Assessment of Residual Cortical Function by Using Tc-99m DMSA SPECT at Follow-Up in Non-Operatively Treated Patients with Traumatic Renal Injuries: A Prospective Single-Centre Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Non-Operative Management for Renal Injury (Indications and Technique of RAE)
2.3. Data Collection and Assessment of Residual Cortical Function
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrone, P.; Perez-Calvo, J.; Brathwaite, C.E.M.; Islam, S.; Joseph, D.K. Traumatic kidney injuries: A systematic review and meta-analysis. Int. J. Surg. 2020, 74, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Moore, E.E.; Kluger, Y.; Biffl, W.; Leppaniemi, A.; Matsumura, Y.; Kim, F.; Peitzman, A.B.; Fraga, G.P.; Sartelli, M.; et al. Kidney and uro-trauma: WSES-AAST guidelines. World J. Emerg. Surg. 2019, 14, 54. [Google Scholar] [CrossRef]
- Santucci, R.A.; Wessells, H.; Bartsch, G.; Descotes, J.; Heyns, C.F.; McAninch, J.W.; Nash, P.; Schmidlin, F. Evaluation and management of renal injuries: Consensus statement of the renal trauma subcommittee. BJU Int. 2004, 93, 937–954. [Google Scholar] [CrossRef]
- Serafetinidis, E.; Campos-Juanatey, F.; Hallscheidt, P.; Mahmud, H.; Mayer, E.; Schouten, N.; Sharma, D.M.; Waterloos, M.; Zimmermann, K.; Kitrey, N.D. Summary Paper of the Updated 2023 European Association of Urology Guidelines on Urological Trauma. Eur. Urol. Focus 2024, 10, 475–485. [Google Scholar] [CrossRef]
- Bryk, D.J.; Zhao, L.C. Guideline of guidelines: A review of urological trauma guidelines. BJU Int. 2016, 117, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Saour, M.; Charbit, J.; Millet, I.; Monnin, V.; Taourel, P.; Klouche, K.; Capdevila, X. Effect of renal angioembolization on post-traumatic acute kidney injury after high-grade renal trauma: A comparative study of 52 consecutive cases. Injury 2014, 45, 894–901. [Google Scholar] [CrossRef]
- Mohsen, T.; El-Assmy, A.; El-Diasty, T. Long-term functional and morphological effects of transcatheter arterial embolization of traumatic renal vascular injury. BJU Int. 2008, 101, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.S.; Green, M.C. Comparison of short- and long-term functional outcome of nonoperatively managed renal injuries in children. J. Pediatr. Surg. 2009, 44, 144–147, discussion 147. [Google Scholar] [CrossRef]
- Liguori, G.; Rebez, G.; Larcher, A.; Rizzo, M.; Cai, T.; Trombetta, C.; Salonia, A. The role of angioembolization in the management of blunt renal injuries: A systematic review. BMC Urol. 2021, 21, 104. [Google Scholar] [CrossRef]
- Aguayo, P.; Fraser, J.D.; Sharp, S.; Holcomb, G.W., 3rd; Ostlie, D.J.; St Peter, S.D. Nonoperative management of blunt renal injury: A need for further study. J. Pediatr. Surg. 2010, 45, 1311–1314. [Google Scholar] [CrossRef]
- Moog, R.; Becmeur, F.; Dutson, E.; Chevalier-Kauffmann, I.; Sauvage, P.; Brunot, B. Functional evaluation by quantitative dimercaptosuccinic Acid scintigraphy after kidney trauma in children. J. Urol. 2003, 169, 641–644. [Google Scholar] [CrossRef]
- Fiard, G.; Rambeaud, J.J.; Descotes, J.L.; Boillot, B.; Terrier, N.; Thuillier, C.; Chodez, M.; Skowron, O.; Berod, A.A.; Arnoux, V.; et al. Long-term renal function assessment with dimercapto-succinic acid scintigraphy after conservative treatment of major renal trauma. J. Urol. 2012, 187, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Pereira Junior, G.A.; Muglia, V.F.; Dos Santos, A.C.; Miyake, C.H.; Nobre, F.; Kato, M.; Simoes, M.V.; de Andrade, J.I. Late evaluation of the relationship between morphological and functional renal changes and hypertension after non-operative treatment of high-grade renal injuries. World J. Emerg. Surg. 2012, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Vali, R.; Armstrong, I.S.; Bar-Sever, Z.; Biassoni, L.; Borgwardt, L.; Brown, J.; Grant, F.D.; Mandell, G.A.; Majd, M.; Nadel, H.R. SNMMI procedure standard/EANM practice guideline on pediatric [99mTc] Tc-DMSA renal cortical scintigraphy: An update. Clin. Transl. Imaging 2022, 10, 173–184. [Google Scholar] [CrossRef]
- Cho, S.G.; Park, K.S.; Kim, J.; Moon, J.B.; Song, H.C.; Kang, T.W.; Yu, S.H. Tc-99m DMSA SPECT for Follow-Up of Non-Operative Treatments in Renal Injuries: A Prospective Single-Center Study. Korean J. Radiol. 2023, 24, 1017–1027. [Google Scholar] [CrossRef]
- Kozar, R.A.; Crandall, M.; Shanmuganathan, K.; Zarzaur, B.L.; Coburn, M.; Cribari, C.; Kaups, K.; Schuster, K.; Tominaga, G.T.; Committee, A.P.A. Organ injury scaling 2018 update: Spleen, liver, and kidney. J. Trauma Acute Care Surg. 2018, 85, 1119–1122. [Google Scholar] [CrossRef]
- Reichkendler, M.H.; Berg, R.M.G.; de Nijs, R.; Norgaard, H.; Schmidt, I.M.; Borgwardt, L. Planar scan vs. SPECT/low-dose CT for estimating split renal function by (99m)Tc-DMSA scintigraphy in children. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 729–733. [Google Scholar] [CrossRef]
- Hidas, G.; Lupinsky, L.; Kastin, A.; Moskovitz, B.; Groshar, D.; Nativ, O. Functional significance of using tissue adhesive substance in nephron-sparing surgery: Assessment by quantitative SPECT of 99m Tc-Dimercaptosuccinic acid scintigraphy. Eur. Urol. 2007, 52, 785–789. [Google Scholar] [CrossRef]
- Sujenthiran, A.; Elshout, P.J.; Veskimae, E.; MacLennan, S.; Yuan, Y.; Serafetinidis, E.; Sharma, D.M.; Kitrey, N.D.; Djakovic, N.; Lumen, N.; et al. Is Nonoperative Management the Best First-line Option for High-grade Renal trauma? A Systematic Review. Eur. Urol. Focus 2019, 5, 290–300. [Google Scholar] [CrossRef]
- Aziz, H.A.; Bugaev, N.; Baltazar, G.; Brown, Z.; Haines, K.; Gupta, S.; Yeung, L.; Posluszny, J.; Como, J.; Freeman, J.; et al. Management of adult renal trauma: A practice management guideline from the eastern association for the surgery of trauma. BMC Surg. 2023, 23, 22. [Google Scholar] [CrossRef]
- Baboudjian, M.; Gondran-Tellier, B.; Panayotopoulos, P.; Hutin, M.; Olivier, J.; Ruggiero, M.; Dominique, I.; Millet, C.; Bergerat, S.; Freton, L.; et al. Factors Predictive of Selective Angioembolization Failure for Moderate- to High-grade Renal Trauma: A French Multi-institutional Study. Eur. Urol. Focus 2022, 8, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Bae, M.; Jeon, C.H.; Hwangbo, L.; Lee, C.M.; Kim, C.W. Volume preservation of a shattered kidney after blunt trauma by superselective renal artery embolization. Diagn. Interv. Radiol. 2022, 28, 72–78. [Google Scholar] [CrossRef]
- Salvatori, F.; Macchini, M.; Rosati, M.; Boscarato, P.; Alborino, S.; Paci, E.; Candelari, R. Endovascular management of vascular renal injuries: Outcomes and comparison between traumatic and iatrogenic settings. Urol. J. 2022, 89, 167–175. [Google Scholar] [CrossRef]
- Lee, P.; Roh, S. Renal embolization for trauma: A narrative review. J. Trauma Inj. 2024, 37, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Chatziioannou, A.; Brountzos, E.; Primetis, E.; Malagari, K.; Sofocleous, C.; Mourikis, D.; Kelekis, D. Effects of superselective embolization for renal vascular injuries on renal parenchyma and function. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 201–206. [Google Scholar] [CrossRef]
- Hagiwara, A.; Sakaki, S.; Goto, H.; Takenega, K.; Fukushima, H.; Matuda, H.; Shimazaki, S. The role of interventional radiology in the management of blunt renal injury: A practical protocol. J. Trauma 2001, 51, 526–531. [Google Scholar] [CrossRef]
- Tasian, G.E.; Aaronson, D.S.; McAninch, J.W. Evaluation of renal function after major renal injury: Correlation with the American Association for the Surgery of Trauma Injury Scale. J. Urol. 2010, 183, 196–200. [Google Scholar] [CrossRef]
- Bergman, S.M.; O’Mailia, J.; Krane, N.K.; Wallin, J.D. Vitamin-A-induced hypercalcemia: Response to corticosteroids. Nephron 1988, 50, 362–364. [Google Scholar] [CrossRef]
- Vozianov, S.; Sabadash, M.; Shulyak, A. Experience of renal artery embolization in patients with blunt kidney trauma. Cent. Eur. J. Urol. 2015, 68, 471–477. [Google Scholar] [CrossRef]
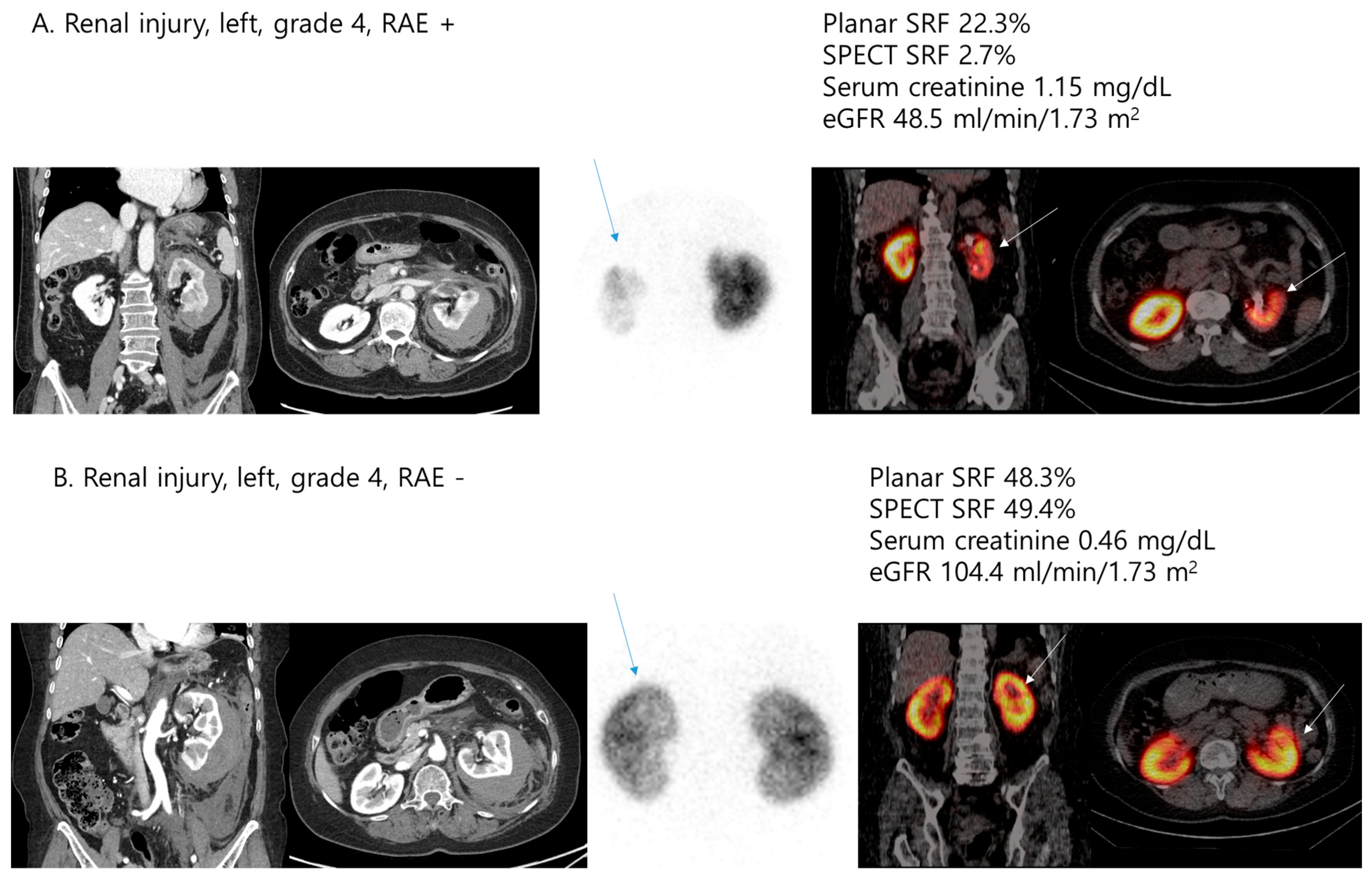
| Variables | n = 59 |
|---|---|
| Age (years) | 49.10 ± 22.67 |
| BMI (kg/m2) | 24.24 ± 4.22 |
| Sex | |
| Male | 35 (59.3%) |
| Female | 24 (40.7%) |
| Comorbidities | |
| Hypertension | 19 (32.2%) |
| Diabetes mellitus | 8 (13.6%) |
| Underlying renal diseases | |
| Cyst | 16 (27.1%) |
| Others | 10 (16.9%) |
| Causes of renal injuries | |
| Traffic accident | 23 (39.0%) |
| Fall-down | 12 (20.3%) |
| Slip-down | 9 (15.3%) |
| Iatrogenic | 12 (20.3%) |
| Others | 3 (5.1%) |
| Injured kidney | |
| Right | 24 (40.7%) |
| Left | 35 (59.3%) |
| AAST grades | |
| 1 | 6 (10.2%) |
| 2 | 10 (16.9%) |
| 3 | 15 (25.4%) |
| 4 | 23 (39.0%) |
| 5 | 5 (8.5%) |
| Concomitant organ injuries | 26 (44.1%) |
| Renal artery endovascular procedure | 20 (33.9%) |
| Renal function | |
| At index injury | |
| Serum creatinine (mg/dL) | 0.97 ± 0.39 |
| eGFR (mL/min/1.73 m2) | 88.87 ± 32.65 |
| Follow-up | |
| Serum creatinine (mg/dL) | 0.81 ± 0.26 |
| eGFR (mL/min/1.73 m2) | 100.00 ± 30.86 |
| Tc-99m DMSA scintigraphy | |
| Planar SRF (%) | 44.39 ± 14.17 |
| SPECT SRF (%) | 41.50 ± 14.73 |
| ICV (mL) | 93.65 ± 44.21 |
| ICU (%) | 11.42 ± 5.49 |
| ICV index, mL/m2 | 53.51 ± 23.14 |
| AAST Renal Injury Grade | ||
|---|---|---|
| Correlation Coefficients | p-Value | |
| Age (years) | 0.247 | 0.059 |
| BMI (kg/m2) | −0.156 | 0.239 |
| Serum creatinine (mg/dL) at follow-up | 0.290 | 0.026 |
| eGFR (mL/min/1.73 m2) at follow-up | −0.336 | 0.009 |
| Concomitant organ injuries | −0.266 | 0.042 |
| Planar SRF (%) | −0.291 | 0.025 |
| SPECT SRF (%) | −0.565 | <0.001 |
| ICV (mL) | −0.551 | <0.001 |
| ICU (%) | −0.450 | <0.001 |
| ICV index (mL/m2) | −0.555 | <0.001 |
| Variable | Low Grade Injuries (n = 31) | High Grade Injuries (n = 28) | p-Value |
|---|---|---|---|
| Age (years) | 46.39 ± 24.45 | 52.11 ± 20.56 | 0.370 a |
| BMI (kg/m2) | 24.63 ± 5.01 | 23.81 ± 3.16 | 0.451 b |
| Sex | 0.461 c | ||
| Male | 17 (54.8%) | 18 (64.3%) | |
| Female | 14 (45.2%) | 10 (35.7%) | |
| Hypertension | 10 (32.3%) | 9 (32.1%) | 0.992 c |
| Diabetes mellitus | 4 (12.9%) | 4 (14.3%) | 1.000 d |
| Causes of renal injuries | 0.053 d | ||
| Traffic accident | 16 (51.6%) | 7 (25.0%) | |
| Fall-down | 8 (25.8%) | 4 (14.3%) | |
| Slip-down | 3 (9.7%) | 6 (21.4%) | |
| Iatrogenic | 3 (9.7%) | 9 (32.1%) | |
| Others | 1 (3.2%) | 2 (7.1%) | |
| Injured kidney | 0.461 c | ||
| Right | 14 (45.2%) | 10 (35.7%) | |
| Left | 17 (54.8%) | 18 (64.3%) | |
| Concomitant organ injuries | 16 (51.6%) | 10 (35.7%) | 0.219 c |
| Renal artery endovascular procedure | 1 (3.2%) | 19 (67.9%) | <0.001 d |
| Renal function at index injury | |||
| Serum creatinine (mg/dL) | 0.87 ± 0.27 | 1.09 ± 0.47 | 0.104 a |
| eGFR (mL/min/1.73 m2) | 96.69 ± 32.29 | 80.20 ± 31.36 | 0.052 b |
| Renal function at follow-up | |||
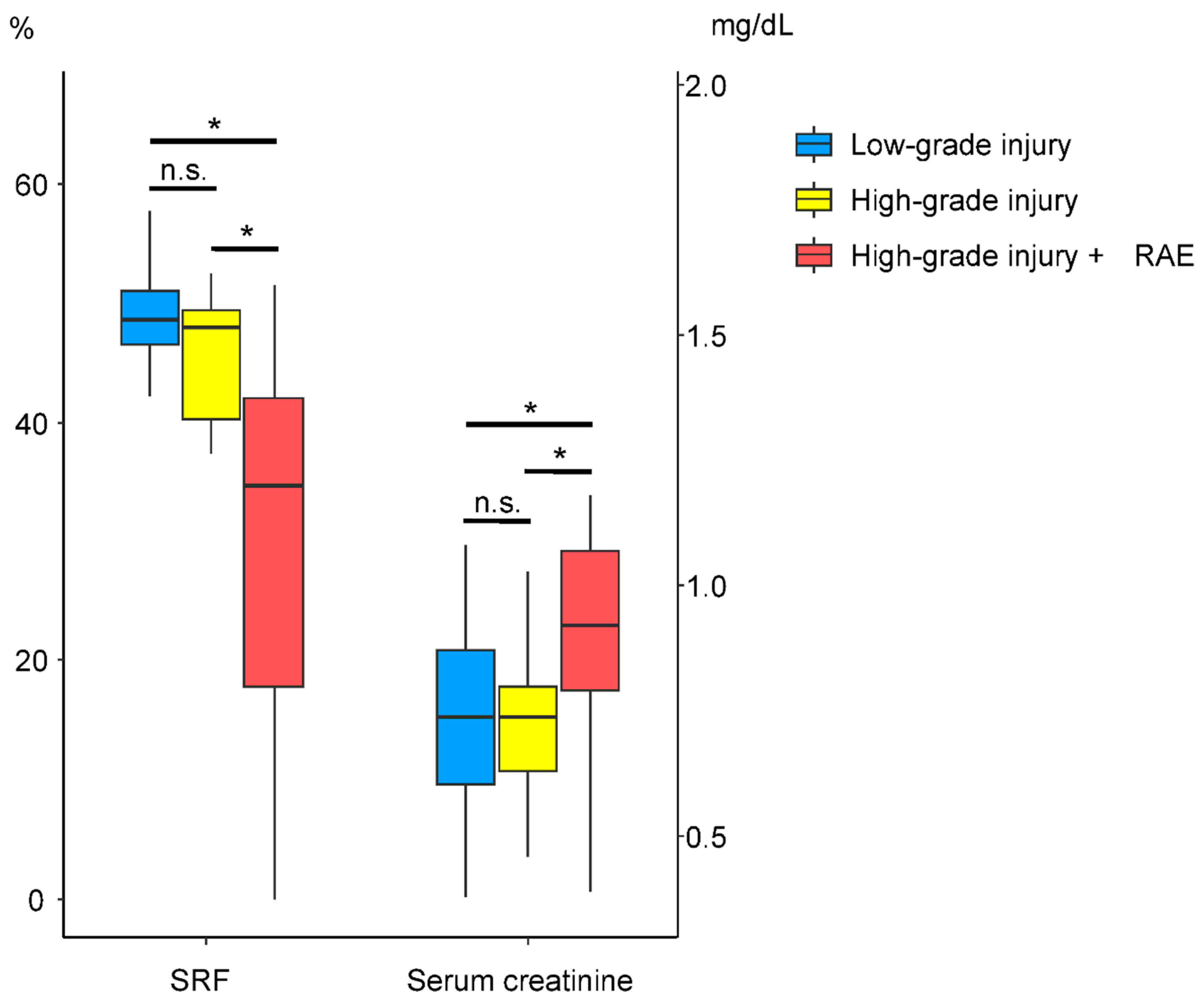
| Serum creatinine (mg/dL) | 0.74 ± 0.19 | 0.89 ± 0.30 | 0.024 a |
| eGFR (mL/min/1.73 m2) | 107.28 ± 30.71 | 91.95 ± 29.49 | 0.056 b |
| DMSA scintigraphy | |||
| Planar SRF (%) | 48.75 ± 8.24 | 39.57 ± 17.61 | 0.022 a |
| SPECT SRF (%) | 48.91 ± 6.38 | 33.30 ± 16.97 | <0.001 a |
| ICV (mL) | 113.07 ± 30.69 | 72.15 ± 47.32 | <0.001 b |
| ICU (%) | 13.41 ± 3.38 | 9.22 ± 6.52 | 0.004 b |
| ICV index (mL/m2) | 64.58 ± 12.55 | 41.27 ± 26.06 | <0.001 b |
| Variable | 3 Months | 1 Year | p-Value |
|---|---|---|---|
| Renal function | |||
| Serum creatinine (mg/dL) | 0.89 ± 0.35 | 0.88 ± 0.24 | 0.346 a |
| eGFR (mL/min/1.73 m2) | 97.22 ± 35.00 | 95.03 ± 31.63 | 0.337 b |
| DMSA scintigraphy | |||
| Planar SRF (%) | 43.28 ± 14.22 | 41.82 ± 15.29 | 0.331 b |
| SPECT SRF (%) | 35.55 ± 16.58 | 35.65 ± 15.69 | 0.798 a |
| ICV (mL) | 73.17 ± 49.33 | 75.14 ± 49.25 | 0.719 b |
| ICU (%) | 9.45 ± 7.08 | 10.21 ± 6.44 | 0.360 b |
| ICV index (mL/m2) | 42.63 ± 26.38 | 44.35 ± 27.30 | 0.563 b |
| Variable | No (n = 39) | Yes (n = 20) | p-Value |
|---|---|---|---|
| Age (years) | 44.74 ± 23.51 | 57.60 ± 18.69 | 0.040 a |
| BMI (kg/m2) | 24.40 ± 4.84 | 23.91 ± 2.69 | 0.620 b |
| Sex | 0.525 c | ||
| Male | 22 (56.4%) | 13 (65.0%) | |
| Female | 17 (43.6%) | 7 (35.0%) | |
| Hypertension | 9 (23.1%) | 10 (50.0%) | 0.036 c |
| Diabetes mellitus | 5 (12.8%) | 3 (15.0%) | 1.000 d |
| Causes of renal injuries | 0.255 d | ||
| Traffic accident | 18 (46.1%) | 5 (25.0%) | |
| Fall-down | 9 (23.1%) | 3 (15.0%) | |
| Slip-down | 4 (10.3%) | 5 (25.0%) | |
| Iatrogenic | 6 (15.4%) | 6 (30.0%) | |
| Others | 2 (5.1%) | 1 (5.0%) | |
| Injured kidney | 0.232 c | ||
| Right | 18 (46.1%) | 6 (30.0%) | |
| Left | 21 (53.9%) | 14 (70.0%) | |
| Concomitant organ injuries | 19 (48.7%) | 7 (35.0%) | 0.315 c |
| Renal function at index | |||
| Serum creatinine (mg/dL) | 0.91 ± 0.37 | 1.10 ± 0.41 | 0.058 a |
| eGFR (mL/min/1.73 m2) | 96.59 ± 34.07 | 73.81 ± 23.89 | 0.010 b |
| Renal function at follow-up | |||
| Serum creatinine (mg/dL) | 0.74 ± 0.21 | 0.94 ± 0.30 | 0.003 a |
| eGFR (mL/min/1.73 m2) | 108.20 ± 31.24 | 84.03 ± 23.43 | 0.004 b |
| DMSA scintigraphy | |||
| Planar SRF (%) | 47.87 ± 8.48 | 37.61 ± 19.90 | 0.036 a |
| SPECT SRF (%) | 47.41 ± 8.73 | 29.98 ± 17.28 | <0.001 a |
| ICV (mL) | 107.96 ± 33.47 | 65.77 ± 49.86 | 0.002 b |
| ICU (%) | 13.12 ± 4.02 | 8.10 ± 6.49 | 0.001 b |
| ICV index (mL/m2) | 61.65 ± 15.09 | 37.64 ± 27.90 | 0.001 b |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Age (years) | 1.02 (0.99–1.04) | 0.129 | Not applicable | |
| BMI (kg/m2) | 1.03 (0.91–1.17) | 0.605 | ||
| Sex | ||||
| Male | Reference | |||
| Female | 1.21 (0.42–3.50) | 0.726 | ||
| Causes of renal injuries | ||||
| Traffic accident | Reference | |||
| Fall-down | 0.94 (0.19–4.70) | 0.944 | ||
| Slip-down | 3.54 (0.71–17.73) | 0.124 | ||
| Iatrogenic | 5.67 (1.24–25.88) | 0.025 | ||
| Others | 1.42 (0.11–18.59) | 0.791 | ||
| Injured kidney | ||||
| Right | Reference | |||
| Left | 2.05 (0.68–6.16) | 0.204 | ||
| AAST renal injury grades | ||||
| Low | 1 (Reference) | 1 (Reference) | ||
| High | 23.33 (5.50–99.04) | <0.001 | 9.50 (1.78–50.61) | 0.008 |
| Concomitant organ injuries | 0.72 (0.25–2.08) | 0.542 | ||
| Renal artery endovascular procedure | 18.29 (4.66–71.76) | <0.001 | 5.15 (1.07–24.88) | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.H.; Kang, T.W.; Park, C.; Cho, S.-G. Assessment of Residual Cortical Function by Using Tc-99m DMSA SPECT at Follow-Up in Non-Operatively Treated Patients with Traumatic Renal Injuries: A Prospective Single-Centre Study. J. Clin. Med. 2025, 14, 6276. https://doi.org/10.3390/jcm14176276
Yu SH, Kang TW, Park C, Cho S-G. Assessment of Residual Cortical Function by Using Tc-99m DMSA SPECT at Follow-Up in Non-Operatively Treated Patients with Traumatic Renal Injuries: A Prospective Single-Centre Study. Journal of Clinical Medicine. 2025; 14(17):6276. https://doi.org/10.3390/jcm14176276
Chicago/Turabian StyleYu, Seong Hyeon, Taek Won Kang, Chan Park, and Sang-Geon Cho. 2025. "Assessment of Residual Cortical Function by Using Tc-99m DMSA SPECT at Follow-Up in Non-Operatively Treated Patients with Traumatic Renal Injuries: A Prospective Single-Centre Study" Journal of Clinical Medicine 14, no. 17: 6276. https://doi.org/10.3390/jcm14176276
APA StyleYu, S. H., Kang, T. W., Park, C., & Cho, S.-G. (2025). Assessment of Residual Cortical Function by Using Tc-99m DMSA SPECT at Follow-Up in Non-Operatively Treated Patients with Traumatic Renal Injuries: A Prospective Single-Centre Study. Journal of Clinical Medicine, 14(17), 6276. https://doi.org/10.3390/jcm14176276





