The Importance of Dental Treatment in Patients Before Radiotherapy, Chemotherapy, and Cardiac Surgeries: A Narrative Review
Abstract
1. Introduction
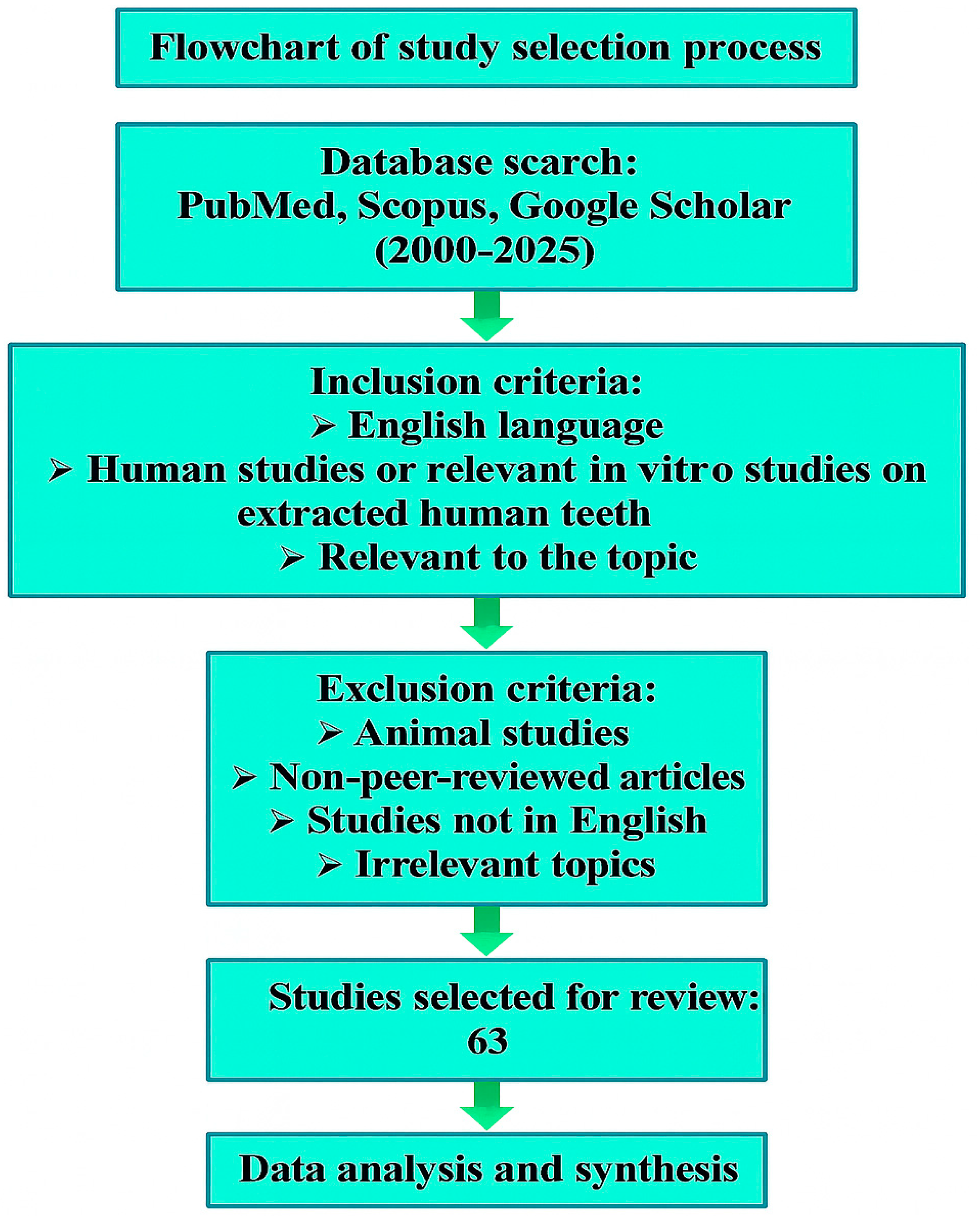
2. Search Strategy and Study Selection
3. Pathophysiology and Identification of Odontogenic Foci
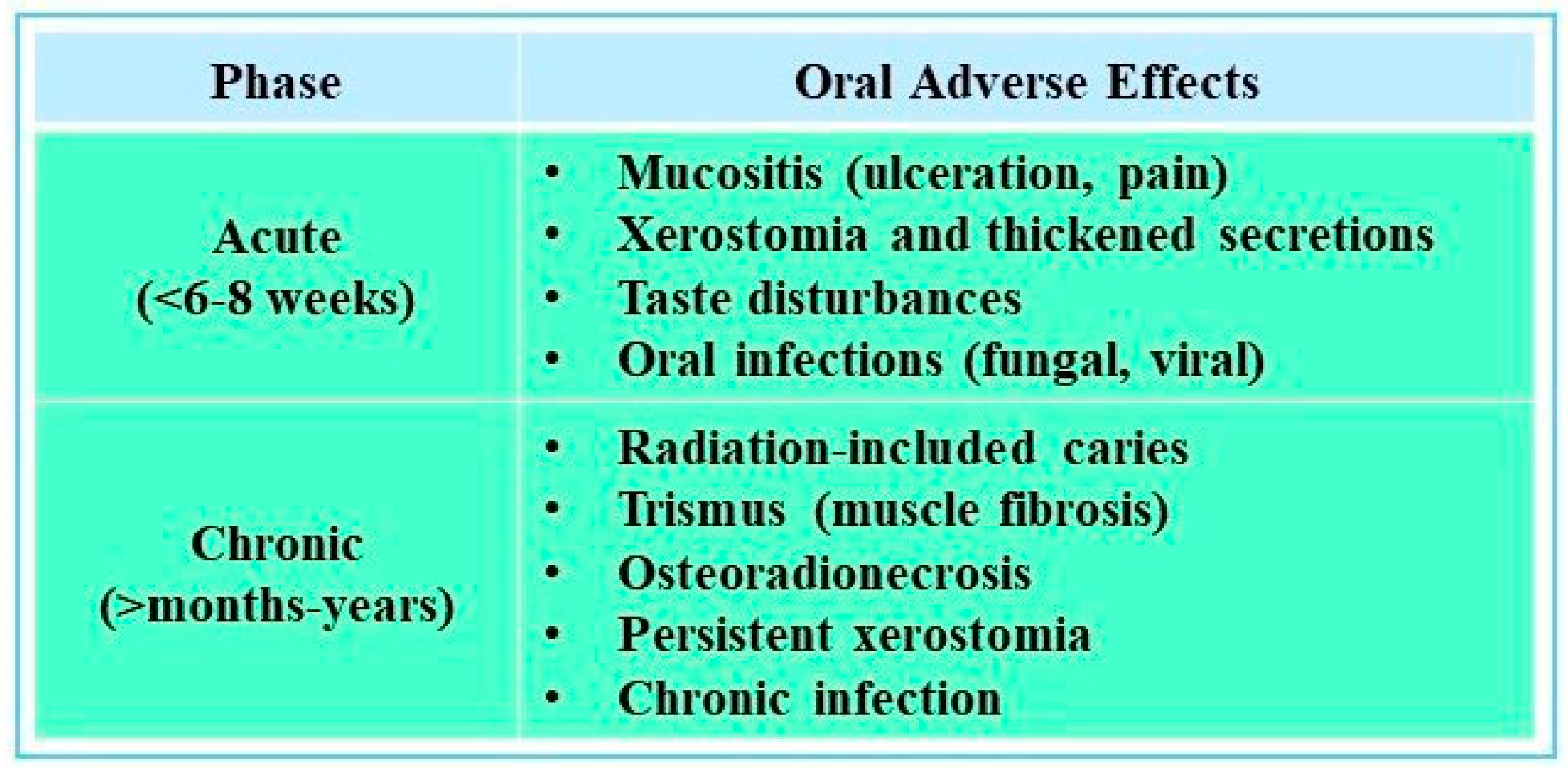
4. Impact of Radiotherapy on Oral Health
4.1. Relationship Between Radiotherapy and Dental Caries
4.2. Importance of Pre-Radiotherapy Dental Assessment and Treatment
4.2.1. Patient Education and Dietary Changes
4.2.2. Fluoride and Remineralizing Agents
4.2.3. The Choice of Dental Restorative Material
4.2.4. Surgical Considerations Pre-RT
5. Impact of Chemotherapy on Oral Health
Role of Pre-Chemotherapy Dental Interventions
6. Oral Health and Cardiovascular Disease
6.1. Profound Dental Caries and Apical Periodontitis as Surgical Risk Factors
6.2. Necessity of Dental Evaluation and Treatment Prior to Surgery
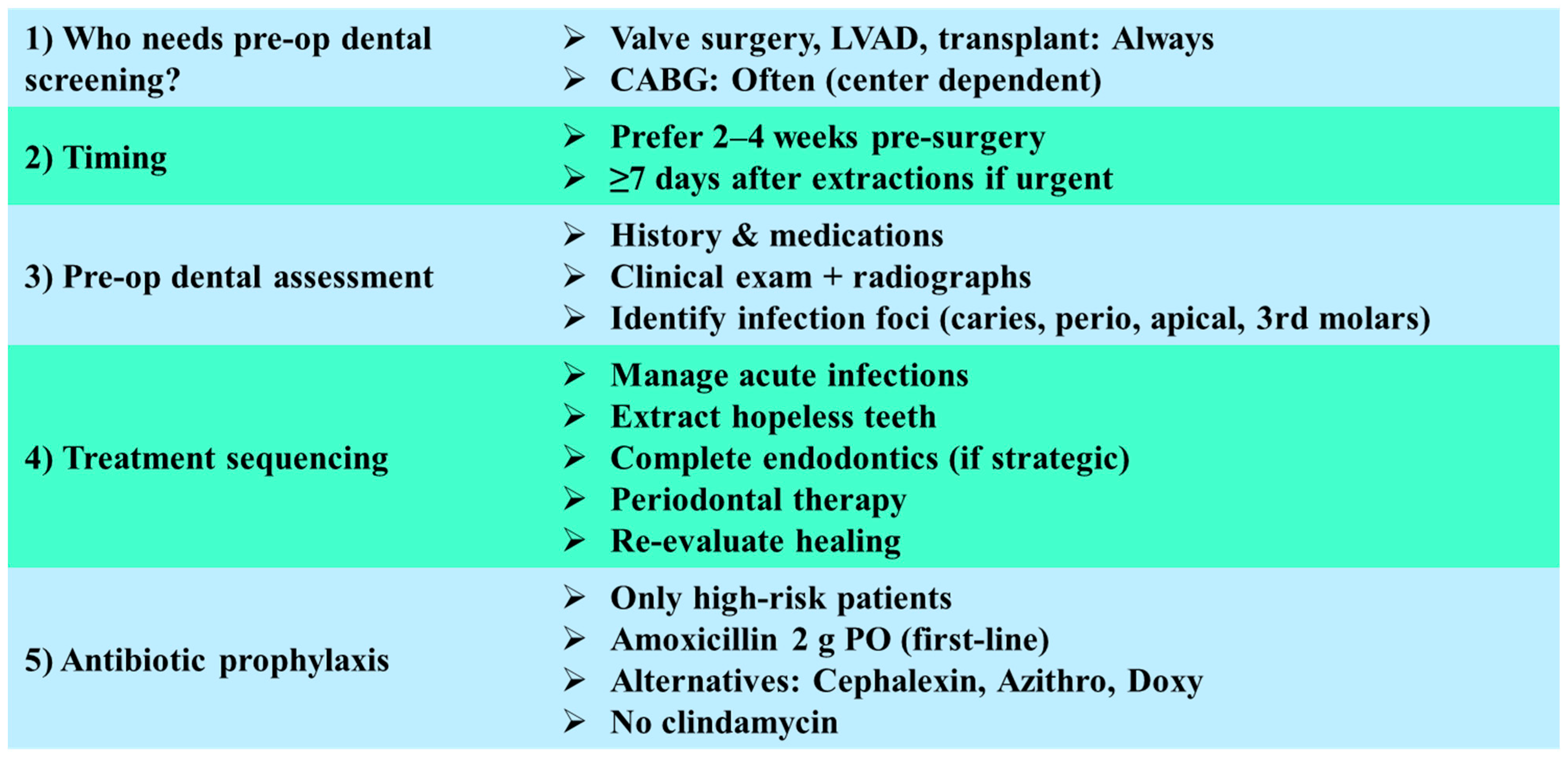
6.2.1. Indications for Preoperative Dental Screening
6.2.2. Timing of Dental Treatment
6.2.3. Scope of Preoperative Dental Assessment
6.2.4. Sequencing of Dental Interventions
- Drainage and management of acute odontogenic infections;
- Extraction of teeth with hopeless prognosis or non-restorable condition;
- Completion of endodontic therapy in teeth with strategic importance and favorable prognosis;
- Periodontal debridement and stabilization of gingival inflammation;
- Re-evaluation of healing status prior to surgical clearance.
6.2.5. Antibiotic Prophylaxis in Dental Procedures
7. Conclusions
- Prioritize acute infections: Conditions such as abscesses, symptomatic apical periodontitis, and severe periodontal disease should be treated promptly, ideally at least 10–14 days before the initiation of surgery or radiotherapy, whenever the medical timeline allows;
- Tailor preventive strategies: Patients scheduled for head and neck radiotherapy should receive targeted preventive measures such as fluoride therapy, dietary counseling, and saliva preserving interventions to reduce the risk of radiation-related caries and osteoradionecrosis. For those undergoing chemotherapy, management should focus on minimizing mucositis and fungal infections, while in cardiovascular patients, preventive care should address bacteremia risk and the potential for infective endocarditis;
- Balance urgency and feasibility: In urgent oncological or cardiac cases, clinicians may need to address acute infections rapidly while deferring management of chronic, asymptomatic conditions. This highlights the importance of interdisciplinary communication between dental, oncology, and cardiology teams;
- Integrate new tools and evidence: Emerging approaches such as adjunctive platelet-rich fibrin (PRF) in extraction sites, advanced imaging for detecting subclinical oral infections, and structured patient education protocols may support more precise, risk-based dental decision making.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Pérez-Mongiovi, D.; Araujo, R. The oral microbiome in health and its implication in oral and systemic diseases. Adv. Appl. Microbiol. 2016, 97, 171–210. [Google Scholar] [CrossRef]
- Marsh, P.D. Contemporary perspective on plaque control. Br. Dent. J. 2012, 212, 601–606. [Google Scholar] [CrossRef]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef]
- Wyszyńska, M.; Nitsze-Wierzba, M.; Czelakowska, A.; Kasperski, J.; Żywiec, J.; Skucha-Nowak, M. An Evidence-Based Review of Application Devices for Nitric Oxide Concentration Determination from Exhaled Air in the Diagnosis of Inflammation and Treatment Monitoring. Molecules 2022, 27, 4279. [Google Scholar] [CrossRef]
- Tassery, H.; Miletic, I.; Turkun, L.S.; Sauro, S.; Gurgan, S.; Banerjee, A.; Basso, M.; Khelafia, S.; Terrer, E.; Pilliol, V.; et al. Preventive management of carious lesions: From non-invasive to micro-invasive operative interventions. Br. Dent. J. 2024, 236, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- White, S.C.; Pharoah, M.J. Oral Radiology: Principles and Interpretation, 7th ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2014; pp. 20–28. ISBN 978-0-323-09633-1. [Google Scholar] [CrossRef][Green Version]
- Eliyas, S.; Al-Khayatt, S.; Porter, R.W.J.; Briggs, P. Dental extractions prior to radiotherapy to the jaws for reducing post-radiotherapy dental complications. Cochrane Database Syst. Rev. 2013, 2013, CD008857. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, B.; Shen, N.; Wu, N.; Sun, J. A systematic review and meta-analysis of the effect of low-level laser therapy (LLLT) on chemotherapy-induced oral mucositis in pediatric and young patients. Eur. J. Pediatr. 2018, 177, 7–17. [Google Scholar] [CrossRef]
- Schrijvers, D.; Cherny, N.I. ESMO clinical practice guidelines on palliative care: Advanced care planning. Ann. Oncol. 2014, 25 (Suppl. S3), iii138–iii142. [Google Scholar] [CrossRef]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef]
- Kruse, C.; Spin-Neto, R.; Evar Kraft, D.C.; Vaeth, M.; Kirkevang, L.L. Diagnostic Accuracy of Cone Beam Computed Tomography Used for Assessment of Apical Periodontitis: An Ex Vivo Histopathological Study on Human Cadavers. Int. Endod. J. 2019, 52, 439–450. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, N.; Weir, M.D.; Reynolds, M.A.; Bai, Y.; Xu, H.H.K. Bioactive Dental Composites and Bonding Agents Having Remineralizing and Antibacterial Characteristics. Dent. Clin. North Am. 2017, 61, 711–733. [Google Scholar] [CrossRef]
- Bali, R.K.; Sharma, P.; Gaba, S.; Kaur, A.; Ghanghas, P. A Review of Complications of Odontogenic Infections. Natl. J. Maxillofac. Surg. 2015, 6, 136–143. [Google Scholar] [CrossRef]
- Ørstavik, D. Essential Endodontology: Prevention and Treatment of Apical Periodontitis, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2020; pp. 1–10. [Google Scholar][Green Version]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Beech, N.; Robinson, S.; Porceddu, S.; Batstone, M. Dental Management of Patients Irradiated for Head and Neck Cancer. Aust. Dent. J. 2014, 59, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.; Newbold, K.; Tanay, M.A.; et al. Parotid-Sparing Intensity Modulated versus Conventional Radiotherapy in Head and Neck Cancer (PARSPORT): A Phase 3 Multicentre Randomised Controlled Trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yahya, N.; Manan, H.A. Quality of Life and Patient-Reported Outcomes Following Proton Therapy for Oropharyngeal Car-cinoma: A Systematic Review. Cancers 2023, 15, 2252. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.T.M.; Aass, H.C.D.; Falk, R.S.; Astrup, G.L.; Helland, Å.; Bjøro, T.; Bjordal, K.; Dale, E.; Hellebust, T.P.; Herlofson, B.B.; et al. Associations between patient-reported late effects and systemic cytokines in long-term survivors of head and neck cancer treated with radiotherapy. J. Cancer Surviv. 2023, 17, 1082–1093. [Google Scholar] [CrossRef]
- Gupta, N.; Pal, M.; Rawat, S.; Grewal, M.S.; Garg, H.; Chauhan, D.; Ahlawat, P.; Tandon, S.; Khurana, R.; Pahuja, A.K.; et al. Radiation-induced dental caries, prevention and treatment—A systematic review. Natl. J. Maxillofac. Surg. 2015, 6, 160–166. [Google Scholar] [CrossRef]
- Müller, V.J.; Belibasakis, G.N.; Bosshard, P.P.; Wiedemeier, D.B.; Bichsel, D.; Rücker, M.; Stadlinger, B. Change of saliva composition with radiotherapy. Arch. Oral Biol. 2019, 106, 104480. [Google Scholar] [CrossRef]
- Lin, A.; Helgeson, E.S.; Treister, N.S.; Schmidt, B.L.; Patton, L.L.; Elting, L.S.; Lalla, R.V.; Brennan, M.T.; Sollecito, T.P. The impact of head and neck radiotherapy on salivary flow and quality of life: Results of the ORARAD study. Oral Oncol. 2022, 127, 105783. [Google Scholar] [CrossRef]
- Silva, A.R.S.; Alves, F.A.; Antunes, A.; Goes, M.F.; Lopes, M.A. Patterns of demineralization and dentin reactions in radiation-related caries. Caries Res. 2009, 43, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.C.; Simon, J.H.; Zhen, W.; Wen, B.C. Long-term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Kawabata, T.; Ando, C.; Sakuma, M.; Aoyama, T.; Ogawa, H.; Yokota, T.; Onozawa, Y.; Mukaigawa, T.; Nishimura, T.; et al. Radiation-induced xerostomia and cariogenic dietary habits. Support Care Cancer 2024, 32, 92. [Google Scholar] [CrossRef] [PubMed]
- Alnaeem, M.M.; Darwish, S.K.; Nashwan, A.J. Radiation-induced xerostomia in head and neck cancers: A comprehensive review for burden and impact on nutrition, sleep, and quality of life. Discov. Med. 2025, 2, 143. [Google Scholar] [CrossRef]
- Sim, C.; Walker, G.D.; Manton, D.J.; Soong, Y.L.; Wee, J.; Adams, G.G.; Reynolds, E.C. Anticariogenic efficacy of a saliva biomimetic in head-and-neck cancer patients undergoing radiotherapy. Aust. Dent. J. 2019, 64, 47–54. [Google Scholar] [CrossRef]
- Shen, P.; Manton, D.J.; Cochrane, N.J.; Walker, G.D.; Yuan, Y.; Reynolds, C.; Reynolds, E.C. Effect of added calcium phosphate on enamel remineralization by fluoride in a randomized controlled in situ trial. J. Dent. 2011, 39, 518–525. [Google Scholar] [CrossRef]
- de Amorim, D.M.G.; Veríssimo, A.H.; Ribeiro, A.K.C.; Souza, R.O.d.A.e.; de Assunção, I.V.; Caldas, M.R.G.R.; Borges, B.C.D. Effects of ionizing radiation on surface properties of current restorative dental materials. J. Mater. Sci. Mater. Med. 2021, 32, 69. [Google Scholar] [CrossRef]
- Atalay, C.; Yazici, A.R. Effect of radiotherapy on the surface roughness and microhardness of contemporary bioactive restorative materials. Support Care Cancer 2024, 32, 295. [Google Scholar] [CrossRef]
- Bennett, M.H.; Feldmeier, J.; Hampson, N.B.; Smee, R.; Milross, C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst. Rev. 2016, 4, CD005005. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, B.; Yang, J.; Wu, M. Effects of platelet-rich fibrin on post-extraction wound healing and wound pain: A meta-analysis. Int. Wound J. 2024, 21, e14654. [Google Scholar] [CrossRef]
- De Pasquale, G.; Mancin, S.; Matteucci, S.; Cattani, D.; Pastore, M.; Franzese, C.; Scorsetti, M.; Mazzoleni, B. Nutritional prehabilitation in head and neck cancer: A systematic review of literature. Clin. Nutr. ESPEN 2023, 58, 326–334. [Google Scholar] [CrossRef]
- Urquhart, O.; DeLong, H.R.; Ziegler, K.M.; Pilcher, L.; Pahlke, S.; Tampi, M.P.; O’Brien, K.K.; Patton, L.L.; Agrawal, N.; Hofstede, T.M.; et al. Effect of Preradiation Dental Intervention on Incidence of Osteoradionecrosis in Patients with Head and Neck Cancer: A Systematic Review and Meta-Analysis. J. Am. Dent. Assoc. 2022, 153, 931–942.e32. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Ponticelli, E.; Clari, M.; Frigerio, S.; De Clemente, A.; Bergese, I.; Scavino, E.; Bernardini, A.; Sacerdote, C. Dysgeusia and health-related quality of life of cancer patients receiving chemotherapy: A cross-sectional study. Eur. J. Cancer Care (Engl.) 2017, 26, e12633. [Google Scholar] [CrossRef]
- Moussa, C.; Estrade, L.; Glomet, J.; Rochefort, G.Y.; Denis, F.; Daou, M.H. The impact of preventive protocols on oral health outcomes in cancer patients undergoing chemotherapy or radiotherapy: A systematic review and meta-analysis. Diseases 2025, 13, 186. [Google Scholar] [CrossRef]
- Brennan, M.T.; Elting, L.S.; Spijkervet, F.K.L. Systematic reviews of oral complications from cancer therapies, Oral Care Study Group, MASCC/ISOO: Methodology and quality of the literature. Support Care Cancer 2010, 18, 979–984. [Google Scholar] [CrossRef]
- Agare, G.I.; Chidike Ezeorba, T.P.; Michael, D.C.; Agbamu, E.; Aghoja, O.C.; Alalor, C.A. Zinc supplementation for mitigating oral mucositis in head and neck cancer patients undergoing radiotherapy and chemoradiotherapy—A systematic review. Clin. Nutr. ESPEN 2025, 67, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Caplan, D.J.; Chasen, J.B.; Krall, E.A.; Cai, J.; Kang, S.; Garcia, R.I.; Offenbacher, S.; Beck, J. Lesions of endodontic origin and risk of coronary heart disease. J. Dent. Res. 2006, 85, 996–1000. [Google Scholar] [CrossRef]
- Robertson, D.; Smith, A.J. The microbiology of the acute dental abscess. J. Med. Microbiol. 2009, 58, 155–162. [Google Scholar] [CrossRef]
- Yong, C.W.; Robinson, A.; Hong, C. Dental evaluation prior to cancer therapy. Front. Oral Health 2022, 3, 876941. [Google Scholar] [CrossRef]
- Schuurhuis, J.M.; Span, L.F.R.; Stokman, M.A.; Van Winkelhoff, A.J.; Vissink, A.; Spijkervet, F.K.L. Effect of leaving chronic oral foci untreated on infectious complications during intensive chemotherapy. Br. J. Cancer 2016, 114, 972–978. [Google Scholar] [CrossRef]
- Spijkervet, F.K.L.; Schuurhuis, J.M.; Stokman, M.A.; Witjes, M.J.H.; Vissink, A. Should Oral Foci of Infection Be Removed Before the Onset of Radiotherapy or Chemotherapy? Oral Dis. 2021, 27, 7–13. [Google Scholar] [CrossRef]
- Zecha, J.A.E.M.; Raber-Durlacher, J.E.; Laheij, A.M.G.A.; Westermann, A.M.; de Lange, J.; Smeele, L.E. The potential contribution of dental foci and oral mucositis to febrile neutropenia in patients treated with myelosuppressive chemotherapy for solid tumors and lymphoma. Front. Oral Health 2022, 3, 940044. [Google Scholar] [CrossRef]
- Lyu, J.; Zhang, Y.; Zhou, R.; Ding, C.; Ye, H.; Fang, Q.; Jiang, C.; Chen, X.; Zhong, L.; Costa, F.O. The effect of periodontal treatments on endothelial function in degrees of periodontitis patients: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0308793. [Google Scholar] [CrossRef]
- León-López, M.; Cabanillas-Balsera, D.; Martín-González, J.; Sánchez-Domínguez, B.; Saúco-Márquez, J.J.; Segura-Egea, J.J. Atherosclerosis and Chronic Apical Periodontitis: Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 1504. [Google Scholar] [CrossRef]
- Koletsi, D.; Iliadi, A.; Tzanetakis, G.N.; Vavuranakis, M.; Eliades, T. Cardiovascular disease and chronic endodontic infection: Is there an association? A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 9111. [Google Scholar] [CrossRef]
- Gomes, M.S.; Blattner, T.C.; Sant’Ana Filho, M.; Grecca, F.S.; Hugo, F.N.; Fouad, A.F.; Reynolds, M.A. Can Apical Periodontitis Modify Systemic Levels of Inflammatory Markers? A Systematic Review and Meta-Analysis. J. Endod. 2013, 39, 1205–1217. [Google Scholar] [CrossRef]
- Cotti, E.; Cairo, F.; Bassareo, P.P.; Fonzar, F.; Venturi, M.; Landi, L.; Parolari, A.; Franco, V.; Fabiani, C.; Barili, F.; et al. Perioperative dental screening and treatment in patients undergoing cardiothoracic surgery and interventional cardiovascular procedures: A consensus report based on RAND/UCLA methodology. Int. Endod. J. 2020, 53, 186–199. [Google Scholar] [CrossRef]
- Al-Abdulla, N.; Bakhsh, A.; Mannocci, F.; Proctor, G.; Moyes, D.; Niazi, S.A. Successful Endodontic Treatment Reduces Serum Levels of Cardiovascular Disease Risk Biomarkers—High-Sensitivity C-Reactive Protein, Asymmetric Dimethylarginine, and Matrix Metalloprotease-2. Int. Endod. J. 2023, 56, 1499–1516. [Google Scholar] [CrossRef]
- Suzuki, H.; Yoshino, K.; Yamashita, Y.; Sugita, N.; Kawabata, T.; Nagao, T. Effects of periodontal treatment on the salivary levels of bacteria associated with periodontal disease. Clin. Exp. Dent. Res. 2019, 5, 485–490. [Google Scholar] [CrossRef]
- Ogawa, M.; Satomi-Kobayashi, S.; Yoshida, N.; Tsuboi, Y.; Komaki, K.; Nanba, N.; Izawa, K.P.; Inoue, T.; Sakai, Y.; Akashi, M.; et al. Impact of Oral Health Status on Postoperative Complications and Functional Recovery After Cardiovascular Surgery. CJC Open 2020, 3, 276–284. [Google Scholar] [CrossRef]
- Lockhart, P.B.; DeLong, H.R.; Lipman, R.D.; Abt, E.; Baddour, L.M.; Colvin, M.; Miller, C.S.; Sollecito, T.; O’Brien, K.; Estrich, C.G.; et al. Effect of dental treatment before cardiac valve surgery: Systematic review and meta-analysis. J. Am. Dent. Assoc. 2019, 150, 739–747.e9. [Google Scholar] [CrossRef]
- Kouwenberg, A.J.; Mensink, G.; Van Geldorp, M.W.A.; Schaap, J.; Bentvelsen, R.G.; Bergsma, J.E. Do dental screening and treatments prior to heart valve interventions help prevent prosthetic valve endocarditis? A systematic review. J. Dent. Probl. Solut. 2022, 9, 055–061. [Google Scholar] [CrossRef]
- Zhou, M.X.; Viozzi, C.F.; Heneberk, O.; Lee, S.K.; Klarich, K.W.; Salinas, T.J. Oral Health Clearance Outcomes for Cardiovascular Surgery. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 121–130. [Google Scholar] [CrossRef]
- Valizadeh, S.; Hosseinzadeh, M. A Review of the Necessity of Preoperative Dental Evaluations in Nonvalvular Open Heart Surgeries. Multidiscip. Cardio-Ann. 2024, 15, e158296. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Baršić, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Iung, B.; Donal, E.; Prendergast, B.; Tornos, P.; Vilacosta, I.; Zamorano, J.L.; Gaemperli, O.; Maggioni, A.P.; et al. 2023 ESC Guidelines for the Management of Endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Thornhill, M.; Michalowicz, B.S.; Noll, J.; Bahrani-Mougeot, F.K.; Sasser, H.C. Poor oral hygiene as a risk factor for infective endocarditis–related bacteremia. J. Clin. Microbiol. 2009, 140, 1238–1244. [Google Scholar] [CrossRef]
- Wilson, W.R.; Gewitz, M.; Lockhart, P.B.; Bolger, A.F.; DeSimone, D.C.; Kazi, D.S.; Couper, D.J.; Beerman, L.B.; Jackson, M.A.; Pahl, E.; et al. Prevention of Viridans Group Streptococcal Infective Endocarditis: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e963–e978. [Google Scholar] [CrossRef]
- Wilson, W.R.; Gewitz, M.; Lockhart, P.B.; Bolger, A.F.; DeSimone, D.C.; Kazi, D.S.; Couper, D.J.; Beerman, L.B.; Jackson, M.A.; Pahl, E.; et al. Prevention of Viridans Group Streptococcal Infective Endocarditis: AHA Scientific Statement. Circulation 2007, 116, 1736–1754. [Google Scholar] [CrossRef] [PubMed]
| Chronic Oral Foci of Infection | Acute Oral Foci of Infection |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafavi, S.; Wyszyńska, M.; Skucha-Nowak, M. The Importance of Dental Treatment in Patients Before Radiotherapy, Chemotherapy, and Cardiac Surgeries: A Narrative Review. J. Clin. Med. 2025, 14, 6330. https://doi.org/10.3390/jcm14176330
Mostafavi S, Wyszyńska M, Skucha-Nowak M. The Importance of Dental Treatment in Patients Before Radiotherapy, Chemotherapy, and Cardiac Surgeries: A Narrative Review. Journal of Clinical Medicine. 2025; 14(17):6330. https://doi.org/10.3390/jcm14176330
Chicago/Turabian StyleMostafavi, Seyedamirreza, Magdalena Wyszyńska, and Małgorzata Skucha-Nowak. 2025. "The Importance of Dental Treatment in Patients Before Radiotherapy, Chemotherapy, and Cardiac Surgeries: A Narrative Review" Journal of Clinical Medicine 14, no. 17: 6330. https://doi.org/10.3390/jcm14176330
APA StyleMostafavi, S., Wyszyńska, M., & Skucha-Nowak, M. (2025). The Importance of Dental Treatment in Patients Before Radiotherapy, Chemotherapy, and Cardiac Surgeries: A Narrative Review. Journal of Clinical Medicine, 14(17), 6330. https://doi.org/10.3390/jcm14176330








