Identifying Frailty Risk in Older Adults: The Predictive Value of Functional Tests and Center-of-Pressure-Based Postural Metrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Consent to Participate
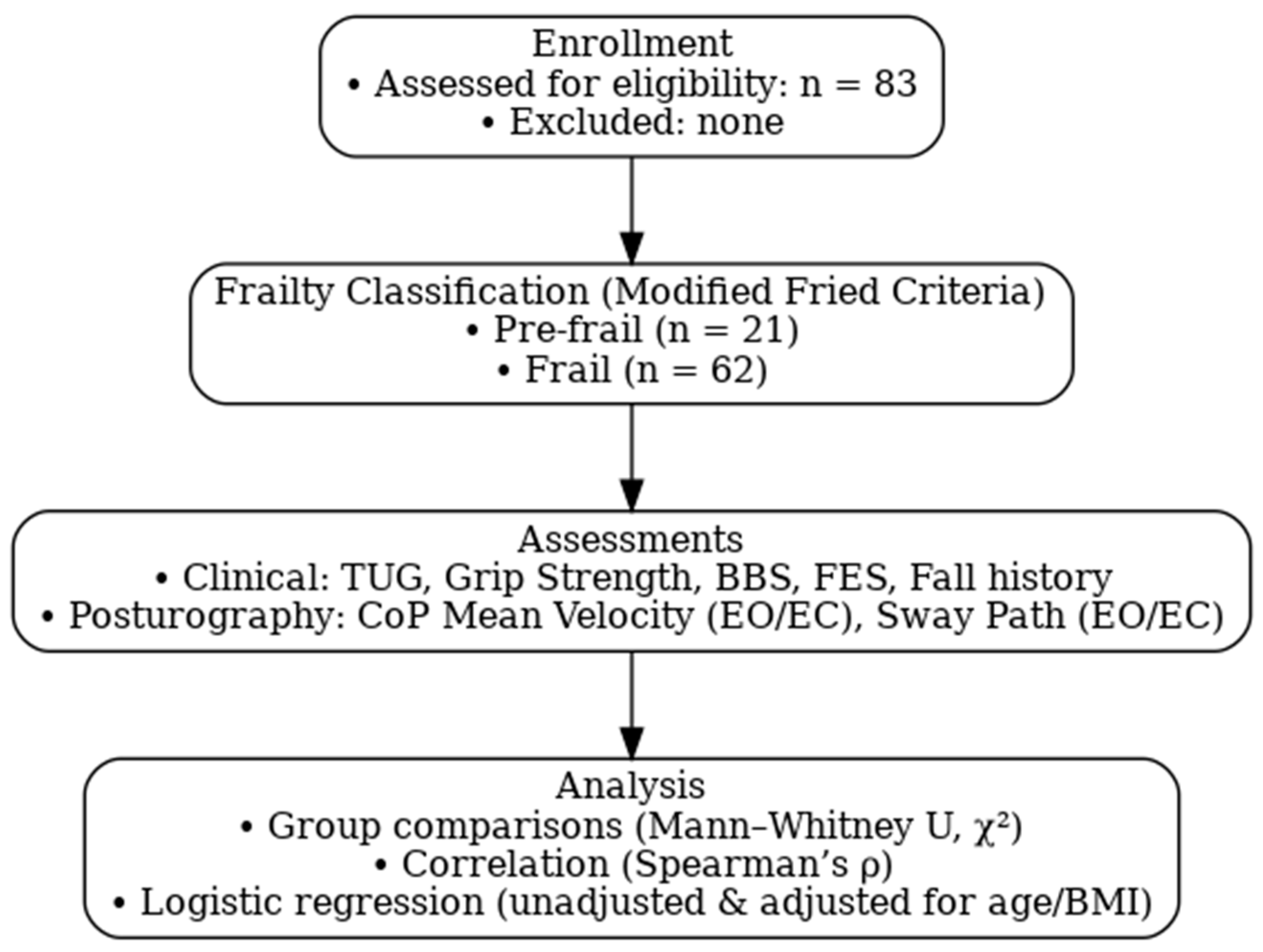
2.2. Participants and Eligibility Criteria
2.3. Outcome Measures
2.3.1. Frailty Index
2.3.2. Physical Function
- i.
- Grip Strength: Measured by a calibrated Jamar hydraulic hand dynamometer. Participants completed three maximal trials with the dominant hand, and the highest value (kg) was recorded.
- ii.
- Timed Up and Go (TUG) [28]: Participants were directed to stand up from a chair, walk 3 m, turn around, return, and sit down. The time to complete the task was recorded.
- iii.
- Berg Balance Scale (BBS) [29]: A 14-item scale assessing functional balance, scored from 0 to 56, with lower scores indicating impaired balance.
- iv.
- Falls Efficacy Scale (FES-I) [30]: A 10-item questionnaire evaluating fear of falling during activity of daily living. Higher scores indicate greater fear and lower balance confidence.
- v.
- Fall History: Participants self-reported the number of falls experienced in the previous 12 months.
2.4. Posturography and Force Plate Measures
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
3.1. Correlation Between Clinical Measures and Postural Control
3.2. Unadjusted Logistic Regression
3.3. Adjusted Logistic Regression
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBS | Berg Balance Scale |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| cm/s | Centimeters per Second |
| CoP | Center of Pressure |
| EC | Eyes Closed |
| EO | Eyes Open |
| FES-I | Falls Efficacy Scale–International |
| kg | Kilogram |
| MV_EC | Mean Velocity with Eyes Closed |
| MV_EO | Mean Velocity with Eyes Open |
| OR | Odds Ratio |
| SP_EC | Sway Path with Eyes Closed |
| SP_EO | Sway Path with Eyes Open |
| TUG | Timed Up and Go |
References
- Fierro-Marrero, J.; Reina-Varona, Á.; Paris-Alemany, A.; La Touche, R. Frailty in geriatrics: A critical review with content analysis of instruments, overlapping constructs, and challenges in diagnosis and prognostic precision. J. Clin. Med. 2025, 14, 1808. [Google Scholar] [CrossRef]
- Long, Q.; Li, Y.; Shi, Z.; Lee, Y.; Mao, L. Investigation of the association between the triglyceride-glucose index and the incidence of frailty among middle-aged and older adults: Evidence from the China health and retirement longitudinal study. Front. Public Health 2025, 13, 1548222. [Google Scholar] [CrossRef]
- O’cAoimh, R.; Sezgin, D.; O’dOnovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Duregon, F.; Vecchiato, M.; Ermolao, A.; Neunhaeuserer, D. Sedentary lifestyle and physical inactivity: A mutual interplay with early and overt frailty. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103971. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.F.; Canever, J.B.; Moreira, B.D.S.; Danielewicz, A.L.; de Avelar, N.C.P. Association between fear of falling and frailty in community-dwelling older adults: A systematic review. Clin. Interv. Aging 2022, 17, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Rockwood, K. Frailty in older adults. N. Engl. J. Med. 2024, 391, 538–548. [Google Scholar] [CrossRef]
- De Luca, V.; Donnoli, C.; Formosa, V.; Carnevale, E.; Bisogno, M.; Patumi, L.; Leonardini, L.; Obbia, P.; Palummeri, E.; Ruatta, M.; et al. Preliminary results of a multidimensional approach to screen for frailty in community-dwelling older adults of eight Italian regions: The SUNFRAIL+ study. Front. Public Health 2025, 13, 1543724. [Google Scholar] [CrossRef]
- Wang, X.-M.; Zhang, Y.-H.; Meng, C.-C.; Fan, L.; Wei, L.; Li, Y.-Y.; Liu, X.-Z.; Lv, S.-C. Scale-based screening and assessment of age-related frailty. Front. Public Health 2024, 12, 1424613. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Wada, Y.; Shojima, K.; Tamaki, K.; Mori, T.; Kusunoki, H.; Onishi, M.; Tsuji, S.; Matsuzawa, R.; Nagai, K.; Sano, K.; et al. Association between timed Up-and-Go test and future changes in the frailty status in a longitudinal study of Japanese Community-Dwelling older adults. Clin. Interv. Aging 2023, 18, 1191–1200. [Google Scholar] [CrossRef]
- Beck Jepsen, D.; Robinson, K.; Ogliari, G.; Montero-Odasso, M.; Kamkar, N.; Ryg, J.; Freiberger, E.; Masud, T. Predicting falls in older adults: An umbrella review of instruments assessing gait, balance, and functional mobility. BMC Geriatr. 2022, 22, 615. [Google Scholar]
- McGarrigle, L.; Yang, Y.; Lasrado, R.; Gittins, M.; Todd, C. A systematic review and meta-analysis of the measurement properties of concerns-about-falling instruments in older people and people at increased risk of falls. Age Ageing 2023, 52, afad055. [Google Scholar] [CrossRef] [PubMed]
- McColl, L.; Strassheim, V.; Linsley, M.; Green, D.; Dunkel, C.; Trundle, H.; Gibbon, J.R.; Parry, S.W. Associations between the Falls Efficacy Scale International (FES-I) and poor strength and balance in community-dwelling older people. Cogent Gerontol. 2024, 3, 2304603. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Borrelli, J.; Komisar, V.; Novak, A.; Maki, B.; King, E. Extending the center of pressure to incorporate handhold forces: Derivation and sample application. J. Biomech. 2020, 104, 109727. [Google Scholar] [CrossRef]
- Mongold, S.J.; Georgiev, C.; Legrand, T.; Yildiran Carlak, E.; Iannotta, A.; Cabaraux, P.; Naeije, G.; Vander Ghinst, M.; Bourguignon, M. Aging-related changes in neuromuscular control strategies and their influence on postural stability. Sci. Rep. 2025, 15, 30127. [Google Scholar] [CrossRef]
- Rizzato, A.; Benazzato, M.; Cognolato, M.; Grigoletto, D.; Paoli, A.; Marcolin, G. Different neuromuscular control mechanisms regulate static and dynamic balance: A center-of-pressure analysis in young adults. Hum. Mov. Sci. 2023, 90, 103120. [Google Scholar] [CrossRef] [PubMed]
- Serra-Prat, M.; Palomera, E. Muscle strength, sarcopenia and frailty associations with balance and gait parameters: A cross-sectional study. Eur. J. Geriatr. Gerontol. 2019, 1, 61–66. [Google Scholar] [CrossRef]
- Rezaei, A.; Bhat, S.G.; Cheng, C.-H.; Pignolo, R.J.; Lu, L.; Kaufman, K.R.; Jan, Y.-K. Age-related changes in gait, balance, and strength parameters: A cross-sectional study. PLoS ONE 2024, 19, e0310764. [Google Scholar] [CrossRef]
- Dallaire, M.; Houde-Thibeault, A.; Bouchard-Tremblay, J.; Wotto, E.A.; Côté, S.; Oliveira, C.S.; Ngomo, S.; da Silva, R.A. Impact of frailty and sex-related differences on postural control and gait in older adults with Parkinson’s Disease. Exp. Gerontol. 2024, 186, 112360. [Google Scholar] [CrossRef]
- Pinloche, L.; Zhang, Q.; Berthouze, S.E.; Monteil, K.; Hautier, C. Physical ability, cervical function, and walking plantar pressure in frail and pre-frail older adults: An attentional focus approach. Front. Aging 2022, 3, 1063320. [Google Scholar] [CrossRef]
- Quijoux, F.; Vienne-Jumeau, A.; Bertin-Hugault, F.; Zawieja, P.; Lefèvre, M.; Vidal, P.-P.; Ricard, D. Center of pressure displacement characteristics differentiate fall risk in older people: A systematic review with meta-analysis. Ageing Res. Rev. 2020, 62, 101117. [Google Scholar] [CrossRef] [PubMed]
- Anzai, E.; Ren, D.; Cazenille, L.; Aubert-Kato, N.; Tripette, J.; Ohta, Y. Random forest algorithms to classify frailty and falling history in seniors using plantar pressure measurement insoles: A large-scale feasibility study. BMC Geriatr. 2022, 22, 746. [Google Scholar]
- Zhuang, Y.; Hong, Z.; Wu, L.; Zou, C.; Zheng, Y.; Chen, L.; Yin, L.; Qin, J. Influence of age on static postural control in adults with type 2 diabetes mellitus: A cross-sectional study. Front. Endocrinol. 2023, 14, 1242700. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Gustafsson, T.; Nyberg, L.; Röijezon, U. Predicting balance impairments in older adults: A wavelet-based center of pressure classification approach. Biomed. Eng. Online 2023, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Tao, X.; Zhu, L.; Qi, W.; Min, X.; Deng, H.; Wei, S.; Zhang, X.; Chang, X. A risk prediction system for depression in middle-aged and older adults grounded in machine learning and visualization technology: A cohort study. Front. Public Health 2025, 13, 1606316. [Google Scholar] [CrossRef]
- Oliosi, E.; Guede-Fernández, F.; Londral, A. Machine learning approaches for the frailty screening: A narrative review. Int. J. Env. Res. Public Health 2022, 19, 8825. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphine, S.; Williams, J.I.; Gayton, D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother. Can. 1989, 41, 304–311. [Google Scholar] [CrossRef]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef]
- Scoppa, F.; Capra, R.; Gallamini, M.; Shiffer, R. Clinical stabilometry standardization: Basic definitions–acquisition interval–sampling frequency. Gait Posture 2013, 37, 290–292. [Google Scholar] [CrossRef]
- Sarvari, M.; Shanbehzadeh, S.; Shavehei, Y.; ShahAli, S. Postural control among older adults with fear of falling and chronic low back pain. BMC Geriatr. 2024, 24, 862. [Google Scholar] [CrossRef]
- Promsri, A.; Pitiwattanakulchai, P.; Saodan, S.; Thiwan, S. Age-Related Changes in Postural Stability in Response to Varying Surface Instability in Young and Middle-Aged Adults. Sensors 2024, 24, 6846. [Google Scholar] [CrossRef] [PubMed]
- Schülein, S.; Sieber, C.C.; Gaßmann, K.-G.; Ritt, M. Frail Older Individuals Maintaining a Steady Standing Position: Associations Between Sway Measurements with Frailty Status Across Four Different Frailty Instruments. Clin. Interv. Aging 2020, 15, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Falk, J.; Strandkvist, V.; Pauelsen, M.; Vikman, I.; Nyberg, L.; Röijezon, U. Increased co-contraction reaction during a surface perturbation is associated with unsuccessful postural control among older adults. BMC Geriatr. 2022, 22, 438. [Google Scholar] [CrossRef] [PubMed]
- Van Humbeeck, N.; Kliegl, R.; Krampe, R.T. Lifespan changes in postural control. Sci. Rep. 2023, 13, 541. [Google Scholar] [CrossRef] [PubMed]
- Wapp, C.; Hager, A.-G.M.; Rikkonen, T.; Hilfiker, R.; Biver, E.; Ferrari, S.; Kröger, H.; Zwahlen, M.; Zysset, P. Validation of a fall rate prediction model for community-dwelling older adults: A combined analysis of three cohorts with 1850 participants. BMC Geriatr. 2024, 24, 287. [Google Scholar] [CrossRef]
- Huang, L.; Shen, X.; Zou, Y.; Wang, Y. Effects of BMI and grip strength on older adults’ falls—A longitudinal study based on CHARLS. Front. Public Health 2024, 12, 1415360. [Google Scholar] [CrossRef]
- Yu, L.; Cao, S.; Song, B.; Hu, Y. Predicting grip strength-related frailty in middle-aged and older Chinese adults using interpretable machine learning models: A prospective cohort study. Front. Public Health 2024, 12, 1489848. [Google Scholar] [CrossRef]
- Wu, K.-Y.; Chen, D.-R.; Chan, C.-C.; Yeh, Y.-P.; Chen, H.-H. Fear of falling as a mediator in the association between social frailty and health-related quality of life in community-dwelling older adults. BMC Geriatr. 2023, 23, 421. [Google Scholar] [CrossRef]
- Baek, W.; Min, A.; Ji, Y.; Park, C.G.; Kang, M. Impact of activity limitations due to fear of falling on changes in frailty in Korean older adults: A longitudinal study. Sci. Rep. 2024, 14, 19121. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Peng, W.; Luo, Y.; Tang, S.; Liu, M. Bidirectional relationship between fear of falling and frailty among community-dwelling older adults: A longitudinal study. Geriatr. Nurs. 2023, 51, 286–292. [Google Scholar] [CrossRef] [PubMed]
| Variable | Pre-Frail (n = 21) | Frail (n = 62) | p-Value |
|---|---|---|---|
| Age (years) | 54.29 (9.44) | 69.10 (8.30) | <0.001 |
| Gender (% male) | 33.3% | 42.0% | 0.661 |
| Height (cm) | 158.95 (8.70) | 167.82 (7.80) | <0.001 |
| Weight (kg) | 76.48 (16.40) | 77.65 (12.21) | 0.192 |
| BMI (kg/m2) | 29.02 (7.05) | 27.68 (4.90) | 0.814 |
| Grip Strength (kg) | 35.73 (6.73) | 26.14 (5.52) | <0.001 |
| No. of Falls | 0.24 (0.44) | 2.71 (1.61) | <0.001 |
| TUG (s) | 11.74 (1.04) | 14.00 (2.29) | <0.001 |
| BBS | 49.84 (6.47) | 42.74 (4.63) | <0.001 |
| FES | 19.13 (18.05) | 27.00 (9.69) | <0.001 |
| MV_EO (cm/s) | 2.81 (2.75) | 9.16 (3.77) | <0.001 |
| MV_EC (cm/s) | 2.97 (2.58) | 8.26 (2.85) | <0.001 |
| SP_EO (cm2) | 3.14 (1.36) | 5.81 (2.16) | <0.001 |
| SP_EC (cm2) | 3.19 (1.24) | 5.26 (1.45) | <0.001 |
| Clinical Measure | Posturography Variable | ρ (Spearman) | 95% CI (Lower–Upper) | p-Value |
|---|---|---|---|---|
| TUG | MV_EO | −0.645 | [−0.758, −0.494] | <0.001 |
| MV_EC | −0.620 | [−0.748, −0.456] | <0.001 | |
| SP_EO | −0.393 | [−0.610, −0.146] | 0.001 | |
| SP_EC | −0.336 | [−0.566, −0.036] | 0.002 | |
| BBS | MV_EO | 0.803 | [0.693, 0.882] | <0.001 |
| MV_EC | 0.720 | [0.593, 0.812] | <0.001 | |
| SP_EO | 0.681 | [0.523, 0.795] | <0.001 | |
| SP_EC | 0.565 | [0.366, 0.715] | <0.001 | |
| FES | MV_EO | −0.395 | [−0.611, −0.149] | <0.001 |
| MV_EC | −0.371 | [−0.594, −0.122] | 0.001 | |
| SP_EO | −0.348 | [−0.579, −0.093] | 0.001 | |
| SP_EC | −0.343 | [−0.570, −0.088] | 0.001 | |
| No of Falls | MV_EO | −0.597 | [−0.732, −0.432] | <0.001 |
| MV_EC | −0.537 | [−0.678, −0.352] | <0.001 | |
| SP_EO | −0.422 | [−0.629, −0.221] | <0.001 | |
| SP_EC | −0.427 | [−0.632, −0.226] | <0.001 |
| Predictor Variable | Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| MV_EO | 0.747 (0.593–0.941) | 0.013 | 0.635 (0.525–0.767) | <0.001 |
| MV_EC | 0.724 (0.546–0.959) | 0.024 | 0.597 (0.481–0.740) | <0.001 |
| SP_EO | 0.616 (0.408–0.931) | 0.021 | 0.432 (0.293–0.636) | <0.001 |
| SP_EC | 0.528 (0.296–0.940) | 0.030 | 0.323 (0.189–0.554) | <0.001 |
| TUG | 2.418 (1.302–4.488) | 0.005 | 2.607 (1.621–4.194) | <0.001 |
| Grip Strength | 0.898 (0.804–1.002) | 0.055 | 0.801 (0.725–0.886) | <0.001 |
| BBS | 0.913 (0.814–1.023) | 0.117 | 0.806 (0.729–0.892) | <0.001 |
| Number of Falls | 7.234 (1.969–26.571) | 0.003 | 3.652 (2.112–6.313) | <0.001 |
| FES | 1.053 (1.005–1.103) | 0.028 | 1.078 (1.016–1.143) | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhasan, H.S. Identifying Frailty Risk in Older Adults: The Predictive Value of Functional Tests and Center-of-Pressure-Based Postural Metrics. J. Clin. Med. 2025, 14, 6266. https://doi.org/10.3390/jcm14176266
Alhasan HS. Identifying Frailty Risk in Older Adults: The Predictive Value of Functional Tests and Center-of-Pressure-Based Postural Metrics. Journal of Clinical Medicine. 2025; 14(17):6266. https://doi.org/10.3390/jcm14176266
Chicago/Turabian StyleAlhasan, Hammad S. 2025. "Identifying Frailty Risk in Older Adults: The Predictive Value of Functional Tests and Center-of-Pressure-Based Postural Metrics" Journal of Clinical Medicine 14, no. 17: 6266. https://doi.org/10.3390/jcm14176266
APA StyleAlhasan, H. S. (2025). Identifying Frailty Risk in Older Adults: The Predictive Value of Functional Tests and Center-of-Pressure-Based Postural Metrics. Journal of Clinical Medicine, 14(17), 6266. https://doi.org/10.3390/jcm14176266





