sagi, S.V.; Kessler, W.R.; Rogers, N.A.; Fischer, M.; Bohm, M.E.; Dewitt, J.M.; Lahr, R.E.; Searight, M.P.; Sullivan, A.W.; et al. A comparison of 2 distal attachment mucosal exposure devices: A noninferiority randomized controlled trial. Gastrointest. Endosc. 2019, 90, 835–840.e831. [Google Scholar] [CrossRef]
- Triantafyllou, K.; Polymeros, D.; Apostolopoulos, P.; Brandao, C.L.; Gkolfakis, P.; Repici, A.; Papanikolaou, I.S.; Dinis-Ribeiro, M.; Alexandrakis, G.; Hassan, C. Endocuff-assisted colonoscopy is associated with a lower adenoma miss rate: A multicenter randomized tandem study. Endoscopy 2017, 49, 1051–1060. [Google Scholar] [CrossRef]
- Rameshshanker, R.; Tsiamoulos, Z.; Wilson, A.; Rajendran, A.; Bassett, P.; Tekkis, P.; Saunders, B.P. Endoscopic cuff-assisted colonoscopy versus cap-assisted colonoscopy in adenoma detection: Randomized tandem study-Detection in Tandem Endocuff Cap Trial (DETECT). Gastrointest. Endosc. 2020, 91, 894–904.e891. [Google Scholar] [CrossRef]
- Facciorusso, A.; Buccino, V.R.; Sacco, R. Endocuff-assisted versus Cap-assisted Colonoscopy in Increasing Adenoma Detection Rate. A Meta-analysis. J. Gastrointestin Liver Dis. 2020, 29, 415–420. [Google Scholar] [CrossRef]
- Quach, D.T.; Nguyen, T.A.; Luu, M.N.; Vo, U.P.-P.; Tran, V.L.-T.; Tran, T.L.-T.; Nguyen, T.D.; Le, N.Q.; Hiyama, T.; Tanaka, S. Endocuff Vision-Assisted Colonoscopy Significantly Improves Adenoma Detection in a Shorter Withdrawal Time Compared with Standard Colonoscopy: A Randomized Controlled Trial. Digestion 2025, 106, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Ngu, W.S.; Bevan, R.; Tsiamoulos, Z.P.; Bassett, P.; Hoare, Z.; Rutter, M.D.; Clifford, G.; Totton, N.; Lee, T.J.; Ramadas, A. Improved adenoma detection with Endocuff Vision: The ADENOMA randomised controlled trial. Gut 2019, 68, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Lui, T.K.; Lam, C.P.; To, E.W.; Ko, M.K.-L.; Tsui, V.W.M.; Liu, K.S.-H.; Hui, C.K.-Y.; Cheung, M.K.-S.; Mak, L.L.-Y.; Hui, R.W.-H.; et al. Endocuff with or Without Artificial Intelligence-Assisted Colonoscopy in Detection of Colorectal Adenoma: A Randomized Colonoscopy Trial. Am. J. Gastroenterol. 2024, 119, 1318–1325. [Google Scholar] [CrossRef]
- Spadaccini, M.; Hassan, C.; Rondonotti, E.; Antonelli, G.; Andrisani, G.; Lollo, G.; Auriemma, F.; Iacopini, F.; Facciorusso, A.; Maselli, R.; et al. Combination of Mucosa-Exposure Device and Computer-Aided Detection for Adenoma Detection During Colonoscopy: A Randomized Trial. Gastroenterology 2023, 165, 244–251.e3. [Google Scholar] [CrossRef]
- Kim, J.H.; Wang, J.; Pence, C.; Magahis, P.; Dawod, E.; Schnoll-Sussman, F.; Sharaiha, R.Z.; Wan, D. GI Genius increases small and right-sided adenoma and sessile serrated lesion detection rate when used with EndoCuff in a real-world setting: A retrospective United States study. Clin. Endosc. 2025, 58, 438–447. [Google Scholar] [CrossRef]
- Premier Applied Sciences® PI. Premier Healthcare Database White Paper: Data That Informs and Performs; Premier Applied Sciences® PI: Charlotte, NC, USA, 2020. [Google Scholar]
- Vojtechova, G.; Ngo, O.; Grega, T.; Kmochova, K.; Voska, M.; Buckova, B.; Majek, O.; Zavoral, M.; Suchanek, S. The conversion factor for predicting adenoma detection rate from polyp detection rate varies according to colonoscopy indication and patient sex. Eur. J. Cancer Prev. 2020, 29, 294–302. [Google Scholar] [CrossRef] [PubMed]
- van Keulen, K.E.; Papanikolaou, I.S.; Mak, T.W.C.; Postolopoulos, P.; Neumann, H.; Delconte, G.; Furnari, M.; Peters, Y.; Lau, J.Y.; Polymeros, D.; et al. Comparison of adenoma miss rate and adenoma detection rate between conventional colonoscopy and colonoscopy with second-generation distal attachment cuff: A multicenter, randomized, back-to-back trial. Gastrointest. Endosc. 2024, 99, 798–808.e3. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Buccino, R.V.; Della Valle, N.; Nacchiero, M.C.; Monica, F.; Cannizzaro, R.; Muscatiello, N. Comparative Efficacy of Colonoscope Distal Attachment Devices in Increasing Rates of Adenoma Detection: A Network & Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 1209–1219.e9. [Google Scholar] [PubMed]
- Aniwan, S.; Vanduangden, K.; Kerr, S.J.; Piyachaturawat, P.; Jangsirikul, S.; Luangsukrerk, T.; Kulpatcharapong, S.; Tiankanon, K.; Kongtab, N.; Wisedopas, N.; et al. Linked color imaging, mucosal exposure device, their combination, and standard colonoscopy for adenoma detection: A randomized trial. Gastrointest. Endosc. 2021, 94, 969–977. [Google Scholar] [CrossRef]
- Pattarajierapan, S.; Tipmanee, P.; Supasiri, T.; Wisedopas, N.; Khomvilai, S. Texture and color enhancement imaging (TXI) plus endocuff vision versus TXI alone for colorectal adenoma detection: A randomized controlled trial. Surg. Endosc. 2023, 37, 8340–8348. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.M.; Tradonsky, A.; Tang, J.; Arnold, R.J.G. Cost-effectiveness of adding Endocuff® to standard colonoscopies for interval colorectal cancer screening. Clin. Outcomes Res. 2019, 11, 487–504. [Google Scholar] [CrossRef] [PubMed]


| Gastroenterologists | Surgeons | |||||
|---|---|---|---|---|---|---|
| Year | SC | EAC | Total | SC | EAC | Total |
| 2018 | 276,101 (99.5%) | 1258 (0.45%) | 277,359 | 70,202 (99.6%) | 251 (0.36%) | 70,453 |
| 2019 | 273,799 (99.5%) | 1314 (0.48%) | 275,113 | 69,340 (99.6%) | 252 (0.36%) | 69,592 |
| 2020 | 166,085 (99.0%) | 1606 (0.96%) | 167,691 | 46,515 (99.4%) | 293 (0.63%) | 46,808 |
| 2021 | 171,683 (99.0%) | 1714 (0.99%) | 173,397 | 47,826 (99.4%) | 283 (0.59%) | 48,109 |
| Gastroenterologists | Surgeons | ||||||
|---|---|---|---|---|---|---|---|
| SC N = 887,669 | EAC N = 5892 | p-Value | SC N = 223,883 | EAC N = 1079 | p-Value | ||
| Mean age ± SD, years | 60.4 ± 8.1 | 59.2 ± 7.8 | <0.001 | 60.3 ± 8.1 | 59.7 ± 7.6 | 0.07 | |
| Age, n (%) | |||||||
| 50–54 | 655,387 (73.8) | 3803 (64.5) | <0.001 | 169,220 (72.4) | 691 (64.0) | <0.001 | |
| 55–64 | 125,576 (14.1) | 1195 (20.3) | 35,595 (15.2) | 228 (21.1) | |||
| 65–69 | 53,754 (6.1) | 493 (8.4) | 14,925 (6.4) | 91 (8.4) | |||
| 70 and over | 52,951 (6.0) | 401 (6.8) | 14,143 (6.0) | 69 (6.4) | |||
| Gender, n (%) | |||||||
| Female | 464,908 (52.4) | 3208 (54.4) | 0.001 | 116,771 (49.9) | 564 (52.3) | 0.29 | |
| Male | 422,146 (47.6) | 2684 (45.6) | 117,087 (50.1) | 515 (47.7) | |||
| Race, n (%) | |||||||
| Black | 122,129 (13.8) | 1896 (32.2) | <0.001 | 23,702 (10.1) | 330 (30.6) | <0.001 | |
| Others | 126,587 (14.3) | 1035 (17.6) | 18,773 (8.0) | 68 (6.3) | |||
| White | 638,952 (72.0) | 2961 (50.3) | 191,408 (81.8) | 681 (63.1) | |||
| Endoscopist Experience Level, n (%) | <0.0001 | <0.0001 | |||||
| ≤500 cases | 175,662 (19.8) | 546 (9.3) | 87,181 (37.3) | 450 (41.7) | |||
| 501–2000 cases | 364,826 (41.1) | 2630 (44.6) | 118,826 (50.8) | 628 (58.2) | |||
| 2000 cases | 347,180 (39.1) | 2716 (46.1) | 27,876 (11.9) | 1 (0.1) | |||
| Variables | EAC Group (N = 6971) | SC Group | p-Value vs. EAC | |||||
|---|---|---|---|---|---|---|---|---|
| All (N = 1,121,551) | Random Selection (N = 6971) | Propensity Score-Matched (N = 20,913) | SC All | SC Random Selection | SC Propensity Score-Matched | |||
| Age, years | <0.0001 | <0.0001 | <0.0001 | |||||
| Mean ± SD | 59.3 ± 7.8 | 60.4 ± 8.1 | 60.3 ± 8.1 | 61.0 ± 7.7 | ||||
| Age Group | <0.0001 | <0.0001 | 0.8006 | |||||
| age 50–54 | 4494 (64.5) | 824,607 (73.5) | 5120 (73.4) | 13,502 (64.6) | ||||
| age 55–64 | 1423 (20.4) | 161,171 (14.4) | 974 (14.0) | 4175 (20.0) | ||||
| age 65–69 | 584 (8.4) | 68,679 (6.1) | 450 (6.5) | 1781 (8.5) | ||||
| age 70 and older | 470 (6.7) | 67,094 (6.0) | 427 (6.1) | 1455 (7.0) | ||||
| Gender | <0.0001 | 0.1269 | 0.8028 | |||||
| Female | 3772 (54.1) | 581,679 (51.9) | 3685 (52.9) | 11,280 (53.9) | ||||
| Male | 3199 (45.9) | 539,233 (48.1) | 3284 (47.1) | 9633 (46.1) | ||||
| Race | <0.0001 | <0.0001 | 0.1703 | |||||
| Black | 2226 (31.9) | 145,831 (13.0) | 888 (12.7) | 6441 (30.8) | ||||
| Other | 1103 (15.8) | 145,360 (13.0) | 926 (13.3) | 3299 (15.8) | ||||
| White | 3642 (52.2) | 830,360 (74.0) | 5157 (74.0) | 11,173 (53.4) | ||||
| Hispanic | <0.0001 | <0.0001 | <0.0001 | |||||
| No | 6246 (89.6) | 883,541 (78.8) | 5560 (79.8) | 19,126 (91.5) | ||||
| Unknown | 311 (4.5) | 159,351 (14.2) | 928 (13.3) | 851 (4.1) | ||||
| Yes | 414 (5.9) | 78,659 (7.0) | 483 (6.9) | 936 (4.5) | ||||
| Marital Status | <0.0001 | 0.0027 | 0.6084 | |||||
| Marry | 4148 (59.5) | 637,978 (56.9) | 4026 (57.8) | 12,307 (58.8) | ||||
| Other | 659 (9.5) | 107,813 (9.6) | 625 (9.0) | 2064 (9.9) | ||||
| Single | 2152 (30.9) | 371,751 (33.1) | 2291 (32.9) | 6513 (31.1) | ||||
| Unknown | 12 (0.2) | 4009 (0.4) | 29 (0.4) | 29 (0.1) | ||||
| Insurance Type | <0.0001 | <0.0001 | 0.8422 | |||||
| Medicare | 2001 (28.7) | 360,008 (32.1) | 2231 (32.0) | 5936 (28.4) | ||||
| Medicaid | 407 (5.8) | 94,894 (8.5) | 612 (8.8) | 1277 (6.1) | ||||
| Commercial | 4309 (61.8) | 608,179 (54.2) | 3785 (54.3) | 12,930 (61.8) | ||||
| Others | 254 (3.6) | 58,470 (5.2) | 343 (4.9) | 770 (3.7) | ||||
| Bed Size | <0.0001 | <0.0001 | <0.0001 | |||||
| 000–099 | 1203 (17.3) | 177,875 (15.9) | 1094 (15.7) | 3742 (17.9) | ||||
| 100–199 | 1068 (15.3) | 206,414 (18.4) | 1294 (18.6) | 3247 (15.5) | ||||
| 200–299 | 2230 (32.0) | 149,994 (13.4) | 910 (13.1) | 6943 (33.2) | ||||
| 300–399 | 802 (11.5) | 158,712 (14.2) | 1011 (14.5) | 1817 (8.7) | ||||
| 400–499 | 24 (0.3) | 141,307 (12.6) | 892 (12.8) | 60 (0.3) | ||||
| 500+ | 1644 (23.6) | 287,249 (25.6) | 1770 (25.4) | 5104 (24.4) | ||||
| Region | <0.0001 | <0.0001 | 0.5703 | |||||
| Midwest | 437 (6.3) | 386,803 (34.5) | 2374 (34.1) | 1396 (6.7) | ||||
| Northeast | 16 (0.2) | 178,199 (15.9) | 1096 (15.7) | 58 (0.3) | ||||
| South | 5815 (83.4) | 434,425 (38.7) | 2749 (39.4) | 17,329 (82.9) | ||||
| West | 703 (10.1) | 122,124 (10.9) | 752 (10.8) | 2130 (10.2) | ||||
| Teaching Hospital | <0.0001 | <0.0001 | 0.0332 | |||||
| Yes | 4617 (66.2) | 618,762 (55.2) | 3881 (55.7) | 14,140 (67.6) | ||||
| No | 2354 (33.8) | 502,789 (44.8) | 3090 (44.3) | 6773 (32.4) | ||||
| Population Density | <0.0001 | 0.0003 | 0.302 | |||||
| Rural | 1447 (20.8) | 202,380 (18.0) | 1278 (18.3) | 4463 (21.3) | ||||
| Urban | 5524 (79.2) | 919,171 (82.0) | 5693 (81.7) | 16,450 (78.7) | ||||
| Endoscopist Experience | <0.0001 | <0.0001 | 0.1345 | |||||
| ≤500 cases | 996 (14.3) | 262,843 (23.4) | 1549 (22.2) | 3131 (15.0) | ||||
| 501–2000 cases | 3258 (46.7) | 483,652 (43.1) | 3050 (43.8) | 9886 (47.3) | ||||
| >2000 cases | 2717 (39.0) | 375,056 (33.4) | 2372 (34.0) | 7896 (37.8) | ||||
| Provider Specialty | <0.0001 | <0.0001 | <0.0001 | |||||
| Surgeons | 1079 (15.5) | 233,883 (20.9) | 1432 (20.5) | 4560 (21.8) | ||||
| Gastroenterologists | 5892 (84.5) | 887,668 (79.1) | 5539 (79.5) | 16,353 (78.2) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheloff, A.Z.; Gross, S.A. Use of Mechanical Enhanced Colonoscopy to Improve Polyp Detection During Colorectal Cancer Screening: A Real-World Healthcare Database Analysis. J. Clin. Med. 2025, 14, 6346. https://doi.org/10.3390/jcm14176346
Cheloff AZ, Gross SA. Use of Mechanical Enhanced Colonoscopy to Improve Polyp Detection During Colorectal Cancer Screening: A Real-World Healthcare Database Analysis. Journal of Clinical Medicine. 2025; 14(17):6346. https://doi.org/10.3390/jcm14176346
Chicago/Turabian StyleCheloff, Abraham Z., and Seth A. Gross. 2025. "Use of Mechanical Enhanced Colonoscopy to Improve Polyp Detection During Colorectal Cancer Screening: A Real-World Healthcare Database Analysis" Journal of Clinical Medicine 14, no. 17: 6346. https://doi.org/10.3390/jcm14176346
APA StyleCheloff, A. Z., & Gross, S. A. (2025). Use of Mechanical Enhanced Colonoscopy to Improve Polyp Detection During Colorectal Cancer Screening: A Real-World Healthcare Database Analysis. Journal of Clinical Medicine, 14(17), 6346. https://doi.org/10.3390/jcm14176346






