Intercostal Artery Screening with Color Doppler Thoracic Ultrasound in Pleural Procedures: A Potential Yet Underexplored Imaging Modality for Minimizing Iatrogenic Bleeding Risk in Interventional Pulmonology
Abstract
1. Introduction
2. Methods
3. Anatomical Overview of Intercostal Arteries: Variations and Clinical Implications
3.1. Gross Anatomy and Common Variants of the Intercostal Arteries
3.2. Radiological Assessment of Intercostal Artery Anatomy
4. Pleural Diseases and Interventional Procedures: Clinical Context and Techniques
4.1. Epidemiology and Clinical Spectrum of Pleural Diseases
4.2. Overview of Interventional Pulmonology Techniques
5. Assessment of Hemorrhagic Risk and Strategies for Prevention in Pleural Interventions
5.1. Overview of Common Complications Associated with Pleural Procedures
5.2. Incidence and Severity of Hemorrhagic Complications in Pleural Interventions
5.3. Procedural Safety Measures and Risk Mitigation for Hemorrhagic Complications in Pleural Interventions
6. Principles and Techniques of Color Doppler Thoracic Ultrasound Imaging
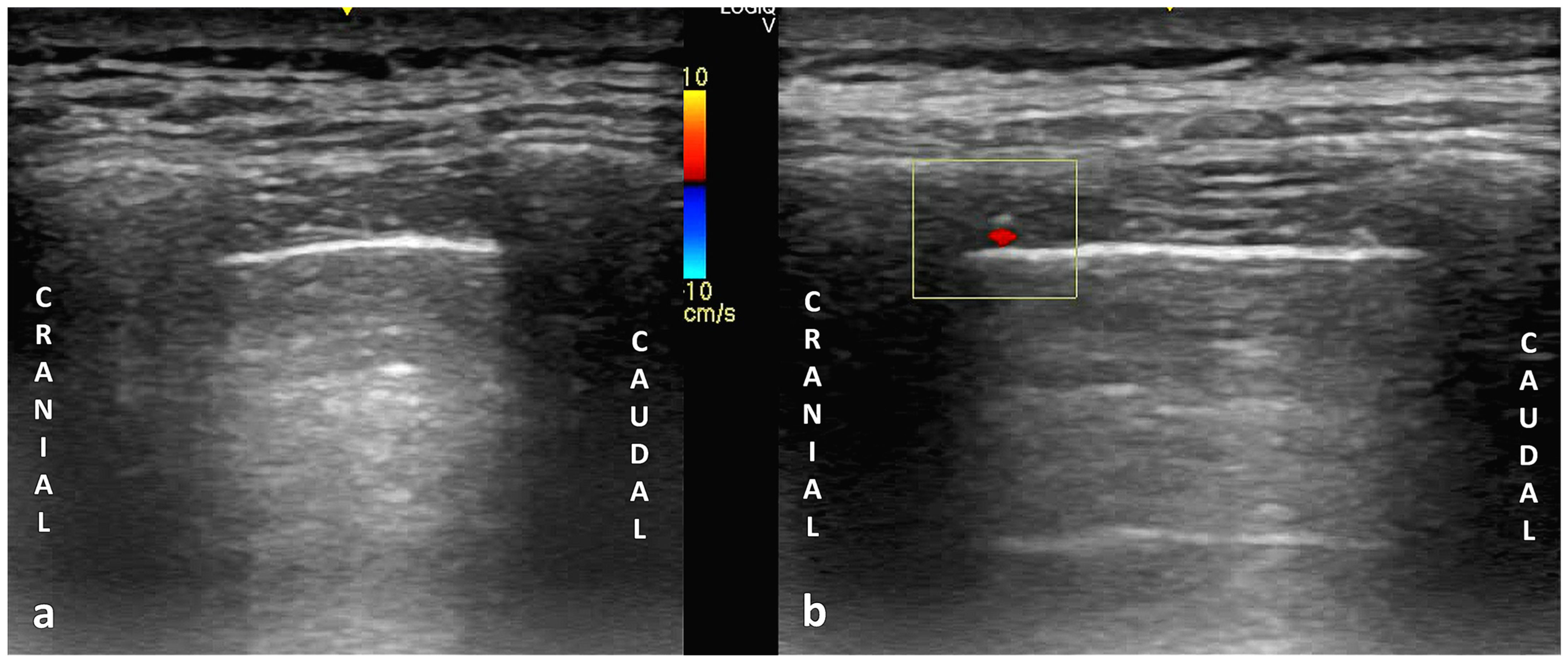
6.1. Principles of Color Doppler Imaging in Thoracic Ultrasound
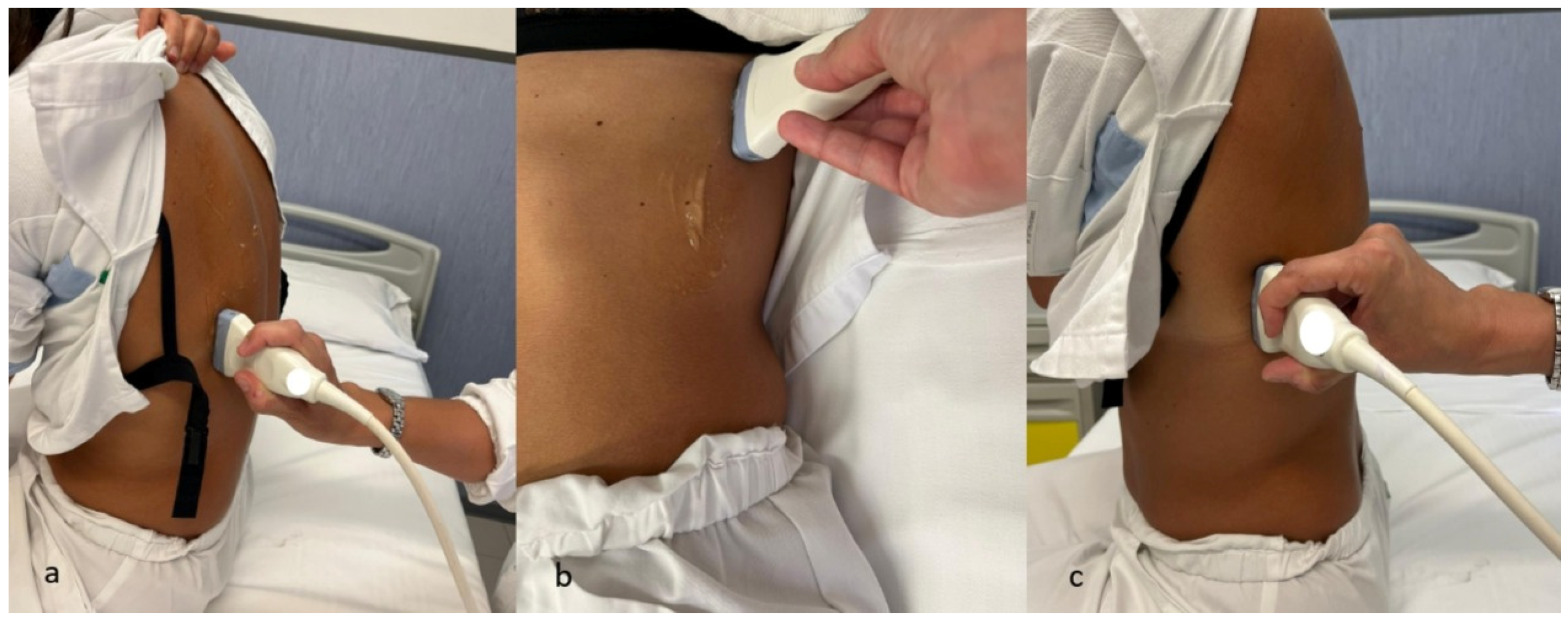
6.2. Technical Parameters in Ultrasonography: Probe Selection, Machine Configuration, and Scanning Techniques
7. Summary and Analysis of Major Studies Assessing Color Doppler Screening Prior to Pleural Procedures
| Study | Year | Design | Population | Procedure Type | Main Findings | Limitations |
|---|---|---|---|---|---|---|
| Koyanagi et al. [13] | 2009 | Observational cohort | 12 healthy young male volunteers | Transthoracic Doppler sonography | Demonstrated feasibility of visualizing intercostal vessels and assessing arterial flow using Doppler ultrasound in healthy subjects. | Small, homogeneous sample; no procedural outcomes evaluated. |
| Salamonsen et al. [14] | 2012 | Prospective cohort | 22 patients undergoing US-guided thoracentesis | Thoracentesis | ICA identified in 74/88 intercostal spaces; most frequently located centrally (28% width); notable anatomical variability observed. | Small sample size; absence of standardized protocol; no outcome assessment. |
| Salamonsen et al. [105] | 2013 | Prospective cohort | 50 patients; LUS + CT angiography | Thoracic ultrasound | Portable US had 86% sensitivity and 30% specificity vs. CT angiography; improved to 95%/97% using a “protected zone” model (ICA in upper 15%); scan time < 1 min; 77% performed by non-radiologists. | No clinical outcome correlation; moderate sample size; limited to one imaging protocol. |
| Bedawi et al. [10] | 2020 | Prospective cohort | 596 patients undergoing pleural procedures | Thoracentesis, pleural biopsy | ICA screening attempted in 95%, successful in 53%; site changed in 16% overall and 30% when ICA-visualized; low complication rate (0.17%); performed by non-radiologists using low-frequency probes. | Observational design; no direct correlation with bleeding risk; linear probes not assessed. |
| Fraser et al. [106] | 2025 | Protocol proposal | Not applicable | Pleural procedures | Introduced the DIVOT protocol for standardized ICA screening using Doppler ultrasound, including patient positioning, probe technique, and safety zones. | No clinical validation; not yet tested in a prospective or multicenter study. |
8. Limitations, Challenges, and Barriers in the Implementation of Color Doppler Thoracic Ultrasound in Pleural Procedures
9. Future Perspectives and Research Priorities
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACCP | American College of Chest Physicians |
| AI | Artificial Intelligence |
| AIA | Anterior Intercostal Artery |
| ALARA | As Low As Reasonably Achievable |
| ATS | American Thoracic Society |
| BTS | British Thoracic Society |
| CDUS | Color Doppler Thoracic Ultrasound |
| CECT | Contrast-Enhanced Computed Tomography |
| CTA | CT Angiography |
| DIC | Disseminated Intravascular Coagulation |
| DIVOT | Doppler Imaging for Vascular Orientation in Thoracic Procedures |
| DOACs | Direct Oral Anticoagulants |
| DSA | Digital Subtraction Angiography |
| ERS | European Respiratory Society |
| Fr | French (catheter size) |
| ICA | Intercostal Artery |
| ICD | Intercostal Chest Drain |
| INR | International Normalized Ratio |
| IPC | Indwelling Pleural Catheter |
| ITA | Internal Thoracic Artery |
| LUS | Lung Ultrasound |
| MHz | Megahertz |
| MI | Mechanical Index |
| MRI | Magnetic Resonance Imaging |
| PD | Power Doppler |
| PE | Pleural Effusion |
| PIA | Posterior Intercostal Artery |
| ROI | Region of Interest |
| TUS | Thoracic Ultrasound |
| US | Ultrasound |
| VATS | Video-Assisted Thoracic Surgery |
References
- Carlucci, P.; Trigiani, M.; Mori, P.A.; Mondoni, M.; Pinelli, V.; Casalini, A.G.; Conte, E.G.; Buggio, G.; Villari, L.; Marchetti, G. Competence in pleural procedures. Panminerva Med. 2019, 61, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Tinè, M.; Daverio, M.; Semenzato, U.; Cocconcelli, E.; Bernardinello, N.; Damin, M.; Saetta, M.; Spagnolo, P.; Balestro, E. Pleural clinic: Where thoracic ultrasound meets respiratory medicine. Front. Med. 2023, 10, 1289221. [Google Scholar] [CrossRef]
- Psallidas, I.; Rahman, N.M. Advances in pleural disease. Eur. Respir. Rev. 2016, 25, 108–109. [Google Scholar] [CrossRef]
- Evison, M.; Blyth, K.G.; Bhatnagar, R.; Corcoran, J.; Saba, T.; Duncan, T.; Hallifax, R.; Ahmed, L.; West, A.; Pepperell, J.C.T.; et al. Providing safe and effective pleural medicine services in the UK: An aspirational statement from UK pleural physicians. BMJ Open Respir. Res. 2018, 5, e000307. [Google Scholar] [CrossRef]
- Corcoran, J.P.; Psallidas, I.; Wrightson, J.M.; Hallifax, R.J.; Rahman, N.M. Pleural procedural complications: Prevention and management. J. Thorac. Dis. 2015, 7, 1058–1067. [Google Scholar] [CrossRef]
- Dunscombe, A.O.; Maskell, N.A. Common iatrogenic pleural complications. Curr. Respir. Care Rep. 2012, 1, 82–90. [Google Scholar] [CrossRef][Green Version]
- Laugsand, E.A.; Xanthoulis, A. Management of a life-threatening intercostal artery bleeding, difficult to visualize in open surgery: A case report. J. Surg. Case Rep. 2020, 2020, rjaa444. [Google Scholar] [CrossRef]
- Psallidas, I.; Helm, E.J.; Maskell, N.A.; Yarmus, L.; Feller-Kopman, D.J.; Gleeson, F.V.; Rahman, N.M. Iatrogenic injury to the intercostal artery: Aetiology, diagnosis and therapeutic intervention. Thorax 2015, 70, 802–804. [Google Scholar] [CrossRef]
- Cantey, E.P.; Walter, J.M.; Corbridge, T.; Barsuk, J.H. Complications of thoracentesis: Incidence, risk factors, and strategies for prevention. Curr. Opin. Pulm. Med. 2016, 22, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Bedawi, E.O.; Talwar, A.; Hassan, M.; McCracken, D.J.; Asciak, R.; Mercer, R.M.; Kanellakis, N.I.; Gleeson, F.V.; Hallifax, R.J.; Wrightson, J.M.; et al. Intercostal vessel screening prior to pleural interventions by the respiratory physician: A prospective study of real world practice. Eur. Respir. J. 2020, 55, 1902245. [Google Scholar] [CrossRef] [PubMed]
- Laursen, C.B.; Clive, A.; Hallifax, R.; Pietersen, P.I.; Asciak, R.; Davidsen, J.R.; Bhatnagar, R.; Bedawi, E.O.; Jacobsen, N.; Coleman, C.; et al. European Respiratory Society statement on thoracic ultrasound. Eur. Respir. J. 2021, 57, 2001519. [Google Scholar] [CrossRef]
- Kanai, M.; Sekiguchi, H. Avoiding vessel laceration in thoracentesis: A role of vascular ultrasound with color Doppler. Chest 2015, 147, e5–e7. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, T.; Kawaharada, N.; Kurimoto, Y.; Ito, T.; Baba, T.; Nakamura, M.; Watanebe, A.; Higami, T. Examination of intercostal arteries with transthoracic Doppler sonography. Echocardiography 2010, 27, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Salamonsen, M.; Ellis, S.; Paul, E.; Steinke, K.; Fielding, D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration 2012, 83, 323–329. [Google Scholar] [CrossRef]
- Roberts, M.E.; Rahman, N.M.; Maskell, N.A.; Bibby, A.C.; Blyth, K.G.; Corcoran, J.P.; Edey, A.; Evison, M.; de Fonseka, D.; Hallifax, R.; et al. British Thoracic Society Guideline for pleural disease. Thorax 2023, 78, 1143–1156. [Google Scholar] [CrossRef]
- Gray, H. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 42nd ed.; Standring, S., Anand, N., Tunstall, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-7020-7705-0. [Google Scholar]
- Donley, E.R.; Holme, M.R.; Loyd, J.W. Anatomy, Thorax, Wall Movements. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK526023/ (accessed on 7 August 2025).
- Dewhurst, C.; O’Neill, S.; O’Regan, K.; Maher, M. Demonstration of the course of the posterior intercostal artery on CT angiography: Relevance to interventional radiology procedures in the chest. Diagn. Interv. Radiol. 2012, 18, 221–224. [Google Scholar] [CrossRef]
- Granger, C.J.; Martin, A.R. Anatomy, Thorax, Superior Intercostal Arteries. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK549847/ (accessed on 7 August 2025).
- Elsy, B. Clinically relevant anatomical variations in the posterior intercostal neurovascular bundle. Folia Morphol. 2025; online first. [Google Scholar] [CrossRef]
- Glenesk, N.L.; Rahman, S.; Lopez, P.P. Anatomy, Thorax, Intercostal Nerves. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK538238/ (accessed on 7 August 2025).
- Hawi, J.S.; Jurjus, R.A.; Daouk, H.S.; Ghazi, M.N.; Basset, C.A.; Cappello, F.; Hajj Hussein, I.; Leone, A.; Jurjus, A.R. A Rare Bilateral Variation in the Branches of the Internal Thoracic Artery: A Case Report. Anatomia 2023, 2, 320–327. [Google Scholar] [CrossRef]
- Agnihotri, G.; Mitra, A. A study on origin, termination, and course characteristics of internal thoracic artery relevant to coronary surgeries and reconstructive procedures. Tzu Chi Med. J. 2022, 34, 348–352. [Google Scholar] [CrossRef]
- Rakuša, M.; Kocbek Šaherl, L. Thiel embalming method used for anatomy dissection as an educational tool in teaching human anatomy, in research, and in training in comparison of different methods for long term preservation. Folia Morphol. 2023, 82, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Nathan, H.; Barkay, M.; Orda, R. Anatomical observations on the origin and course of the aortic intercostal arteries. J. Thorac. Cardiovasc. Surg. 1970, 59, 372–375. [Google Scholar] [CrossRef]
- Khan, S.; Haust, M.D. Variations in the aortic origin of intercostal arteries in man. Anat. Rec. 1979, 195, 545–552. [Google Scholar] [CrossRef]
- Da Rocha, R.P.; Vengjer, A.; Blanco, A.; de Carvalho, P.T.; Mongon, M.L.D.; Fernandes, G.J.M. Size of the collateral intercostal artery in adults: Anatomical considerations in relation to thoracocentesis and thoracoscopy. Surg. Radiol. Anat. 2002, 24, 23–26. [Google Scholar] [CrossRef]
- Shimizu, S.; Tanaka, R.; Kan, S.; Suzuki, S.; Kurata, A.; Fujii, K. Origins of the segmental arteries in the aorta: An anatomic study for selective catheterization with spinal arteriography. AJNR Am. J. Neuroradiol. 2005, 26, 922–928. [Google Scholar] [PubMed]
- Kocbek, L.; Krajnc, I.; Anderhuber, F. Anatomical variations of the posterior intercostal arteries and the thoracic vertebral artery. J. Int. Med. Res. 2011, 39, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, E.; Olinger, A. Posterior intercostal artery tortuosity and collateral branch points: A cadaveric study. Folia Morphol. 2012, 71, 245–251. [Google Scholar]
- Kocbek, L.; Rakuša, M. Common trunk of the posterior intercostal arteries from the thoracic aorta: Anatomical variation, frequency, and importance in individuals. Surg. Radiol. Anat. 2018, 40, 465–470. [Google Scholar] [CrossRef]
- Šaherl, L.K.; Gosak, M.; Rakuša, M. Identification and quantitative analysis of branching networks of the posterior intercostal arteries. Anat. Sci. Int. 2020, 95, 508–515. [Google Scholar] [CrossRef]
- Fanselow, N.R.; Wallace, N.; Sehi, D.; Coomar, L.; Martin, J.; Tan, Y.; Daly, D.T. A Case of Multiple Posterior Intercostal Artery Common Trunks in Conjunction with Additional Arterial Variations. Case Rep. Surg. 2021, 2021, 7430752. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Trieu, J.; Ridley, L. Radiological review of intercostal artery: Anatomical considerations when performing procedures via intercostal space. J. Med. Imaging Radiat. Oncol. 2010, 54, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Helm, E.J.; Rahman, N.M.; Talakoub, O.; Fox, D.L.; Gleeson, F.V. Course and variation of the intercostal artery by CT scan. Chest 2013, 143, 634–639. [Google Scholar] [CrossRef]
- Yoshioka, K.; Niinuma, H.; Ehara, S.; Nakajima, T.; Nakamura, M.; Kawazoe, K. MR angiography and CT angiography of the artery of Adamkiewicz: State of the art. Radiographics 2006, 26 (Suppl. S1), S63–S73. [Google Scholar] [CrossRef]
- Maskell, N.A.; Laursen, C.B.; Lee, Y.C.G. Pleural Disease, 1st ed.; European Respiratory Society: Sheffield, UK, 2020. [Google Scholar]
- Light, R.W. Pleural diseases, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; ISBN 978-0-7817-6957-0. [Google Scholar]
- Feller-Kopman, D.; Light, R. Pleural Disease. N. Engl. J. Med. 2018, 378, 740–751. [Google Scholar] [CrossRef]
- Vakil, E.; Taghizadeh, N.; Tremblay, A. The Global Burden of Pleural Diseases. Semin. Respir. Crit. Care Med. 2023, 44, 417–425. [Google Scholar] [CrossRef]
- Mummadi, S.R.; Stoller, J.K.; Lopez, R.; Kailasam, K.; Gillespie, C.T.; Hahn, P.Y. Epidemiology of Adult Pleural Disease in the United States. Chest 2021, 160, 1534–1551. [Google Scholar] [CrossRef]
- Bodtger, U.; Hallifax, R.J. Epidemiology: Why is pleural disease becoming more common? In Pleural Disease; Maskell, N.A., Laursen, C.B., Lee, Y.C.G., Rahman, N.M., Eds.; European Respiratory Society: Sheffield, UK, 2020. [Google Scholar] [CrossRef]
- Nicholson, M.J.; Manley, C.; Ahmad, D. Thoracentesis for the Diagnosis and Management of Pleural Effusions: The Current State of a Centuries-Old Procedure. J. Respir. 2023, 3, 208–222. [Google Scholar] [CrossRef]
- Ferreiro, L.; Suárez-Antelo, J.; Toubes, M.E.; Valdés, L. Thoracentesis in Primary Care. Semergen 2019, 45, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Shen-Wagner, J.; Gamble, C.; MacGilvray, P. Pleural Effusion: Diagnostic Approach in Adults. Am. Fam. Physician 2023, 108, 464–475. [Google Scholar]
- Sorino, C.; Feller-Kopman, D.; Mei, F.; Mondoni, M.; Agati, S.; Marchetti, G.; Rahman, N.M. Chest Tubes and Pleural Drainage: History and Current Status in Pleural Disease Management. J. Clin. Med. 2024, 13, 6331. [Google Scholar] [CrossRef]
- Anderson, D.; Chen, S.A.; Godoy, L.A.; Brown, L.M.; Cooke, D.T. Comprehensive Review of Chest Tube Management: A Review. JAMA Surg. 2022, 157, 269. [Google Scholar] [CrossRef] [PubMed]
- Porcel, J.M. Chest Tube Drainage of the Pleural Space: A Concise Review for Pulmonologists. Tuberc. Respir. Dis. 2018, 81, 106. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Bonifazi, M.; Rota, M.; Cirilli, L.; Grilli, M.; Duranti, C.; Zuccatosta, L.; Bedawi, E.O.; McCracken, D.; Gasparini, S.; et al. Diagnostic Yield and Safety of Image-Guided Pleural Biopsy: A Systematic Review and Meta-Analysis. Respiration 2021, 100, 77–87. [Google Scholar] [CrossRef]
- Benamore, R.E.; Scott, K.; Richards, C.J.; Entwisle, J.J. Image-guided pleural biopsy: Diagnostic yield and complications. Clin. Radiol. 2006, 61, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Cagle, P.T.; Allen, T.C. Pathology of the pleura: What the pulmonologists need to know. Respirology 2011, 16, 430–438. [Google Scholar] [CrossRef]
- Boy, D. Ultrasound-guided pleural biopsy. Eurasian J. Pulmonol. 2023, 25, 1–11. [Google Scholar] [CrossRef]
- Mondoni, M.; Saderi, L.; Trogu, F.; Terraneo, S.; Carlucci, P.; Ghelma, F.; Centanni, S.; Sotgiu, G. Medical thoracoscopy treatment for pleural infections: A systematic review and meta-analysis. BMC Pulm. Med. 2021, 21, 127. [Google Scholar] [CrossRef]
- Pinelli, V.; Clive, A.O. Medical thoracoscopy in 2020: Essential and future techniques. In Pleural Disease; Maskell, N.A., Laursen, C.B., Lee, Y.C.G., Rahman, N.M., Eds.; European Respiratory Society: Sheffield, UK, 2020. [Google Scholar] [CrossRef]
- Avasarala, S.K.; Lentz, R.J.; Maldonado, F. Medical Thoracoscopy. Clin. Chest Med. 2021, 42, 751–766. [Google Scholar] [CrossRef]
- Bhatnagar, R.; Corcoran, J.P.; Maldonado, F.; Feller-Kopman, D.; Janssen, J.; Astoul, P.; Rahman, N.M. Advanced medical interventions in pleural disease. Eur. Respir. Rev. 2016, 25, 199–213. [Google Scholar] [CrossRef]
- Luzzi, V.; Lindahl, A.L.; Tomassetti, S. Indwelling pleural catheters and medical thoracoscopy. Breathe 2025, 21, 240251. [Google Scholar] [CrossRef]
- Marchi, G.; Cucchiara, F.; Gregori, A.; Biondi, G.; Guglielmi, G.; Serradori, M.; Gherardi, M.; Gabbrielli, L.; Pistelli, F.; Carrozzi, L. Thoracic Ultrasound for Pre-Procedural Dynamic Assessment of Non-Expandable Lung: A Non-Invasive, Real-Time and Multifaceted Diagnostic Tool. J. Clin. Med. 2025, 14, 2062. [Google Scholar] [CrossRef] [PubMed]
- Marchi, G. The road less travelled: Thoracic ultrasound, advanced imaging, and artificial intelligence for early diagnosis of non-expandable lung in malignant pleural effusion. Breathe, 2025; in press. [Google Scholar]
- Baguneid, A.; Wijayaratne, T.; Aujayeb, A.; Panchal, R. The Evolution of the Indwelling Pleural Catheter. Pulm. Ther. 2025, 11, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, N.; Fantin, A.; Palou-schwartzbaum, M.; Viterale, G.; Crisafulli, E.; Sartori, G.; Aujayeb, A.; Patrucco, F.; Patruno, V. Exploring the efficacy and advancements of medical pleurodesis: A comprehensive review of current research. Breathe 2024, 20, 240002. [Google Scholar] [CrossRef]
- Mierzejewski, M.; Korczynski, P.; Krenke, R.; Janssen, J.P. Chemical pleurodesis—A review of mechanisms involved in pleural space obliteration. Respir. Res. 2019, 20, 247. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, R.; Piotrowska, H.E.G.; Laskawiec-Szkonter, M.; Kahan, B.C.; Luengo-Fernandez, R.; Pepperell, J.C.T.; Evison, M.D.; Holme, J.; Al-Aloul, M.; Psallidas, I.; et al. Effect of Thoracoscopic Talc Poudrage vs Talc Slurry via Chest Tube on Pleurodesis Failure Rate Among Patients with Malignant Pleural Effusions: A Randomized Clinical Trial. JAMA 2020, 323, 60. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zayas, G.; Molina, S.; Ost, D.E. Sensitivity and complications of thoracentesis and thoracoscopy: A meta-analysis. Eur. Respir. Rev. 2022, 31, 220053. [Google Scholar] [CrossRef] [PubMed]
- Sundaralingam, A.; Bedawi, E.O.; Harriss, E.K.; Munavvar, M.; Rahman, N.M. The Frequency, Risk Factors, and Management of Complications from Pleural Procedures. Chest 2022, 161, 1407–1425. [Google Scholar] [CrossRef]
- Hernandez, M.C.; El Khatib, M.; Prokop, L.; Zielinski, M.D.; Aho, J.M. Complications in tube thoracostomy: Systematic review and meta-analysis. J. Trauma Acute Care Surg. 2018, 85, 410–416. [Google Scholar] [CrossRef]
- Williams, J.G.; Lerner, A.D. Managing complications of pleural procedures. J. Thorac. Dis. 2021, 13, 5242–5250. [Google Scholar] [CrossRef]
- Iyer, N.P.; Reddy, C.B.; Wahidi, M.M.; Lewis, S.Z.; Diekemper, R.L.; Feller-Kopman, D.; Gould, M.K.; Balekian, A.A. Indwelling Pleural Catheter versus Pleurodesis for Malignant Pleural Effusions. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2019, 16, 124–131. [Google Scholar] [CrossRef]
- Bedawi, E.O.; Ricciardi, S.; Hassan, M.; Gooseman, M.R.; Asciak, R.; Castro-Añón, O.; Armbruster, K.; Bonifazi, M.; Poole, S.; Harris, E.K.; et al. ERS/ESTS statement on the management of pleural infection in adults. Eur. Respir. J. 2023, 61, 2201062. [Google Scholar] [CrossRef]
- Zeiler, J.; Idell, S.; Norwood, S.; Cook, A. Hemothorax: A Review of the Literature. Clin. Pulm. Med. 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Azfar Ali, H.; Lippmann, M.; Mundathaje, U.; Khaleeq, G. Spontaneous Hemothorax. Chest 2008, 134, 1056–1065. [Google Scholar] [CrossRef]
- Mahmood, K.; Shofer, S.L.; Moser, B.K.; Argento, A.C.; Smathers, E.C.; Wahidi, M.M. Hemorrhagic complications of thoracentesis and small-bore chest tube placement in patients taking clopidogrel. Ann. Am. Thorac. Soc. 2014, 11, 73–79. [Google Scholar] [CrossRef]
- DiVietro, M.L.; Huggins, J.T.; Angotti, L.B.; Kummerfeldt, C.E.; Nestor, J.E.; Doelken, P.; Sahn, S.A. Pleural Fluid Analysis in Chronic Hemothorax: A Mimicker of Infection. Clin. Med. Insights Case Rep. 2015, 8, 71–76. [Google Scholar] [CrossRef]
- Ault, M.J.; Rosen, B.T.; Scher, J.; Feinglass, J.; Barsuk, J.H. Thoracentesis outcomes: A 12-year experience. Thorax 2015, 70, 127–132. [Google Scholar] [CrossRef]
- Puchalski, J.T.; Argento, A.C.; Murphy, T.E.; Araujo, K.L.B.; Pisani, M.A. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann. Am. Thorac. Soc. 2013, 10, 336–341. [Google Scholar] [CrossRef]
- Aljundi, L.; Chaar, A.; Boshara, P.; Shiari, A.; Gennaoui, G.; Noori, Z.; Girard, C.; Szpunar, S.; Franco-Elizondo, R. Incidence of bleeding in patients on different anticoagulants and antiplatelet therapies undergoing thoracentesis. BMJ Open Respir. Res. 2021, 8, e000874. [Google Scholar] [CrossRef] [PubMed]
- Giri, M.; Dai, H.; Guo, S.; Li, Y.; He, L.; Zhuang, R. Efficacy and Safety of Pleural Cryobiopsy vs. Forceps Biopsy for Evaluation of Undiagnosed Pleural Effusion: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 847146. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.M.; Ali, N.J.; Brown, G.; Chapman, S.J.; Davies, R.J.O.; Downer, N.J.; Gleeson, F.V.; Howes, T.Q.; Treasure, T.; Singh, S.; et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010, 65 (Suppl. S2), ii54–60. [Google Scholar] [CrossRef] [PubMed]
- Mowery, N.T.; Gunter, O.L.; Collier, B.R.; Diaz, J.J.; Haut, E.; Hildreth, A.; Holevar, M.; Mayberry, J.; Streib, E. Practice management guidelines for management of hemothorax and occult pneumothorax. J. Trauma 2011, 70, 510–518. [Google Scholar] [CrossRef]
- Meyer, D.M.; Jessen, M.E.; Wait, M.A.; Estrera, A.S. Early evacuation of traumatic retained hemothoraces using thoracoscopy: A prospective, randomized trial. Ann. Thorac. Surg. 1997, 64, 1396–1400; discussion 1400–1401. [Google Scholar] [CrossRef]
- Velmahos, G.C.; Demetriades, D.; Chan, L.; Tatevossian, R.; Cornwell, E.E.; Yassa, N.; Murray, J.A.; Asensio, J.A.; Berne, T.V. Predicting the need for thoracoscopic evacuation of residual traumatic hemothorax: Chest radiograph is insufficient. J. Trauma 1999, 46, 65–70. [Google Scholar] [CrossRef]
- DuBose, J.; Inaba, K.; Demetriades, D.; Scalea, T.M.; O’Connor, J.; Menaker, J.; Morales, C.; Konstantinidis, A.; Shiflett, A.; Copwood, B.; et al. Management of post-traumatic retained hemothorax: A prospective, observational, multicenter AAST study. J. Trauma Acute Care Surg. 2012, 72, 11–22; discussion 22–24; quiz 316. [Google Scholar] [CrossRef] [PubMed]
- Oğuzkaya, F.; Akçali, Y.; Bilgin, M. Videothoracoscopy versus intrapleural streptokinase for management of post traumatic retained haemothorax: A retrospective study of 65 cases. Injury 2005, 36, 526–529. [Google Scholar] [CrossRef]
- Skeete, D.A.; Rutherford, E.J.; Schlidt, S.A.; Abrams, J.E.; Parker, L.A.; Rich, P.B. Intrapleural tissue plasminogen activator for complicated pleural effusions. J. Trauma 2004, 57, 1178–1183. [Google Scholar] [CrossRef]
- Stiles, P.J.; Drake, R.M.; Helmer, S.D.; Bjordahl, P.M.; Haan, J.M. Evaluation of chest tube administration of tissue plasminogen activator to treat retained hemothorax. Am. J. Surg. 2014, 207, 960–963. [Google Scholar] [CrossRef]
- Feller-Kopman, D.J.; Reddy, C.B.; DeCamp, M.M.; Diekemper, R.L.; Gould, M.K.; Henry, T.; Iyer, N.P.; Lee, Y.C.G.; Lewis, S.Z.; Maskell, N.A.; et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, 839–849. [Google Scholar] [CrossRef]
- Simoff, M.J.; Lally, B.; Slade, M.G.; Goldberg, W.G.; Lee, P.; Michaud, G.C.; Wahidi, M.M.; Chawla, M. Symptom management in patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e455S–e497S. [Google Scholar] [CrossRef]
- Hibbert, R.M.; Atwell, T.D.; Lekah, A.; Patel, M.D.; Carter, R.E.; McDonald, J.S.; Rabatin, J.T. Safety of ultrasound-guided thoracentesis in patients with abnormal preprocedural coagulation parameters. Chest 2013, 144, 456–463. [Google Scholar] [CrossRef]
- Keeling, D.; Tait, R.C.; Watson, H. British Committee of Standards for Haematology Peri-operative management of anticoagulation and antiplatelet therapy. Br. J. Haematol. 2016, 175, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Linder, K.; Epelbaum, O. Percutaneous pleural drainage in patients taking clopidogrel: Real danger or phantom fear? J. Thorac. Dis. 2018, 10, 5162–5169. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Jensen, J.A.; Nielsen, M.B. Ultrasonic colour Doppler imaging. Interface Focus 2011, 1, 490–502. [Google Scholar] [CrossRef]
- Carroll, D.; Knipe, H.; Bickle, I. Color Flow Doppler (Ultrasound). 2019. Available online: https://radiopaedia.org/articles/67339 (accessed on 5 August 2025).
- Magee, P. Essential notes on the physics of Doppler ultrasound. BJA Educ. 2020, 20, 112–113. [Google Scholar] [CrossRef]
- Carroll, D.; Chieng, R.; Howden, W. Spectral Doppler (Ultrasound). 2019. Available online: https://radiopaedia.org/articles/67204 (accessed on 5 August 2025).
- Kitamura, F.; Agolah, D.; Haouimi, A. Power Doppler. 2014. Available online: https://radiopaedia.org/articles/30430 (accessed on 5 August 2025).
- Görg, C.; Bert, T.; Görg, K.; Heinzel-Gutenbrunner, M. Colour Doppler ultrasound mapping of chest wall lesions. Br. J. Radiol. 2005, 78, 303–307. [Google Scholar] [CrossRef]
- Williamson, J.P.; Grainge, C.; Parameswaran, A.; Twaddell, S.H. Thoracic Ultrasound: What Non-radiologists Need to Know. Curr. Pulmonol. Rep. 2017, 6, 39–47. [Google Scholar] [CrossRef]
- Nathani, A.; Keshishyan, S.; Cho, R.J. Advancements in Interventional Pulmonology: Harnessing Ultrasound Techniques for Precision Diagnosis and Treatment. Diagnostics 2024, 14, 1604. [Google Scholar] [CrossRef]
- Corcoran, J.P.; Hew, M.; Maldonado, F.; Koegelenberg, C.F.N. Ultrasound-guided procedures. In Thoracic Ultrasound; Laursen, C.B., Rahman, N.M., Volpicelli, G., Eds.; European Respiratory Society: Sheffield, UK, 2018. [Google Scholar] [CrossRef]
- Colares, P.d.F.B.; Mafort, T.T.; Sanches, F.M.; Monnerat, L.B.; Menegozzo, C.A.M.; Mariani, A.W. Thoracic ultrasound: A review of the state-of-the-art. J. Bras. Pneumol. 2024, 50, e20230395. [Google Scholar] [CrossRef]
- Bartolomé-Solanas, A.; Porta-Vilaró, M.; Soler-Perromat, J.C.; Del Amo, M.; García-Diez, A.I.; Radalov, I.; Cornellas, L.; Pomés Lopez, I.; Isern-Kebschull, J.; Tomás, X. Narrative review of chest wall ultrasound: A practical approach. Quant. Imaging Med. Surg. 2024, 14, 7983–8000. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, H.; Arahata, M.; Temaru, R.; Ishizaka, S.; Minami, S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern. Med. 2010, 49, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Demi, L.; Wolfram, F.; Klersy, C.; De Silvestri, A.; Ferretti, V.V.; Muller, M.; Miller, D.; Feletti, F.; Wełnicki, M.; Buda, N.; et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. J. Ultrasound Med. 2023, 42, 309–344. [Google Scholar] [CrossRef] [PubMed]
- Wraight, W.M.; Tweedie, D.J.; Parkin, I.G. Neurovascular anatomy and variation in the fourth, fifth, and sixth intercostal spaces in the mid-axillary line: A cadaveric study in respect of chest drain insertion. Clin. Anat. 2005, 18, 346–349. [Google Scholar] [CrossRef]
- Salamonsen, M.; Dobeli, K.; McGrath, D.; Readdy, C.; Ware, R.; Steinke, K.; Fielding, D. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology 2013, 18, 942–947. [Google Scholar] [CrossRef]
- Fraser, A.; Brenner, D.S.; Coghlan, M.; Andrade, H.; Haouili, M.; Carlos, W.G.; Jackson, E. The Sound of Safety: DIVOT (Doppler Imaging for Vascular Orientation in Thoracic Procedures) Protocol. POCUS J. 2025, 10, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Asciak, R.; Bedawi, E.O.; Bhatnagar, R.; Clive, A.O.; Hassan, M.; Lloyd, H.; Reddy, R.; Roberts, H.; Rahman, N.M. British Thoracic Society Clinical Statement on pleural procedures. Thorax 2023, 78, s43–s68. [Google Scholar] [CrossRef] [PubMed]
- Kloth, C.; Kratzer, W.; Schmidberger, J.; Beer, M.; Clevert, D.A.; Graeter, T. Ultrasound 2020—Diagnostics & Therapy: On the Way to Multimodal Ultrasound: Contrast-Enhanced Ultrasound (CEUS), Microvascular Doppler Techniques, Fusion Imaging, Sonoelastography, Interventional Sonography. Rofo 2021, 193, 23–32. [Google Scholar] [CrossRef]
- Ewertsen, C.; Săftoiu, A.; Gruionu, L.G.; Karstrup, S.; Nielsen, M.B. Real-Time Image Fusion Involving Diagnostic Ultrasound. Am. J. Roentgenol. 2013, 200, W249–W255. [Google Scholar] [CrossRef]
- Bazot, M.; Spagnoli, F.; Guerriero, S. Magnetic resonance imaging and ultrasound fusion technique in gynecology. Ultrasound Obstet. Gyne. 2022, 59, 141–145. [Google Scholar] [CrossRef]
- Gross, J.S.; Yaeger, A.; Tchelepi, H.; Matcuk, G.R. Ultrasound Fusion: Applications in Musculoskeletal Imaging. Life 2023, 13, 1278. [Google Scholar] [CrossRef]
- Sakakibara, J.; Nagashima, T.; Fujimoto, H.; Takada, M.; Ohtsuka, M. A review of MRI (CT)/US fusion imaging in treatment of breast cancer. J. Med. Ultrason. (2001) 2023, 50, 367–373. [Google Scholar] [CrossRef]
- Piazza, M.; Colacchio, E.C.; Bilato, M.J.; Squizzato, F.; Antonello, M. Combining fusion-imaging and intravascular ultrasound guidance to facilitate transcatheter electrosurgical septostomy through preexisting entry tears during endovascular repair of dissecting aneurysms. J. Vasc. Surg. Cases Innov. Tech. 2025, 11, 101818. [Google Scholar] [CrossRef]
- Safai Zadeh, E.; Weide, J.; Dietrich, C.F.; Trenker, C.; Koczulla, A.R.; Görg, C. Diagnostic Accuracy of B-Mode- and Contrast-Enhanced Ultrasound in Differentiating Malignant from Benign Pleural Effusions. Diagnostics 2021, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Safai Zadeh, E.; Görg, C.; Dietrich, C.F.; Görlach, J.; Alhyari, A.; Trenker, C. Contrast-Enhanced Ultrasound for Evaluation of Pleural Effusion: A Pictorial Essay. J. Ultrasound Med. 2022, 41, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.S.; Lee, J.J.; Taha, A.A.; Avgerinos, E.; Chaer, R.A. Vascular applications of contrast-enhanced ultrasound imaging. J. Vasc. Surg. 2017, 66, 266–274. [Google Scholar] [CrossRef]
- Abou Ali, A.N.; Fittipaldi, A.; Rocha-Neves, J.; Ruaro, B.; Benedetto, F.; Al Ghadban, Z.; Simon, G.; Lepidi, S.; D’Oria, M. Clinical applications of contrast-enhanced ultrasound in vascular surgery: State-of-the-art narrative and pictorial review. JVS-Vasc. Insights 2025, 3, 100254. [Google Scholar] [CrossRef]
- Marchi, G.; Mercier, M.; Cefalo, J.; Salerni, C.; Ferioli, M.; Candoli, P.; Gori, L.; Cucchiara, F.; Cenerini, G.; Guglielmi, G.; et al. Advanced imaging techniques and artificial intelligence in pleural diseases: A narrative review. Eur. Respir. Rev. 2025, 34, 240263. [Google Scholar] [CrossRef]
- Addala, D.N.; Rahman, N.M. Man versus Machine in Pleural Diagnostics: Does Artificial Intelligence Provide the Solution? Ann. ATS 2024, 21, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Jang, B.; Gu, K.-M.; Park, Y.S.; Kim, Y.-G.; Cho, J. Differential Diagnosis of Pleural Effusion Using Machine Learning. Ann. ATS 2024, 21, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-Y.; Yin, S.-M.; Shao, M.-M.; Yi, F.-S.; Shi, H.-Z. Machine learning-based Diagnostic model for determining the etiology of pleural effusion using Age, ADA and LDH. Respir. Res. 2025, 26, 170. [Google Scholar] [CrossRef]
- Causio, F.A.; DE Angelis, L.; Diedenhofen, G.; Talio, A.; Baglivo, F. Workshop Participants Perspectives on AI use in medicine: Views of the Italian Society of Artificial Intelligence in Medicine. J. Prev. Med. Hyg. 2024, 65, E285–E289. [Google Scholar] [CrossRef]
- Ishiwata, T.; Yasufuku, K. Artificial intelligence in interventional pulmonology. Curr. Opin. Pulm. Med. 2024, 30, 92–98. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, A.; Bharat, S.; Zheng, M. Automatic ultrasound vessel segmentation with deep spatiotemporal context learning. arXiv 2021, arXiv:2111.02461. [Google Scholar] [CrossRef]
- Nimmagadda, N.; Aboian, E.; Kiang, S.; Fischer, U. The role of artificial intelligence in vascular care. JVS-Vasc. Insights 2025, 3, 100179. [Google Scholar] [CrossRef]
- Alsharqi, M.; Edelman, E.R. Artificial Intelligence in Cardiovascular Imaging and Interventional Cardiology: Emerging Trends and Clinical Implications. J. Soc. Cardiovasc. Angiogr. Interv. 2025, 4, 102558. [Google Scholar] [CrossRef] [PubMed]
- Föllmer, B.; Williams, M.C.; Dey, D.; Arbab-Zadeh, A.; Maurovich-Horvat, P.; Volleberg, R.H.J.A.; Rueckert, D.; Schnabel, J.A.; Newby, D.E.; Dweck, M.R.; et al. Roadmap on the use of artificial intelligence for imaging of vulnerable atherosclerotic plaque in coronary arteries. Nat. Rev. Cardiol. 2024, 21, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Barash, Y.; Livne, A.; Klang, E.; Sorin, V.; Cohen, I.; Khaitovich, B.; Raskin, D. Artificial Intelligence for Identification of Images with Active Bleeding in Mesenteric and Celiac Arteries Angiography. Cardiovasc. Intervent Radiol. 2024, 47, 785–792. [Google Scholar] [CrossRef]
- Tran, Z.; Wilkinson, M.C.; Guardamondo, G.; El-Farra, M.H.; Peetz, A.B.; Tomihama, R.T.; Kiang, S.C. Narrative review on applications of artificial intelligence in vascular trauma. JVS-Vasc. Insights 2025, 3, 100268. [Google Scholar] [CrossRef]
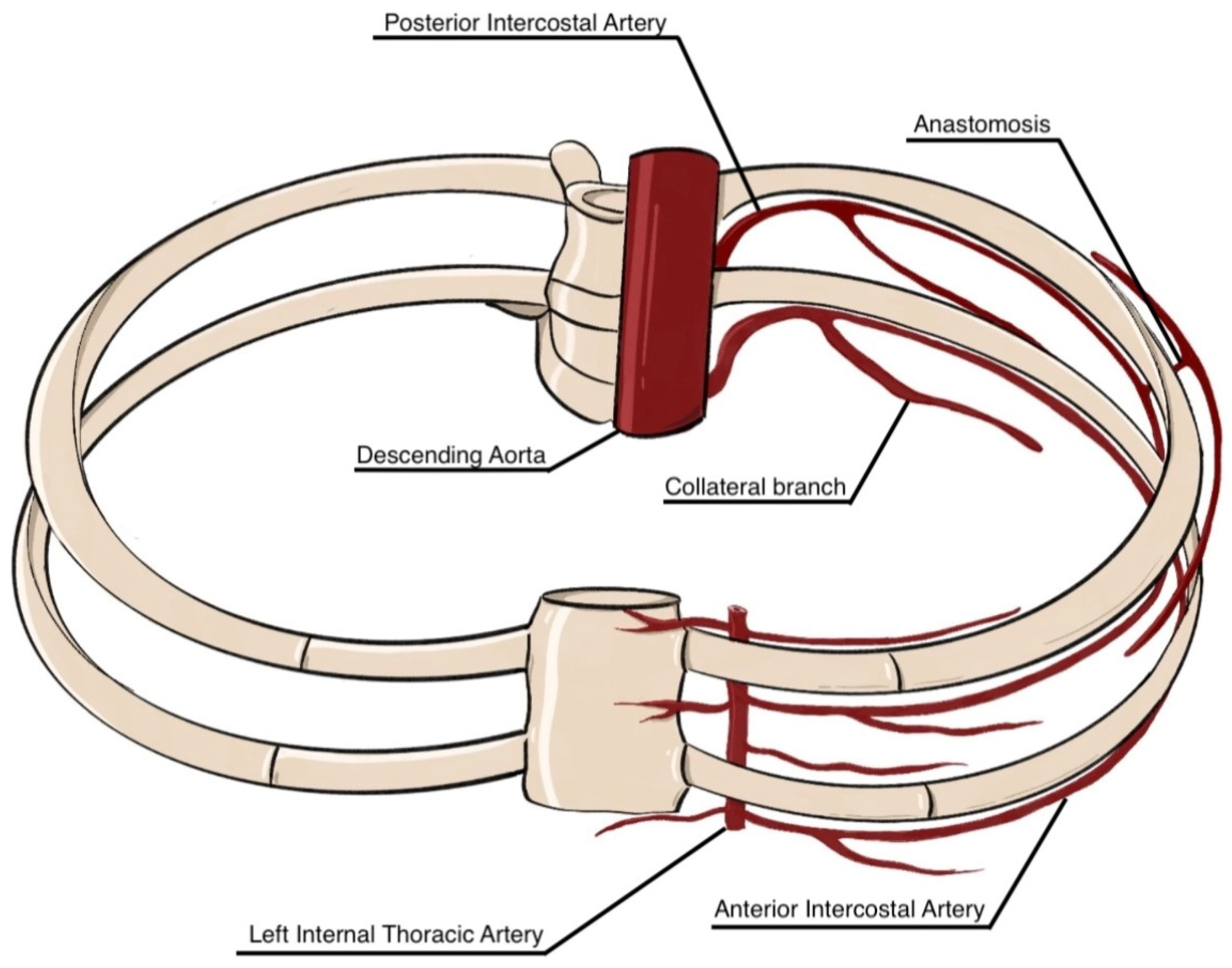
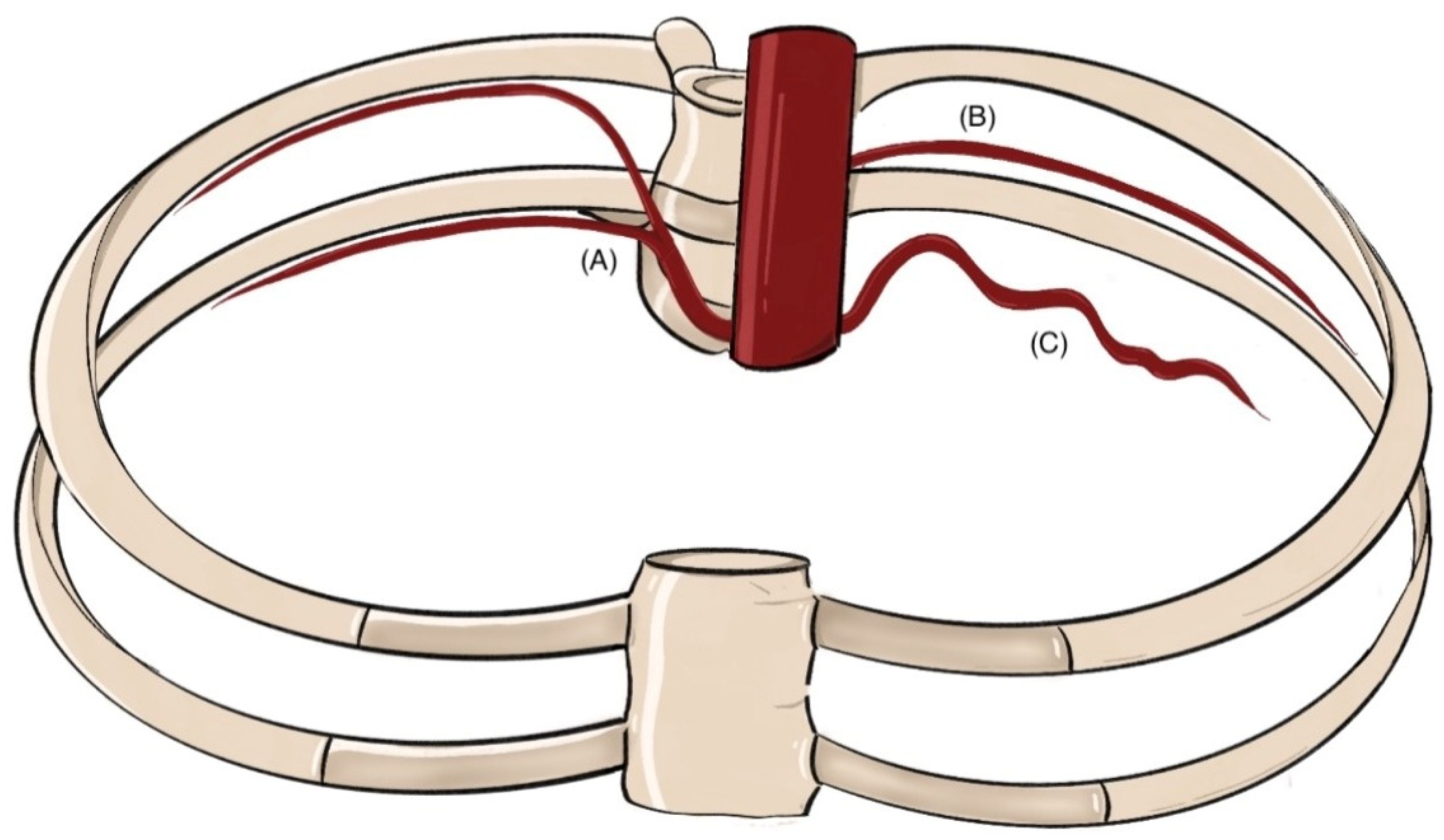



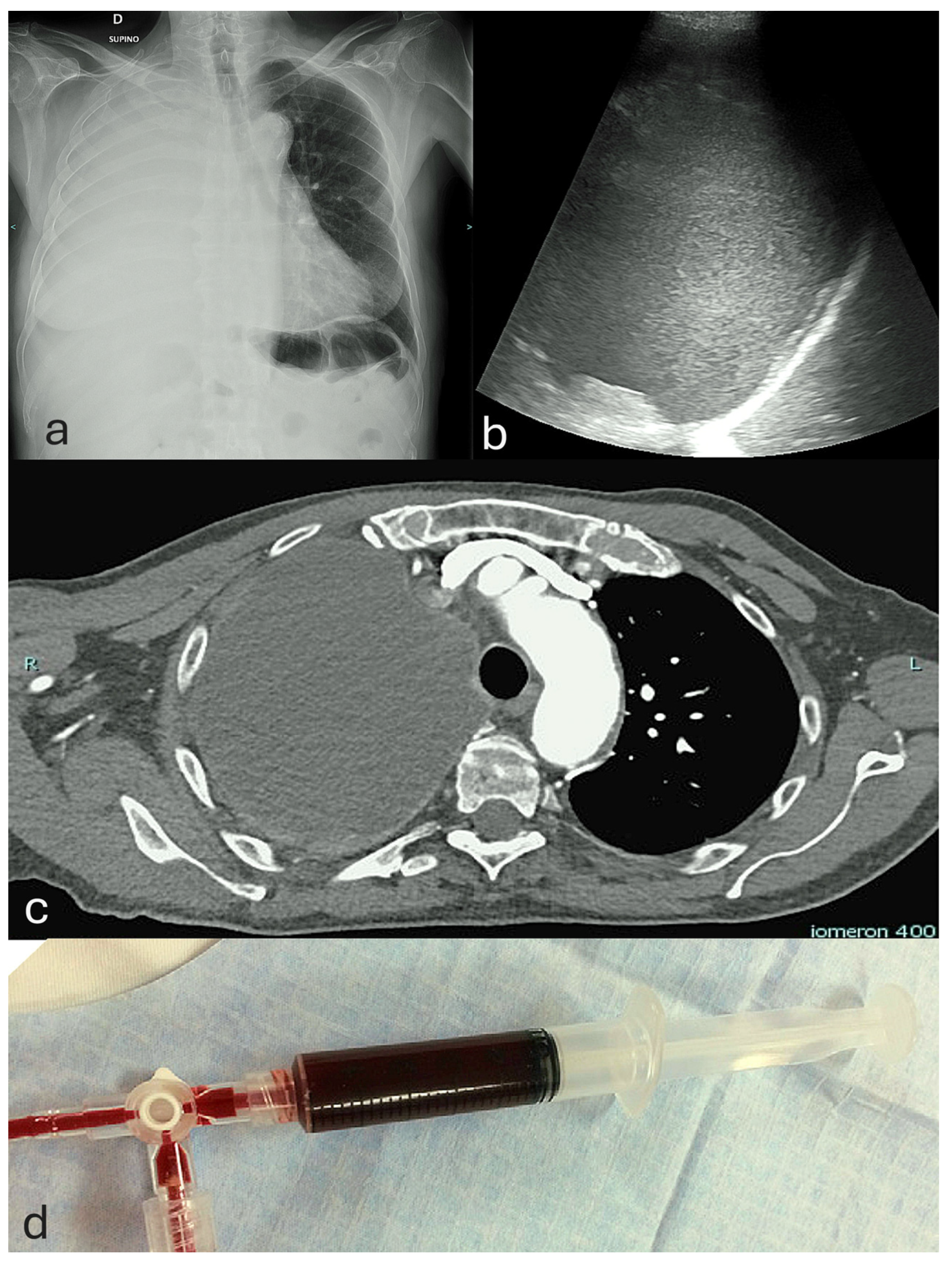
| Authors | Year | Cadaveric Specimens (n) | Age (Mean) | Observed Anatomical Variations in PIAs |
|---|---|---|---|---|
| Nathan et al. [25] | 1970 | 50 | NA | Location of the orifices Course variations |
| Khan and Haust [26] | 1979 | 79 | 7.5 years | Size and location of the orifices Numerosity variations (common trunks) Complete or incomplete division of a single artery beyond its origin |
| Porto da Rocha et al. [27] | 2002 | 90 | 40 years | Origin, size, and topographic relationships of the collateral intercostal arteries and of the posterior intercostal arteries |
| Shimizu et al. [28] | 2005 | 5 | NA | Location of the orifices |
| Kocbek et al. [29] | 2011 | 44 | NA | Origin and course |
| Shurtleff and Olinger [30] | 2012 | 29 | 71 years | Tortuosity Point of origin of collateral branches Dimension of collateral branches |
| Kocbek and Rakuša [31] | 2018 | 44 | NA | Numerosity variations (common trunks) and their prevalence |
| Kocbek Šaherl et al. [32] | 2020 | 43 | NA | Density, position, and networking of collateral branches |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchi, G.; Cinquini, S.; Tannura, F.; Guglielmi, G.; Gelli, R.; Pantano, L.; Cenerini, G.; Wandael, V.; Vivaldi, B.; Coltelli, N.; et al. Intercostal Artery Screening with Color Doppler Thoracic Ultrasound in Pleural Procedures: A Potential Yet Underexplored Imaging Modality for Minimizing Iatrogenic Bleeding Risk in Interventional Pulmonology. J. Clin. Med. 2025, 14, 6326. https://doi.org/10.3390/jcm14176326
Marchi G, Cinquini S, Tannura F, Guglielmi G, Gelli R, Pantano L, Cenerini G, Wandael V, Vivaldi B, Coltelli N, et al. Intercostal Artery Screening with Color Doppler Thoracic Ultrasound in Pleural Procedures: A Potential Yet Underexplored Imaging Modality for Minimizing Iatrogenic Bleeding Risk in Interventional Pulmonology. Journal of Clinical Medicine. 2025; 14(17):6326. https://doi.org/10.3390/jcm14176326
Chicago/Turabian StyleMarchi, Guido, Sara Cinquini, Francesco Tannura, Giacomo Guglielmi, Riccardo Gelli, Luca Pantano, Giovanni Cenerini, Valerie Wandael, Beatrice Vivaldi, Natascia Coltelli, and et al. 2025. "Intercostal Artery Screening with Color Doppler Thoracic Ultrasound in Pleural Procedures: A Potential Yet Underexplored Imaging Modality for Minimizing Iatrogenic Bleeding Risk in Interventional Pulmonology" Journal of Clinical Medicine 14, no. 17: 6326. https://doi.org/10.3390/jcm14176326
APA StyleMarchi, G., Cinquini, S., Tannura, F., Guglielmi, G., Gelli, R., Pantano, L., Cenerini, G., Wandael, V., Vivaldi, B., Coltelli, N., Martinelli, G., Celi, A., Fanni, S. C., Serradori, M., Gherardi, M., Gabbrielli, L., Pistelli, F., & Carrozzi, L. (2025). Intercostal Artery Screening with Color Doppler Thoracic Ultrasound in Pleural Procedures: A Potential Yet Underexplored Imaging Modality for Minimizing Iatrogenic Bleeding Risk in Interventional Pulmonology. Journal of Clinical Medicine, 14(17), 6326. https://doi.org/10.3390/jcm14176326









