Evolution of Aortic Root Preservation After Type A Acute Aortic Dissection Repair: Impact of Root-Residual Aortic Dissection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Procedure
2.3. Endpoints
2.4. Root-RAD Diagnostic and Follow-Up CT Protocol
2.5. Follow-Up Transthoracic Echocardiography
2.6. Aortic Root Reintervention
2.7. Statistical Analysis
3. Results
3.1. Baseline and Procedure Data
3.2. Risk Factors for Root-RAD
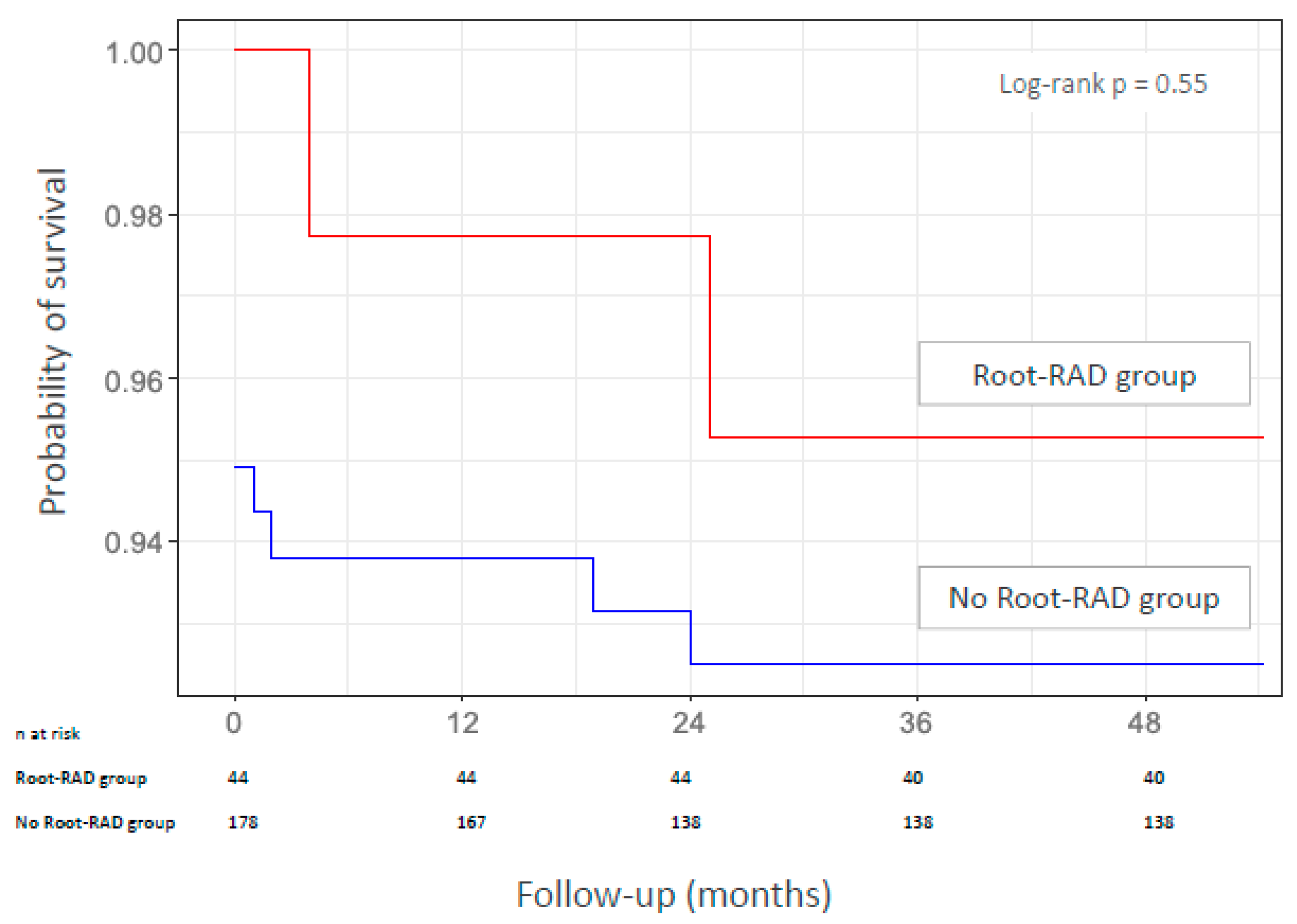
3.3. Mortality During Follow-Up
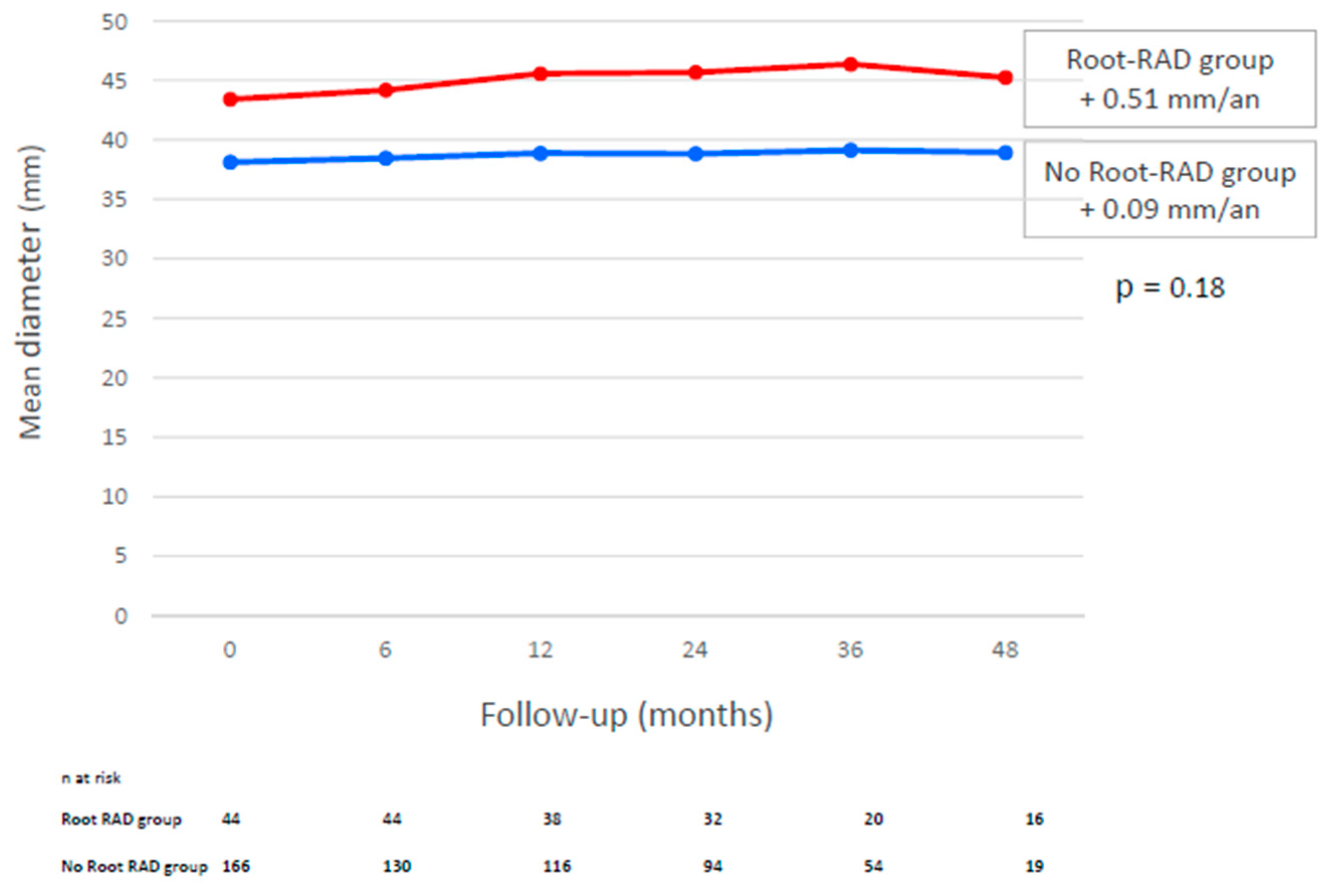
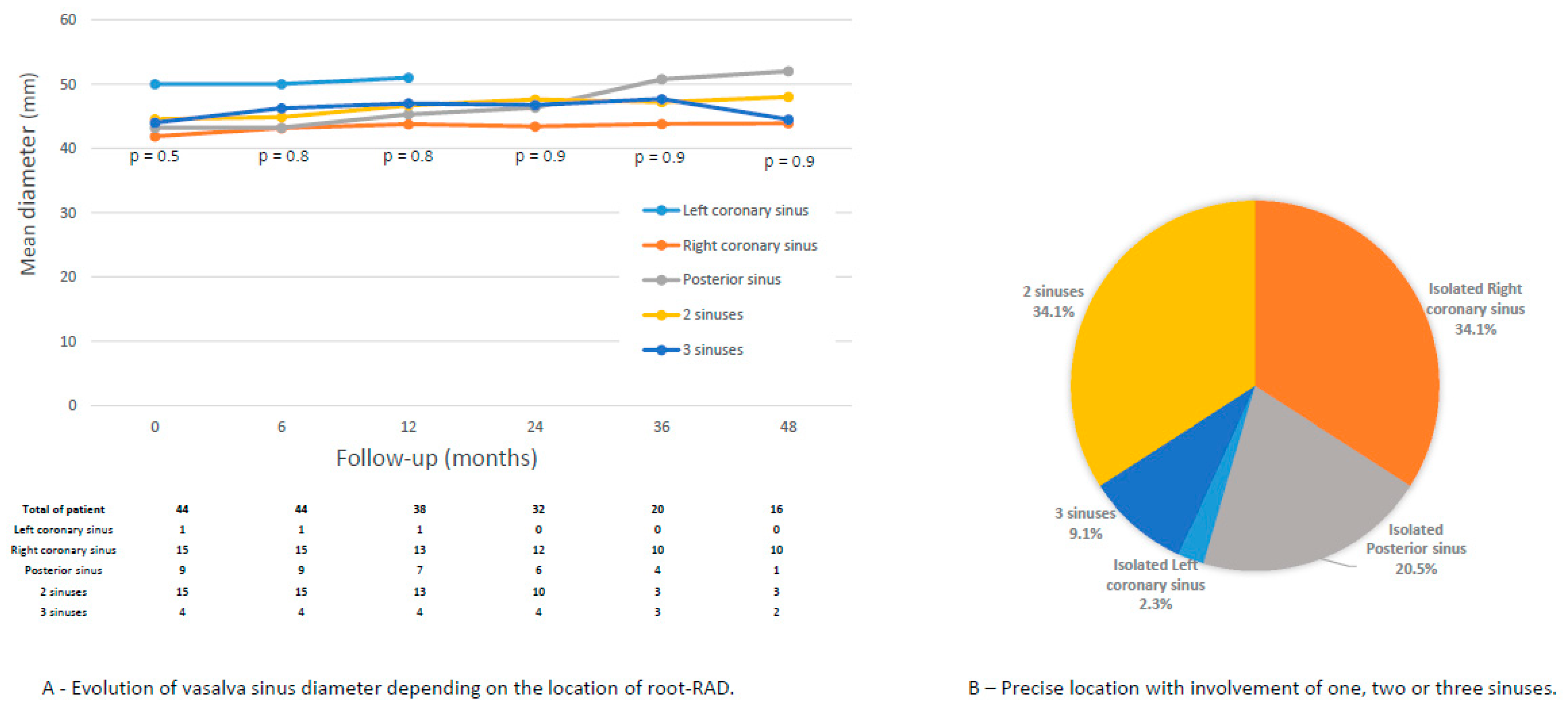
3.4. Increase in Root Diameter During Follow-Up
3.5. Aortic Root Reintervention
3.6. Aortic Regurgitation and Risk Factors
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Aortic cross-clamp |
| AR | Aortic regurgitation |
| AV | Aortic valve |
| CA | Circulatory arrest |
| CABG | Coronary artery bypass graft |
| CPB | Cardiopulmonary bypass |
| CI | Confidence interval |
| CT | Computed tomography |
| Root-RAD | Root residual aortic dissection |
| SCAR | Supracoronary aortic replacement |
| TAAD | Type A aortic dissection |
| TEVAR | Total endovascular aortic repair |
References
- Gouveia, E.; Melo, R.; Mourão, M.; Caldeira, D.; Alves, M.; Lopes, A.; Duarte, A.; Fernandes, E.; Fernandes, R.; Mendes Pedro, L. A systematic review and meta-analysis of the incidence of acute aortic dissections in population-based studies. J. Vasc. Surg. 2022, 75, 709–720. [Google Scholar] [CrossRef]
- Berretta, P.; Patel, H.J.; Gleason, T.G.; Sundt, T.M.; Myrmel, T.; Desai, N.; Korach, A.; Panza, A.; Bavaria, J.; Khoynezhad, A.; et al. IRAD experience on surgical type A acute dissection patients: Results and predictors of mortality. Ann. Cardiothorac. Surg. 2016, 5, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.; Isselbacher, E.M.; Bossone, E.; Gleason, T.G.; Eusanio, M.D.; Sechtem, U.; Ehrlich, M.P.; Trimarchi, S.; Braverman, A.C.; Myrmel, T.; et al. Insights from the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 2018, 137, 1846–1860. [Google Scholar] [PubMed]
- Pape, L.A.; Awais, M.; Woznicki, E.M.; Suzuki, T.; Trimarchi, S.; Evangelista, A.; Myrmel, T.; Larsen, M.; Harris, K.M.; Greason, K.; et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection. J. Am. Coll. Cardiol. 2015, 66, 350–358. [Google Scholar]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. Available online: https://www.ahajournals.org/doi/10.1161/CIR.0000000000001106 (accessed on 25 August 2025). [CrossRef]
- Trimarchi, S.; Nienaber, C.A.; Rampoldi, V.; Myrmel, T.; Suzuki, T.; Mehta, R.H.; Bossone, E.; Cooper, J.V.; Smith, D.E.; Menicanti, L.; et al. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J. Thorac. Cardiovasc. Surg. 2005, 129, 112–122. [Google Scholar] [CrossRef]
- Di Eusanio, M.; Trimarchi, S.; Peterson, M.D.; Myrmel, T.; Hughes, G.C.; Korach, A.; Sundt, T.M.; Di Bartolomeo, R.; Greason, K.; Khoynezhad, A.; et al. Root Replacement Surgery Versus More Conservative Management During Type A Acute Aortic Dissection Repair. Ann. Thorac. Surg. 2014, 98, 2078–2084. [Google Scholar] [CrossRef]
- Wang, Z.; Greason, K.L.; Pochettino, A.; Schaff, H.V.; Suri, R.M.; Stulak, J.M.; Dearani, J.A. Long-term outcomes of survival and freedom from reoperation on the aortic root or valve after surgery for acute ascending aorta dissection. J. Thorac. Cardiovasc. Surg. 2014, 148, 2117–2122. [Google Scholar] [CrossRef]
- Castrovinci, S.; Pacini, D.; Di Marco, L.; Berretta, P.; Cefarelli, M.; Murana, G.; Alfonsi, J.; Pantaleo, A.; Leone, A.; Di Eusanio, M.; et al. Surgical management of aortic root in type A acute aortic dissection: A propensity-score analysis. Eur. J. Cardiothorac. Surg. 2016, 50, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.H.; Richardt, D.; Diwoky, M.; Auer, C.; Bucsky, B.; Nasseri, B.; Klotz, S. Survival and reoperation after valve-sparing root replacement and root repair in acute type A dissection. J. Thorac. Cardiovasc. Surg. 2018, 156, 2076–2082.e2. [Google Scholar] [CrossRef]
- Jormalainen, M.; Kesävuori, R.; Raivio, P.; Vento, A.; Mustonen, C.; Honkanen, H.P.; Rosato, S.; Simpanen, J.; Teittinen, K.; Biancari, F.; et al. Long-term outcomes after ascending aortic replacement and aortic root replacement for type A aortic dissection. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.R.; Sundt, T.M.; Pasque, M.K.; Barner, H.B.; Huddleston, C.B.; Damiano, R.J.; Gay, W.A. Does the extent of proximal or distal resection influence outcome for type A dissections? Ann. Thorac. Surg. 2001, 71, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.T.; Miller, D.C.; Mitchell, R.S.; Oyer, P.E.; Moore, K.A.; Robbins, R.C.; Shumway, N.E.; Reitz, B.A. Acute type a aortic dissection complicated by aortic regurgitation: Composite valve graft versus separate valve graft versus conservative valve repair. J. Thorac. Cardiovasc. Surg. 2003, 126, 1978–1985. [Google Scholar] [CrossRef]
- Hagan, P.G.; Nienaber, C.A.; Isselbacher, E.M.; Bruckman, D.; Karavite, D.J.; Russman, P.L.; Evangelista, A.; Fattori, R.; Suzuki, T.; Oh, J.K.; et al. The International Registry of Acute Aortic Dissection (IRAD): New Insights into an Old Disease. JAMA 2000, 283, 897. [Google Scholar] [CrossRef]
- Ikeno, Y.; Yokawa, K.; Yamanaka, K.; Inoue, T.; Tanaka, H.; Okada, K.; Okita, Y. The fate of aortic root and aortic regurgitation after supracoronary ascending aortic replacement for acute type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2021, 161, 483–493.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.K.; Qiu, Z.H.; Fang, G.H.; Wu, X.J.; Chen, L.W. Reported outcomes after aortic valve resuspension for acute type A aortic dissection: A systematic review and meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 331–338. [Google Scholar] [CrossRef]
- Sabik, J.F.; Lytle, B.W.; Blackstone, E.H.; McCarthy, P.M.; Loop, F.D.; Cosgrove, D.M. Long-term effectiveness of operations for ascending aortic dissections. J. Thorac. Cardiovasc. Surg. 2000, 119, 946–964. [Google Scholar] [CrossRef]
- Chiu, P.; Trojan, J.; Tsou, S.; Goldstone, A.B.; Woo, Y.J.; Fischbein, M.P. Limited root repair in acute type A aortic dissection is safe but results in increased risk of reoperation. J. Thorac. Cardiovasc. Surg. 2018, 155, 1–7.e1. [Google Scholar] [CrossRef]
- Kallenbach, K.; Oelze, T.; Salcher, R.; Hagl, C.; Karck, M.; Leyh, R.G.; Haverich, A. Evolving Strategies for Treatment of Acute Aortic Dissection Type A. Circulation 2004, 110 (Suppl. S1), II243-9. Available online: https://www.ahajournals.org/doi/10.1161/01.CIR.0000138948.14144.d6 (accessed on 19 July 2025).
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Rivière, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar]
- Rylski, B.; Beyersdorf, F.; Blanke, P.; Boos, A.; Hoffmann, I.; Dashkevich, A.; Siepe, M. Supracoronary ascending aortic replacement in patients with acute aortic dissection type A: What happens to the aortic root in the long run? J. Thorac. Cardiovasc. Surg. 2013, 146, 285–290. [Google Scholar] [CrossRef]
- Valdis, M.; Adams, C.; Chu, M.W.A.; Kiaii, B.; Guo, L. Comparison of outcomes of root replacement procedures and supracoronary techniques for surgical repair of acute aortic dissection. Can. J. Surg. 2017, 60, 198–204. [Google Scholar] [CrossRef][Green Version]
- Beckmann, E.; Martens, A.; Pertz, J.; Kaufeld, T.; Umminger, J.; Hanke, J.S.; Schmitto, J.D.; Cebotari, S.; Haverich, A.; Shrestha, M.L. Valve-sparing David I procedure in acute aortic type A dissection: A 20-year experience with more than 100 patients. Eur. J. Cardiothorac. Surg. 2017, 52, 319–324. [Google Scholar] [CrossRef]
- Vendramin, I.; Lechiancole, A.; Piani, D.; Deroma, L.; Tullio, A.; Sponga, S.; Milano, A.D.; Onorati, F.; Bortolotti, U.; Livi, U. Type A acute aortic dissection with ≥40-mm aortic root: Results of conservative and replacement strategies at long-term follow-up. Eur. J. Cardiothorac. Surg. 2021, 59, 1115–1122. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Pan, E.; Gudbjartsson, T.; Ahlsson, A.; Fuglsang, S.; Geirsson, A.; Hansson, E.C.; Hjortdal, V.; Jeppsson, A.; Järvelä, K.; Mennander, A.; et al. Low rate of reoperations after acute type A aortic dissection repair from The Nordic Consortium Registry. J. Thorac. Cardiovasc. Surg. 2018, 156, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Westaby, S.; Saito, S.; Katsumata, T. Acute type A dissection: Conservative methods provide consistently low mortality. Ann. Thorac. Surg. 2002, 73, 707–713. [Google Scholar] [CrossRef]
- Dell’Aquila, A.M.; Concistrè, G.; Gallo, A.; Pansini, S.; Piccardo, A.; Passerone, G.; Regesta, T. Fate of the preserved aortic root after treatment of acute type A aortic dissection: 23-year follow-up. J. Thorac. Cardiovasc. Surg. 2013, 146, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Magouliotis, D.E.; Sicouri, S.; Baudo, M.; Cabrucci, F.; Yamashita, Y.; Ramlawi, B. Comparative outcomes of aortic root preservation versus root replacement for acute type A aortic dissection: Balancing survival, durability, and reoperation. Hellenic J. Cardiol. 2025; epub ahead of print. [Google Scholar] [CrossRef]
- Yang, B.; Norton, E.L.; Hobbs, R.; Farhat, L.; Wu, X.; Hornsby, W.E.; Kim, K.M.; Patel, H.J.; Deeb, G.M. Short- and long-term outcomes of aortic root repair and replacement in patients undergoing acute type A aortic dissection repair: Twenty-year experience. J. Thorac. Cardiovasc. Surg. 2019, 157, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H.; Tabata, M.; Fukui, T.; Takanashi, S. Surgical Strategy and Outcome for Aortic Root in Patients Undergoing Repair of Acute Type A Aortic Dissection. Ann. Thorac. Surg. 2016, 101, 1464–1469. [Google Scholar] [CrossRef]
- Movsowitz, H.D.; Levine, R.A.; Hilgenberg, A.D.; Isselbacher, E.M. Transesophageal echocardiographic description of the mechanisms of aortic regurgitation in acute type A aortic dissection: Implications for aortic valve repair. J. Am. Coll. Cardiol. 2000, 36, 884–890. [Google Scholar] [CrossRef]
- Patel, P.A.; Bavaria, J.E.; Ghadimi, K.; Gutsche, J.T.; Vallabhajosyula, P.; Ko, H.A.; Desai, N.D.; Mackay, E.; Weiss, S.J.; Augoustides, J.G.T. Aortic Regurgitation in Acute Type-A Aortic Dissection: A Clinical Classification for the Perioperative Echocardiographer in the Era of the Functional Aortic Annulus. J. Cardiothorac. Vasc. Anesth. 2018, 32, 586–597. [Google Scholar] [CrossRef]
- Pessotto, R.; Santini, F.; Pugliese, P.; Montalbano, G.; Luciani, G.B.; Faggian, G.; Bertolini, P.; Mazzucco, A. Preservation of the aortic valve in acute type A dissection complicated by aortic regurgitation. Ann. Thorac. Surg. 1999, 67, 2010–2013. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; Soustelle, C.; Houël, R.; Hillion, M.L.; Loisance, D. Risk factor analysis for proximal and distal reoperations after surgery for acute type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2002, 123, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wu, J.; Xie, E.; Luo, X.; Chen, J.F.; Gao, W.; Jiang, W.; Qiu, J.; Zhao, R.; Yu, C. Surgical Management and Outcomes of the Aortic Root in Acute Type A Aortic Dissection. Ann. Thorac. Surg. 2020, 110, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Bojko, M.M.; Assi, R.; Bavaria, J.E.; Suhail, M.; Habertheuer, A.; Hu, R.W.; Harmon, J.; Milewski, R.K.; Desai, N.D.; Szeto, W.Y.; et al. Midterm outcomes and durability of sinus segment preservation compared with root replacement for acute type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2022, 163, 900–910.e2. [Google Scholar] [CrossRef]
- Gaudry, M.; Porto, A.; Guivier-Curien, C.; Blanchard, A.; Bal, L.; Resseguier, N.; Omnes, V.; De Masi, M.; Ejargue, M.; Jacquier, A.; et al. Results of a prospective follow-up study after type A aortic dissection repair: A high rate of distal aneurysmal evolution and reinterventions. Eur. J. Cardiothorac. Surg. 2021, 61, 152–159. [Google Scholar] [CrossRef]
| Overall n = 210 (100%) | No Root-RAD n = 166 (79.0%) | Root-RAD n = 44 (21.0%) | p Value | |
|---|---|---|---|---|
| Male sex, n (%) | 148 (66.7) | 120 (67.4) | 29 (65.9) | 0.46 |
| Age (years), mean (SD) | 65.8 (11.0) | 65.3 (11.7) | 67.7 (11.0) | 0.20 |
| Hypertension, n (%) | 140 (66.7) | 113 (68.1) | 27 (61.4) | 0.47 |
| Dyslipidemia, n (%) | 40 (19.0) | 33 (19.9) | 7 (15.9) | 0.67 |
| Smoking, n (%) | 47 (22.4) | 38 (22.9) | 9 (20.5) | 0.84 |
| Diabetes, n (%) | 6 (2.9) | 5 (3.0) | 1 (2.3) | 1.00 |
| COPD, n (%) | 13 (6.2) | 8 (4.8) | 5 (11.4) | 0.15 |
| CAD, n (%) | 17 (8.1) | 12 (7.2) | 5 (11.4) | 0.36 |
| Peripheral vascular disease, n (%) | 7 (3.3) | 4 (2.4) | 3 (6.8) | 0.16 |
| Renal failure, n (%) | 1 (0.5) | 1 (0.6) | 0 (0) | 1.00 |
| Marfan syndrome, n (%) | 7 (3.3) | 4 (2.2) | 3 (6.8) | 0.16 |
| Bicuspid aortic valve, n (%) | 5 (2.4) | 4 (2.4) | 1 (2.3) | 1.00 |
| Redo cardiac surgery, n (%) | 6 (2.9) | 5 (3.0) | 1 (2.3) | 1.00 |
| Concomitant procedure, n (%) | ||||
| Innominate artery debranching | 43 (20.5) | 39 (23.5) | 4 (9.1) | 0.04 |
| Aortic valve replacement | 7 (3.3) | 4 (2.4) | 3 (6.8) | 0.16 |
| Mean CPB time, min | 148.8 ± 42.5 | 151.4 ± 43.6 | 142.0 ± 42.5 | 0.27 |
| Mean ACC time, min | 83.6 ± 33.3 | 85.6 ± 33.4 | 78.0 ± 33.3 | 0.08 |
| Mean CA time, min | 24.4 ± 11.4 | 25.2 ± 11.2 | 21.9 ± 11.4 | 0.20 |
| Age, Years | Location of Root-RAD | Delay of Reintervention | Indication of Reintervention | Reintervention | Follow-Up | |
|---|---|---|---|---|---|---|
| Patient 1 | 79 | Isolated right coronary sinus | 18 months | Severe AR, aortic root 61 mm | Modified Bentall procedure | No postoperative complication. Alive at 2 years. |
| Patient 2 | 68 | 2 sinuses (right and posterior) | 4 years | Severe AR, aortic root 50 mm, aneurysm in the descending aorta | Hybrid treatment: SCAR with aortic valve resuspension, supra-aortic trunk reimplantation, TEVAR | No postoperative complication. Alive at 1 year. |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Risk of severe AR | ||||
| Root-RAD | 19.6 (1.6–2.7) | 0.05 | 4.48 (0.2–7.3) | 0.27 |
| Location of root-RAD: right coronary sinus | 28.2 (2.2–3.9) | 0.03 | 4.48 (0.2–7.3) | 0.27 |
| Mean diameter of aortic root at discharge | 1.3 (1.0–1.8) | 0.02 | 0.8 (0.4–1.4) | 0.21 |
| Maximum diameter of aortic root | 1.4 (1.1–2.0) | 0.006 | 1.48 (1.0–2.5) | 0.018 |
| Risk of moderate AR | ||||
| Chronic obstructive pulmonary disease | 14.0 (3.4–55.1) | <0.001 | 5.5 (0.7–66.5) | 0.09 |
| Marfan syndrome | 10.5 (1.7–51.6) | 0.005 | 4.1 (0.2–2.9) | 0.31 |
| Root-RAD | 10.1 (12.5–13.1) | 0.002 | 23.0 (2.8–2.9) | 0.009 |
| Mean diameter of aortic root at discharge | 1.4 (1.2–1.7) | <0.001 | 1.4 (1.1–2.1) | 0.02 |
| Maximum diameter of aortic root | 1.2 (1.1–1.4) | <0.001 | 0.9 (0.7–1.1) | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto, A.; Court, A.; Barral, P.A.; Boucekine, M.; Imbert, L.; Gariboldi, V.; Jacquier, A.; Collart, F.; Theron, A.; Gaudry, M. Evolution of Aortic Root Preservation After Type A Acute Aortic Dissection Repair: Impact of Root-Residual Aortic Dissection. J. Clin. Med. 2025, 14, 6274. https://doi.org/10.3390/jcm14176274
Porto A, Court A, Barral PA, Boucekine M, Imbert L, Gariboldi V, Jacquier A, Collart F, Theron A, Gaudry M. Evolution of Aortic Root Preservation After Type A Acute Aortic Dissection Repair: Impact of Root-Residual Aortic Dissection. Journal of Clinical Medicine. 2025; 14(17):6274. https://doi.org/10.3390/jcm14176274
Chicago/Turabian StylePorto, Alizee, Axel Court, Pierre Antoine Barral, Mohamed Boucekine, Laura Imbert, Vlad Gariboldi, Alexis Jacquier, Frederic Collart, Alexis Theron, and Marine Gaudry. 2025. "Evolution of Aortic Root Preservation After Type A Acute Aortic Dissection Repair: Impact of Root-Residual Aortic Dissection" Journal of Clinical Medicine 14, no. 17: 6274. https://doi.org/10.3390/jcm14176274
APA StylePorto, A., Court, A., Barral, P. A., Boucekine, M., Imbert, L., Gariboldi, V., Jacquier, A., Collart, F., Theron, A., & Gaudry, M. (2025). Evolution of Aortic Root Preservation After Type A Acute Aortic Dissection Repair: Impact of Root-Residual Aortic Dissection. Journal of Clinical Medicine, 14(17), 6274. https://doi.org/10.3390/jcm14176274






