Propeller Flaps for Acute Lower Limb Reconstruction After Trauma: Evidence from a Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- Original clinical studies (prospective, retrospective, case series or selected case reports);
- Focused on the use of propeller flaps for soft tissue reconstruction following traumatic injuries in the lower limb;
- Human studies;
- Articles written in English;
- Available as a full text.
- Cadaveric or purely anatomical studies;
- Experimental studies on animals;
- Studies where the propeller flap was used for non-traumatic indications (e.g., oncologic, pressure ulcers, burns, chronic wounds, diabetic foot);
- Reviews, letters, commentaries, expert opinions, and conference abstracts without a full text;
- Articles that did not specify the surgical technique or used ambiguous terminology (e.g., general “local flap” without mention of propeller configuration).
2.1. Data Extraction
2.2. Quality Assessment
2.3. Outcome Definitions
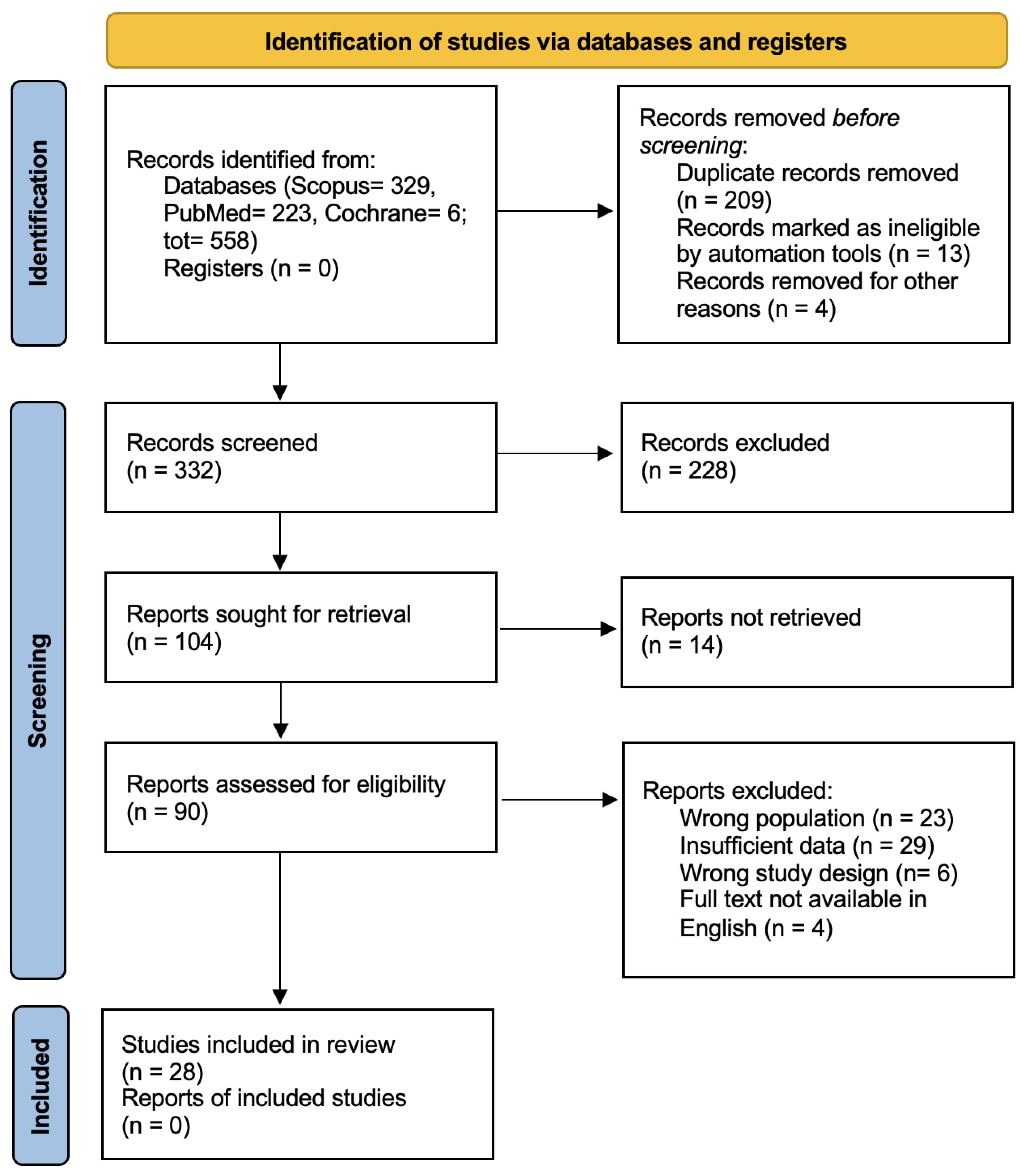
3. Results
- Insufficient data (n = 29),
- Wrong population (n = 23),
- Unsuitable study design (n = 6),
- Full text not available in English (n = 4).
3.1. Descriptive Analysis
3.2. Clinical Outcomes
3.2.1. Flaps Survival and Failure
3.2.2. Postoperative Complications
3.2.3. Donor Site Management
3.2.4. Reintervention
3.2.5. Hospitalization
3.2.6. Follow-Up Duration
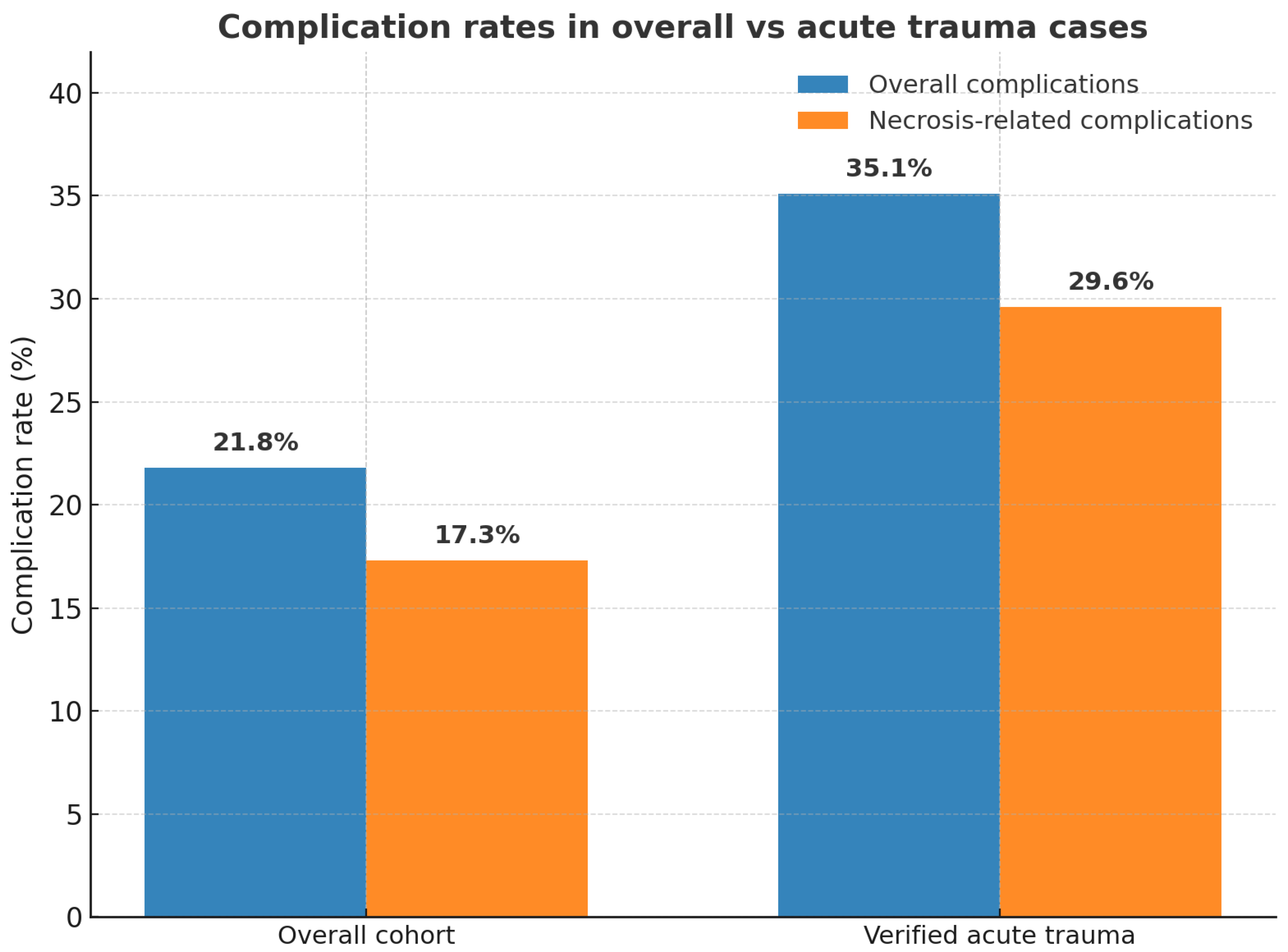
3.2.7. Verified Acute Trauma Subgroup
3.3. Functional Outcomes and Patient Satisfaction
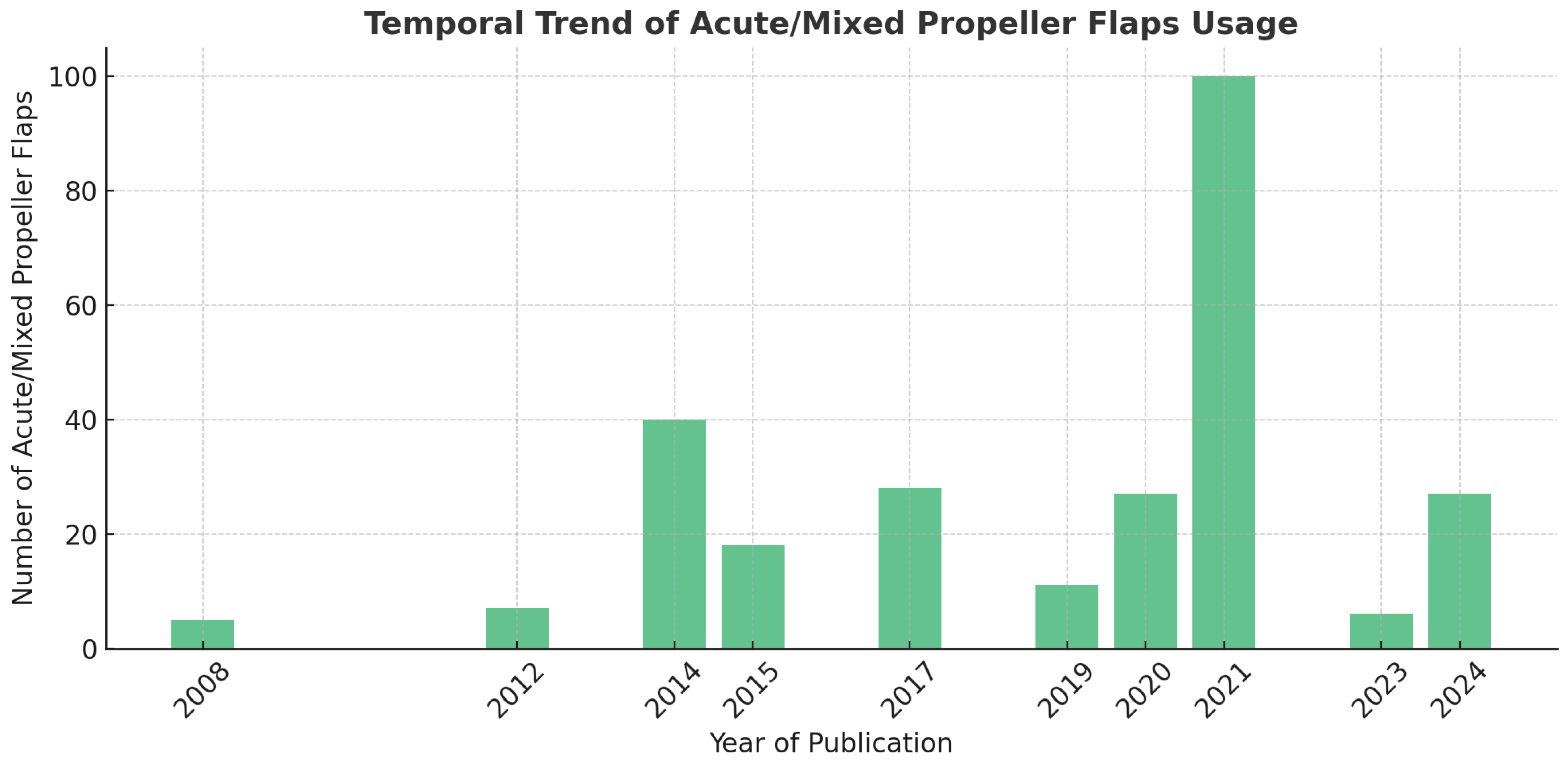
3.4. Trends in Propeller Flap Usage
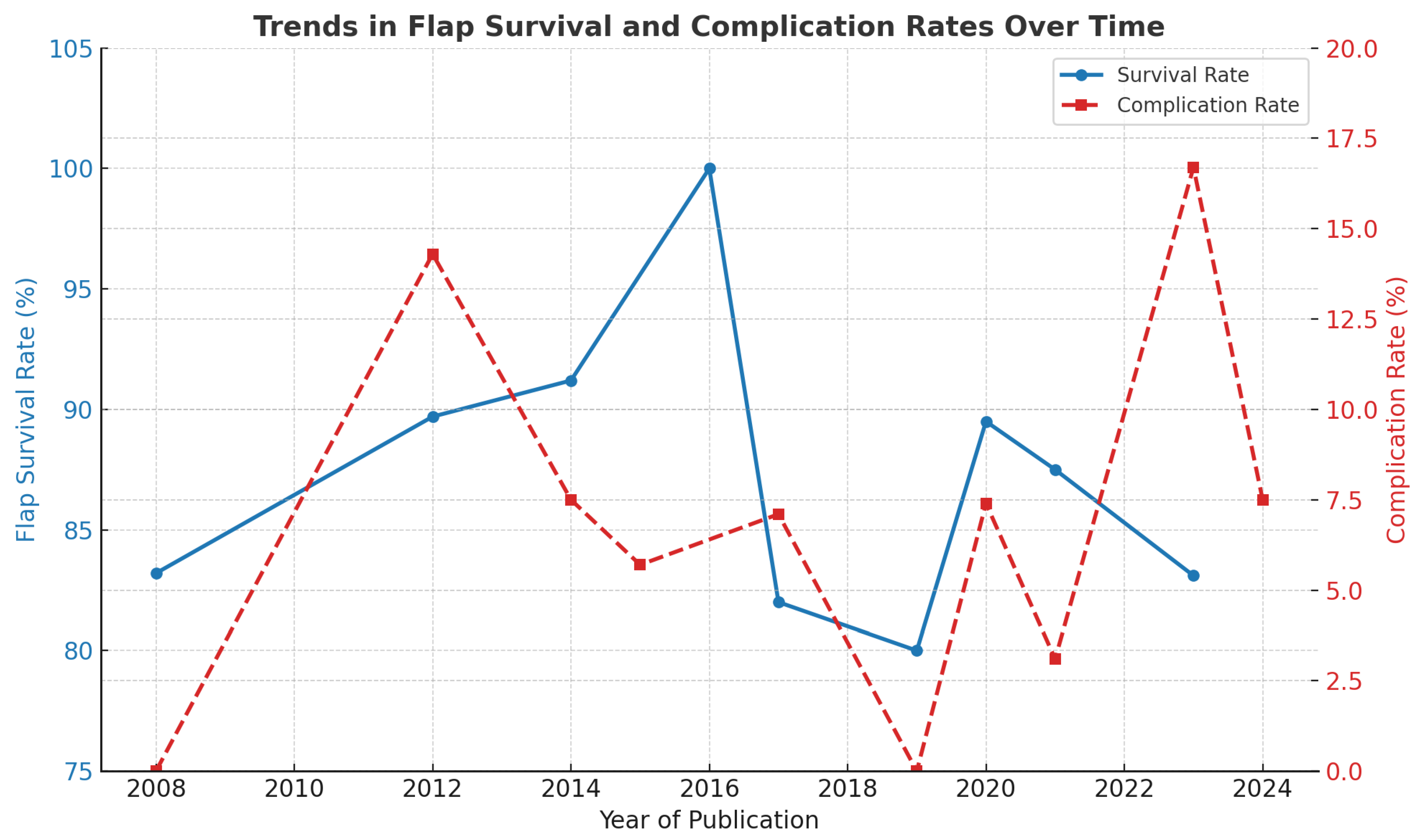
3.5. Complication Rates over Time
3.6. Flap Survival
3.7. Acute vs. Overall Complication Rate
3.8. Technical Factors Influencing Complications
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godina, M. Early microsurgical reconstruction of complex trauma of the extremities. Plast. Reconstr. Surg. 1986, 78, 285–292. [Google Scholar] [CrossRef]
- Gir, P.; Cheng, A.; Oni, G.; Mojallal, A.; Saint-Cyr, M. Pedicled-perforator (propeller) flaps for lower extremity defects: A systematic review. J. Reconstr. Microsurg. 2012, 28, 595–601. [Google Scholar] [CrossRef]
- Koshima, I.; Itoh, S.; Nanba, Y.; Tsutsui, T.; Takahashi, Y. Medial and lateral malleolar perforator flaps for repair of defects around the ankle. Ann. Plast. Surg. 2003, 51, 579–583. [Google Scholar] [CrossRef]
- Ong, Y.S.; Levin, L.S. Lower limb salvage in trauma. Plast. Reconstr. Surg. 2010, 125, 582–588. [Google Scholar] [CrossRef]
- Mathes, S.J.; Nahai, F. Reconstructive Surgery: Principles, Anatomy, and Technique; Churchill Livingstone: London, UK, 1997. [Google Scholar]
- Gopal, S.; Majumder, S.; Batchelor, A.G.; Knight, S.L.; De Boer, P.; Smith, R.M. Fix and flap: The radical orthopaedic and plastic treatment of severe open fractures of the tibia. J. Bone Joint Surg. Br. 2000, 82, 959–966. [Google Scholar] [CrossRef]
- Teo, T.C. The propeller flap concept. Clin. Plast. Surg. 2010, 37, 615–626. [Google Scholar] [CrossRef]
- Geddes, C.R.; Morris, S.F.; Neligan, P.C. Perforator flaps: Evolution, classification, and applications. Ann. Plast. Surg. 2003, 50, 90–99. [Google Scholar] [CrossRef]
- Saint-Cyr, M.; Schaverien, M.V.; Rohrich, R.J. Perforator flaps: History, controversies, physiology, anatomy, and use in reconstruction. Plast Reconstr Surg. 2009, 123 (Suppl. S4), 132e–145e. [Google Scholar] [CrossRef]
- Hallock, G.G. Lower extremity muscle perforator flaps for lower extremity reconstruction. Plast. Reconstr. Surg. 2004, 114, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Masia, J.; Moscatiello, F.; Pons, G.; Fernandez, M.; Lopez, S.; Serret, P. Our experience in lower limb reconstruction with perforator flaps. Ann. Plast. Surg. 2007, 58, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Hyakusoku, H.; Yamamoto, T.; Fumiiri, M. The propeller flap method. Br. J. Plast. Surg. 1991, 44, 53–54. [Google Scholar] [CrossRef]
- Hyakusoku, H.; Ono, S. The Propeller Flap Method—30 Years of Experience and Its Place in Reconstruction. Semin. Plast. Surg. 2020, 34, 133–138. [Google Scholar]
- Pignatti, M.; Ogawa, R.; Hallock, G.G.; Mateev, M.; Georgescu, A.V.; Balakrishnan, G.; Ono, S.; Cubison, T.C.S.; D’Arpa, S.; Koshima, I.; et al. The “Tokyo” consensus on propeller flaps. Plast. Reconstr. Surg. 2011, 127, 716–722. [Google Scholar] [CrossRef]
- Rad, A.N.; Singh, N.K.; Rosson, G.D. Peroneal artery perforator-based propeller flap reconstruction of the lateral distal lower extremity after tumor extirpation: Case report and literature review. Microsurgery 2008, 28, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Bekara, F.; Herlin, C.; Somda, S.; de Runz, A.; Grolleau, J.L.; Chaput, B. Free versus perforator-pedicled propeller flaps in lower extremity reconstruction: What is the safest coverage? A meta-analysis. Microsurgery. 2018, 38, 109–119. [Google Scholar] [CrossRef]
- Lese, I.; Grobbelaar, A.O.; Sabau, D.; Georgescu, A.V.; Constantinescu, M.A.; Olariu, R. The Propeller Flap for Traumatic Distal Lower-Limb Reconstruction: Risk Factors, Pitfalls, and Recommendations. J. Bone Joint Surg. Am. 2020, 102, 510–518. [Google Scholar] [CrossRef]
- Saint-Cyr, M.; Wong, C.; Schaverien, M.; Mojallal, A.; Rohrich, R.J. The perforasome theory: Vascular anatomy and clinical implications. Plast. Reconstr. Surg. 2009, 124, 1529–1544. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Hamilton, S.A.; Fairburn, N.; Rao, P.; Quaba, A.A. Lower limb reconstruction using the islanded posterior tibial artery perforator flap. Plast. Reconstr. Surg. 2010, 125, 1735–1743. [Google Scholar] [CrossRef]
- De Blacam, C.; Colakoglu, S.; Ogunleye, A.A.; Curtis, M.S.; Tobias, A.M.; Lee, B.T. Risk factors associated with complications in lower-extremity reconstruction with the distally based sural flap: A systematic review and pooled analysis. Ann. Plast. Surg. 2009, 63, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Bonetti, M.; Kasmirski, J.A.; Liu, H.Y.; Corcos, A.C.; Ziembicki, J.A.; Stofman, G.M.; Egro, F.M. Impact of Microsurgical Reconstruction Timing on the Risk of Free Flap Loss in Acute Burns: Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2024, 12, e6025. [Google Scholar] [CrossRef] [PubMed]
- Guillier, D.; Raffoul, W.; Egloff, D.V.; Hohlfeld, J.; di Summa, P.G. Lower limb reconstruction involving osteosynthesis material: A retrospective study on propeller flaps outcomes. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, M.; Pasqualini, M.; Governa, M.; Bruti, M.; Rigotti, G. Propeller flaps for leg reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 777–783. [Google Scholar] [CrossRef]
- Innocenti, M.; Menichini, G.; Baldrighi, C.; Delcroix, L.; Vignini, L.; Tos, P. Are there risk factors for complications of perforator-based propeller flaps for lower-extremity reconstruction? Clin. Orthop. Relat. Res. 2014, 472, 2276–2286. [Google Scholar] [CrossRef]
- National Heart, Lung, and Blood Institute (NHLBI). Study Quality Assessment Tools. NHLBI, NIH. 2014. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 4 June 2025).
- Karki, D.; Narayan, R.P. The versatility of perforator-based propeller flap for reconstruction of distal leg and ankle defects. Plast. Surg. Int. 2012, 2012, 303247. [Google Scholar] [CrossRef][Green Version]
- Mateev, M.A.; Kuokkanen, H.O.M. Reconstruction of soft tissue defects in the extremities with a pedicled perforator flap: Series of 25 patients. J. Plast. Surg. Hand Surg. 2012, 46, 32–36. [Google Scholar] [CrossRef]
- Shin, I.S.; Lee, D.W.; Rah, D.K.; Lee, W.J. Reconstruction of pretibial defect using pedicled perforator flaps. Arch. Plast. Surg. 2012, 39, 360–366. [Google Scholar] [CrossRef]
- Chang, S.M.; Wang, X.; Huang, Y.G.; Zhu, X.Z.; Tao, Y.L.; Zhang, Y.Q. Distally based perforator propeller sural flap for foot and ankle reconstruction: A modified flap dissection technique. Ann. Plast. Surg. 2014, 72, 340–345. [Google Scholar] [CrossRef]
- Rogers, A.D.; Dos Passos, G. Propeller flaps for lower limb trauma. South. Afr. J. Surg. 2014, 52, 105–107. [Google Scholar] [CrossRef]
- Zheng, J.; Liao, H.; Li, J.; Zhuo, L.; Ren, G.; Zhang, P.; Hu, J. Double-pedicle propeller flap for reconstruction of the foot and ankle: Anatomical study and clinical applications. J. Int. Med. Res. 2019, 47, 4775–4786. [Google Scholar] [CrossRef] [PubMed]
- Ademola, S.A.; Michael, A.I.; Oladeji, F.J.; Mbaya, K.M.; Oyewole, O. Propeller Flap for Complex Distal Leg Reconstruction: A Versatile Alternative when Reverse Sural Artery Flap is Not Feasible. J. Surg. Tech. Case Rep. 2015, 7, 23–27. [Google Scholar] [CrossRef][Green Version]
- Kang, J.S.; Choi, H.J.; Tak, M.S. Reconstruction of Heel With Propeller Flap in Postfasciotomy and Popliteal Artery Revascularization State. Int. J. Low. Extrem. Wounds 2016, 15, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Lu, S.; Wang, C.; Wen, G.; Han, P.; Chai, Y. Single perforator greater saphenous neuro-veno-fasciocutaneous propeller flaps for lower extremity reconstructions. ANZ J. Surg. 2017, 87, E40–E45. [Google Scholar] [CrossRef]
- Shen, L.; Liu, Y.; Zhang, C.; Guo, Q.; Huang, W.; Wong, K.K.L.; Chang, S. Peroneal perforator pedicle propeller flap for lower leg soft tissue defect reconstruction: Clinical applications and treatment of venous congestion. J. Int. Med. Res. 2017, 45, 1074–1089. [Google Scholar] [CrossRef]
- Cajozzo, M.; Toia, F.; Innocenti, A.; Tripoli, M.; Zabbia, G.; D’Arpa, S.; Cordova, A. Retrospective Analysis in Lower Limb Reconstruction: Propeller Perforator Flaps versus Free Flaps. J. Reconstr. Microsurg. 2017, 33 (Suppl. S1), S34–S39. [Google Scholar] [CrossRef]
- Balakrishnan, T.M.; Ramkumar, J.; Jaganmohan, J. Ad hoc posterior tibial vessels perforator propeller flaps for the reconstruction of lower third leg soft- tissue defects. Indian. J. Plast. Surg. 2017, 50, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Chaput, B.; Bertheuil, N.; Grolleau, J.L.; Bekara, F.; Carloni, R.; Laloze, J.; Herlin, C. Comparison of propeller perforator flap and venous supercharged propeller perforator flap in reconstruction of lower limb soft tissue defect: A prospective study. Microsurgery 2018, 38, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, M.; Dell’Acqua, I.; Famiglietti, M.; Vignini, L.; Menichini, G.; Ghezzi, S. Free perforator flaps vs propeller flaps in lower limb reconstruction: A cost/effectiveness analysis on a series of 179 cases. Injury 2019, 50 (Suppl. S5), S11–S16. [Google Scholar] [CrossRef]
- Franchi, A.; Fritsche, E.; Scaglioni, M.F. Sequential propeller flaps in the treatment of post-traumatic soft tissue defects of the lower limb—a case series. Injury 2020, 51, 2922–2929. [Google Scholar] [CrossRef]
- Valente, A.S.; de Borba, D.F.; de Resende, D.R.; Resende, M.R.; Goulart, R.G.; Lima, S.J. Use of Propeller Flap in the Coverage of Soft-Tissue Injury in the Lower Limb. Rev. Bras. Ortop. 2021, 56, 192–197. [Google Scholar]
- Eldahshoury, T.; Cacciola, R.; El-Gazzar, K. Safety and Vascular Impact of Perforator Propeller Flaps during Distal Lower Limb Reconstruction. Plast. Reconstr. Surg. Glob. Open. 2021, 9, e3993. [Google Scholar] [CrossRef] [PubMed]
- Tapan, M.; Özkan, Ö.; Özkan, Ö. Versatility of the Peroneal Perforator Propeller Sural Flap for Various Types of Injuries in the Ankle and Foot Regions. Ann. Plast. Surg. 2021, 87, e121–e128. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lin, F.; Ma, Y.; Wang, J.; Zhou, M.; Rui, Y. Predictors of the surgical outcome of propeller perforator flap reconstruction, focusing on the effective safe distance between the perforator and the wound edge. BMC Musculoskelet. Disord. 2021, 22, 643. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.J.; Shieh, S.J. Propeller posterior tibial artery perforator flap for reconstruction of the medial malleolar defect: A case report. Formosan J. Surg. 2023, 56, 153–155. [Google Scholar] [CrossRef]
- Gatto, A.; Giacomini, G.; Cavalli, E.M.; Pajardi, G.E.; Marchesi, A. Immediate Soft Tissue Reconstruction in Lower Limb Traumas Using Propeller Perforator Flaps. Ann. Plast. Surg. 2023, 91, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Humnekar, A.; Kala, P.C.; Dixit, P.K.; Katrolia, D.; Karmakar, S.; Singla, P.; Singh, A.P. Propeller vs. free fasciocutaneous flap in reconstruction of complex lower limb defects-A prospective study. J. Plast. Reconstr. Aesthet. Surg. 2024, 93, 235–241. [Google Scholar] [CrossRef]
- Ota, M.; Motomiya, M.; Watanabe, N.; Shimoda, K.; Iwasaki, N. Clinical outcomes of perforator-based propeller flaps versus free flaps in soft tissue reconstruction for lower leg and foot trauma: A retrospective single-centre comparative study. BMC Musculoskelet. Disord. 2024, 25, 297. [Google Scholar] [CrossRef]
- Franchi, A.; Matarazzo, S.; Valdatta, L.; Jung, F. Propeller Flaps and Potential Lymphatic Damage. Plast. Reconstr. Surg. Glob. Open 2024, 12, e6324. [Google Scholar] [CrossRef]
- Binkley, J.M.; Stratford, P.W.; Lott, S.A.; Riddle, D.L. The Lower Extremity Functional Scale (LEFS): Scale development, measurement properties, and clinical application. Phys. Ther. 1999, 79, 371–383. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar]
- Horta, R.; Valença-Filipe, R.; Nascimento, R.; Monteiro, D.; Silva, A.; Amarante, J.M. Perforator-based propeller flap with venous axial supercharging for reconstruction of a leg defect. Injury 2014, 45, 2118–2119. [Google Scholar] [CrossRef]
- Bindu, A.; Ahmad, Q.G.; Jaiswal, D.; Gour, V.; Yadav, P.; Mathews, S.; Kumar, V.; Mantri, M.; Shankhdhar, V.K. Double free-style perforator propeller flaps for large posterior trunk defects post sarcoma excision. Indian J. Plast. Surg. 2025. [Google Scholar] [CrossRef]
- Chaput, B.; Mojallal, A.; Bertheuil, N.; Carloni, R.; Herlin, C.; Sinna, R.; Grolleau, J.; Garrido, I. Delayed procedure in propeller perforator flap: Defining the venous perforasome. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 286–289. [Google Scholar] [CrossRef]
- Özalp, B.; Aydınol, M. Perforator-based propeller flaps for leg reconstruction in pediatric patients. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, e205–e211. [Google Scholar] [CrossRef] [PubMed]
- Muntean, V.; Florescu, I.P.; Cosgarea, M. Preoperative Color Doppler Ultrasonography in Planning Perforator Flaps. Chirurgia 2012, 107, 59–62. [Google Scholar]
- Giunta, R.E.; Geisweid, A.; Feller, A.M. The value of preoperative Doppler sonography for planning free perforator flaps. Plast. Reconstr. Surg. 2000, 105, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Hallock, G.G. Classification of flaps according to their blood supply. J. Reconstr. Microsurg. 2014, 30, 53–64. [Google Scholar]
- Frey, J.D.; Salibian, A.A.; Karp, N.S.; Choi, M. Perforator flap terminology: A survey of microsurgeons to propose a standardized nomenclature. Microsurgery 2019, 39, 521–527. [Google Scholar]
| Author | Study Type | Patient Age (Mean) | Number of Flaps | Etiology of Defect | Location of Defect | Size of the Defect (Longest Side; Mean Area or Range) | Timing of Reconstruction (Acute/Delayd/Mixed) | Flap Type | Source Vessel (Number and Vessel) | Flap Rotation Angle (Grades or Range) | Size of Flap (Longest Side; Mean Area or Range) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pignatti, 2008 [24] | prospective | 52.5 | 6 | 5 trauma, 1 hardware exposure | 3 middle third leg, 1 lower third leg, 1 knee, 1 medial malleolus | 9.8 cm; 89.83 cm2 | mixed | FC AC | NR | 90°, 135°, 180° | 17.8 cm; 216.67 cm2 |
| Karki, 2012 [27] | retrospective | 38 | 20 | 20 trauma | 10 medial malleolus, 7 lateral malleolus, 3 lower third leg | 14–35 cm2 | NR | FC | 14 PTA, 6 PA | 180° | NR |
| Mateev, 2012 [28] | case series | 37 | 11 | 4 trauma, 3 burn, 2 infection, 2 tumor excision | 10 lower third leg, 1 foot | NR | mixed | FC | PTA, PA, LMA, DPA | 180° | 52 cm2 |
| Shin, 2012 [29] | retrospective | 54.3 | 8 | 3 trauma, 2 ulcer, 2 tumor, 1 post-surgical | leg | 5–10 cm | mixed | FC | 5 PTA, 3 PA | 90–180° | 15cm; 135 cm2 |
| Chang, 2014 [30] | case series | 43 | 12 | 6 trauma, 4 infection, 1 tumor excision, 1 pressure sore | 5 foot, 1 lower third leg | 9–66 cm2 | mixed | FC | 4 PTA, 2 PA | 180° | 15.1 cm; 117.78 cm2 |
| Innocenti, 2014 [25] | retrospective | 54 | 66 | 27 trauma, 18 tumor excision, 17 post-surgical | knee, lower third leg, the Achilles region | NR | mixed | FC AC | PTA, ATA, PFA, PA, LCFA, MSGA, LPCA, MPA | <90°, 91–180° | 10–375 cm2 |
| Rogers, 2014 [31] | prospective | 28.9 | 7 | 7 trauma | 3 lateral malleolus, 1 medial malleolus, 2 lower third leg, 1 middle third leg | 20.75 cm2 | mixed | FC | 4 PTA, 7 PA | 90−180° | NR |
| Zheng, 2014 [32] | case series | 37 | 5 | 4 trauma, 1 tumor excision | 5 knee | 18.2 cm; 29.76–191.1 cm2 | NR | FC | DGA | 180° | 104.96 cm2 |
| Ademola, 2015 [33] | case report | 34 | 2 | gunshot | leg | 10 cm | acute | FC | PTA | 90–180° | NR |
| Kang, 2015 [34] | case report | 45 | 1 | trauma | right heel | 4 cm; 16 cm2 | acute | FC | PTA | 180° | NR |
| Zhong, 2015 [35] | case series | 40 | 15 | trauma | leg, foot | NR | acute | FC | PTA | NR | 17 cm; 92.5 cm2 |
| Shen, 2016 [36] | retrospective | 39.7 | 36 | trauma | 12 lower third leg, 24 foot | 8–120 cm2 | NR | FC | PA | 180° | 50-612 cm2 |
| Cajozzo, 2017 [37] | retrospective | 74 | 17 | 9 trauma, 6 tumor excision, 1 infection, 1 diabetic ulcer | 8 lower third leg, 6 middle third of leg, 2 popliteal fossa, 1 knee | 8 cm | mixed | FC | 8 PTA, 7 PA, 2 ATA | 90°, 180° | 24–130 cm2 |
| Balakrishnan, 2017 [38] | retrospective | 38 | 22 | 19 trauma, 2 hardware exposure, 1 bite | lower third leg | mainly small- and medium- sized defects | mixed | FC | PTA | 180° | 9.7 cm; 35.09 cm2 |
| Chaput, 2018 [39] | retrospective | 52.5 | 60 | 38 trauma, 14 infection, 5 tumor excision, 3 burn | 13 middle third leg, 27 lower third leg, 10 foot | NR | NR | FC | 33 PTA, 27 PFA | 120° | 48–58 cm2 |
| Innocenti, 2019 [40] | retrospective | 53 | 79 | 40 trauma, 21 tumor excision, 4 infection, 14 unknown | NR | NR | NR | NR | 36 PTA, 11 PA, 8 PA, 8 PFA, 5 ATA, 4 MPA, 4 CFA, 3 others | NR | 68 cm2 |
| Zheng, 2019 [32] | prospective | 34.94 | 18 | 11 trauma, 3 poor healing, 2 infection, 1 ulcer, 1 tumor excision | 15 foot, 3 malleolar | NR | mixed | AC | PA | NR | 14.3 cm; 53.36 cm2 |
| Franchi, 2020 [41] | case series | 54 | 16 | 8 trauma | 4 lower third leg, 2 foot, 2 thigh | 6 cm; 46.4 cm2 | mixed | FC | 7 PA, 2 PFA, 2 dbLCFA, 3 PTA, 1 ATA, 1 MSA | 150–180° | 12.3 cm; 73.4 cm2 |
| Lese, 2020 [17] | retrospective | 60 | 26 | 8 trauma, 9 post-surgical, 9 infection | lower third leg | 9–36 cm2 | mixed | NR | 12 PTA, 14 PA | 150–180° | 68–144 cm2 |
| Valente, 2020 [42] | retrospective | 36.4 | 14 | trauma | 2 middle third leg, 12 lower third leg | 9 cm2 | mixed | FC | ATA, PTA | 180° | 29 cm2 |
| Eldahshoury, 2021 [43] | retrospective | 45.5 | 23 | 20 trauma, 2 infection, 1 tumor excision | 20 lower third leg, 3 foot | 15–154 cm2 | NR | FC | 11 PTA, 12 PA | NR | NR |
| Guillier, 2021 [23] | retrospective | 55.4 | 21 | 18 trauma, 3 infection | 2 lateral malleolus, 3 foot, 1 middle third leg, 1 medial malleolus, 3 knee, 4 upper third leg, 5 middle third leg, 2 lower third leg | 29.8 cm2 | mixed | FC | NR | 90–180° | NR |
| Tapan, 2021 [44] | case series | 37.5 | 11 | 5 trauma, 1 gunshot, 1 ulcer, 1 tumor, 1 burn, 2 post-surgical | 7 ankle, 4 foot | 5–10 cm | mixed | FC | PA | 90–180° | 16 cm; 208 cm2 |
| Wang, 2021 [45] | retrospective | 36.5 | 82 | 62 trauma, 20 infection | 11 middle third leg, 32 lower third leg, 31 medial malleolus, 8 lateral malleolus | 10.6 cm | mixed | FC | 12 ATA, 62 PTA, 8 PA | <150°, 151–180° | 15.6 cm; 60.7 cm2 |
| Chiang, 2023 [46] | case report | 20 | 1 | trauma | leg | 12 cm; 72 cm2 | acute | FC | PTA | 90–180° | 12 cm; 60 cm2 |
| Gatto, 2023 [47] | retrospective | 33.8 | 5 | trauma | 4 lateral malleolus, 1 middle third leg | 5–10 cm | acute | FC | 4 ATA, 1 PA | 90–170 ° | 12 cm |
| Humnekar, 2024 [48] | controlled randommize trial | 31.53 | 17 | 12 trauma | 7 foot, 8 lower third leg, 1 upper third leg, 1 thigh | 20.60 cm2 | mixed | FC | PA, DPA, PTA, PFA | 180° | 41–55 cm2 |
| Ota, 2024 [49] | retrospective | 58 | 18 | 6 trauma,9 post-surgical, 3 infection | 3 middle third leg,13 lower third leg, 2 foot | 24–80 cm2 | acute | FC | PTA, PA | NR | NR |
| Author | Number of Flaps | Survived Flaps | Complications (Yes/No) | Total Necrosis | Partial Necrosis | Other Complications |
|---|---|---|---|---|---|---|
| Pignatti, 2008 [24] | 6 | 5 | Y | 0 | 1 | 0 |
| Karki, 2012 [27] | 20 | 19 | Y | 0 | 1 | 1 wound dehiscence |
| Mateev, 2012 [28] | 11 | 10 | Y | 1 | 0 | 0 |
| Shin, 2012 [29] | 12 | 5 | Y | 0 | 1 | 0 |
| Chang, 2014 [30] | 66 | 65 | Y | 1 | 7 | 0 |
| Innocenti, 2014 [25] | 7 | 7 | Y | 0 | 1 | 0 |
| Rogers, 2014 [31] | 5 | 5 | N | 0 | 0 | 0 |
| Zheng, 2014 [32] | 2 | 2 | Y | 0 | 0 | 0 |
| Ademola, 2015 [33] | 1 | 1 | N | 0 | 0 | 0 |
| Kang, 2015 [34] | 15 | 11 | Y | 0 | 1 | 1 infection |
| Zhong, 2015 [35] | 36 | 36 | Y | 0 | 9 | 1 hematoma, 1 infection |
| Shen, 2016 [36] | 22 | 19 | Y | 0 | 0 | 1 hematoma |
| Cajozzo, 2017 [37] | 17 | 13 | Y | 0 | 4 | 2 wound dehiscence, 1 infection |
| Balakrishnan, 2017 [38] | 60 | 41 | Y | 3 | 10 | 1 infection |
| Chaput, 2018 [39] | 79 | 76 | Y | 3 | 13 | 4 wound dehiscence |
| Innocenti, 2019 [40] | 18 | 11 | Y | 0 | 1 | 2 wound dehiscence |
| Zheng, 2019 [32] | 16 | 14 | Y | 0 | 2 | 1 hematoma |
| Franchi, 2020 [41] | 26 | 23 | Y | 1 | 2 | NR |
| Lese, 2020 [17] | 14 | 12 | Y | 0 | 2 | 1 wound dehiscence, 2 hematoma |
| Valente, 2020 [42] | 23 | 21 | Y | 1 | 1 | 1 wound dehiscence |
| Eldahshoury, 2021 [43] | 21 | 13 | Y | 1 | 6 | 1 wound dehiscence, 1 hematoma |
| Guillier, 2021 [23] | 11 | 9 | Y | 1 | 1 | 0 |
| Tapan, 2021 [44] | 8 | 6 | Y | 0 | 0 | 0 |
| Wang, 2021 [45] | 82 | 65 | Y | 6 | 11 | 11 infection |
| Chiang, 2023 [46] | 1 | 1 | N | 0 | 0 | 0 |
| Gatto, 2023 [47] | 5 | 4 | N | 0 | 1 | 0 |
| Humnekar, 2024 [48] | 18 | 6 | Y | 2 | 7 | NR |
| Ota, 2024 [49] | 17 | 14 | Y | 3 | 3 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matarazzo, S.; Corsini, B.; Cozzi, S.; Tellarini, A.; Valdatta, L.; Paganini, F. Propeller Flaps for Acute Lower Limb Reconstruction After Trauma: Evidence from a Systematic Review. J. Clin. Med. 2025, 14, 6288. https://doi.org/10.3390/jcm14176288
Matarazzo S, Corsini B, Cozzi S, Tellarini A, Valdatta L, Paganini F. Propeller Flaps for Acute Lower Limb Reconstruction After Trauma: Evidence from a Systematic Review. Journal of Clinical Medicine. 2025; 14(17):6288. https://doi.org/10.3390/jcm14176288
Chicago/Turabian StyleMatarazzo, Sara, Beatrice Corsini, Silvia Cozzi, Annachiara Tellarini, Luigi Valdatta, and Ferruccio Paganini. 2025. "Propeller Flaps for Acute Lower Limb Reconstruction After Trauma: Evidence from a Systematic Review" Journal of Clinical Medicine 14, no. 17: 6288. https://doi.org/10.3390/jcm14176288
APA StyleMatarazzo, S., Corsini, B., Cozzi, S., Tellarini, A., Valdatta, L., & Paganini, F. (2025). Propeller Flaps for Acute Lower Limb Reconstruction After Trauma: Evidence from a Systematic Review. Journal of Clinical Medicine, 14(17), 6288. https://doi.org/10.3390/jcm14176288





