Glycemic Analysis and Stratification of Pediatric Patients with Type 1 Diabetes Using isCGM in Southern Spain: Insights from the Andiacare Digital Platform
Abstract
1. Introduction
- (I)
- Time in very low hypoglycemia (<54 mg/dL);
- (II)
- Time in low hypoglycemia (54–70 mg/dL);
- (III)
- Time in high hyperglycemia (180–250 mg/dL) and;
- (IV)
- Time in very high hyperglycemia (>250 mg/dL).
- Zone A (Green; 0–20);
- Zone B (Yellow; 21–40);
- Zone C (Orange; 41–60);
- Zone D (Light red; 61–80);
- Zone E (Dark red; 81–100).
2. Materials and Methods
2.1. Study Design
2.2. Cohort Description
2.3. Ethics
2.4. Patient and Public Involvement
2.5. Andiacare Platform
- File format detection and loading of structured patient data.
- Filtering patients by quality of their data, proceeding to eliminate those that contain erroneous entries or missing data in fields marked as essential. To ensure the quality of the analyzed data, automatic exclusion criteria were applied through the Andiacare platform. Patients with sensor usage time < 70% were excluded.
- ATTD19 Target Check: Following the ATTD19 targets for time in range, under range, or over range, a simple compliance or non-compliance check is performed for each patient.
- Calculation of statistics of the main fields, being able to segment sub-cohorts by age ranges, health centers of origin or objective criteria ATTD19.
- Patient classification: the system applies as many algorithms as requested.
- (I)
- Green: TIR > 70%, TBR1 < 4% and TAR1 < 25%.
- (II)
- Yellow: 40% < TIR ≤ 70% or 4% < TBR1 < 11% or 25% ≤ TAR1 < 50%.
- (III)
- Orange: 25% ≤ TIR < 40% or 11% ≤ TBR1 < 20% or 50% ≤ TAR1 < 75%.
- (IV)
- Red: TIR < 25% or TBR1 > 20% or TAR1 ≥ 75%.
2.6. Statistical Analysis
3. Results
3.1. Description of the Glucometric Data
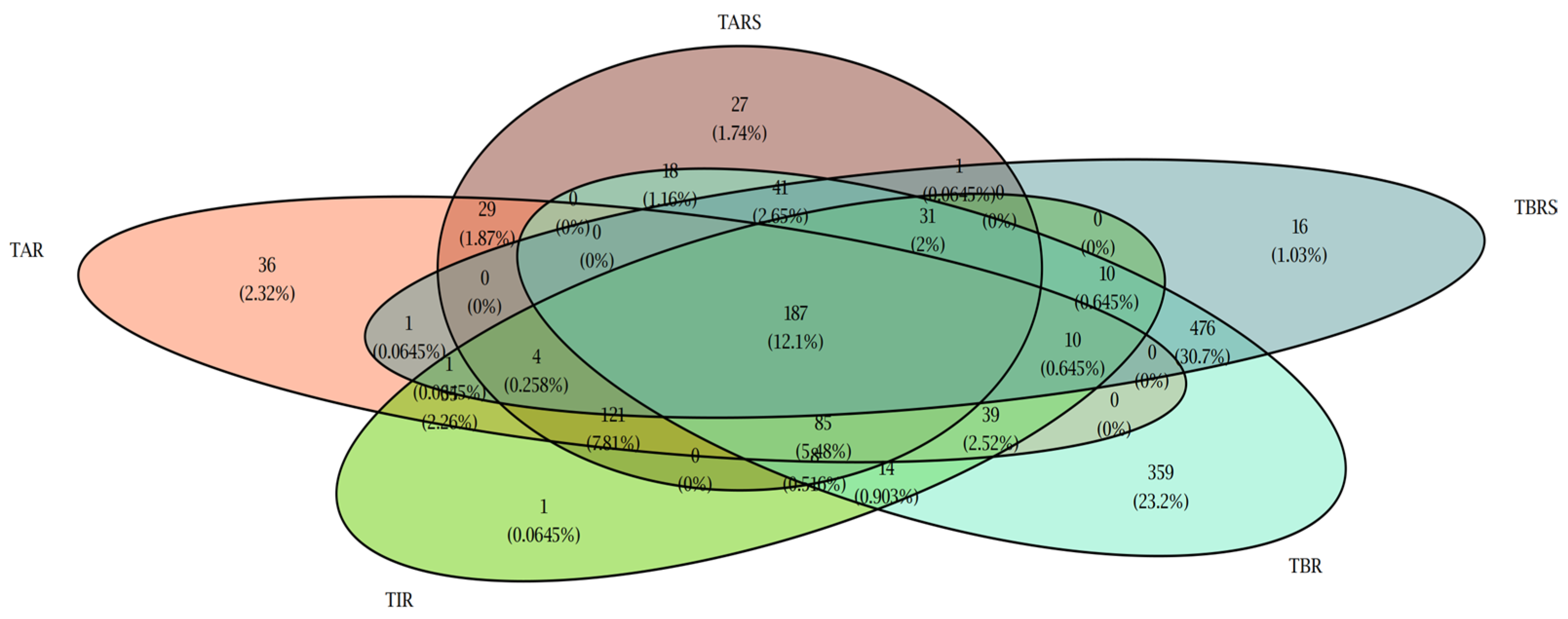
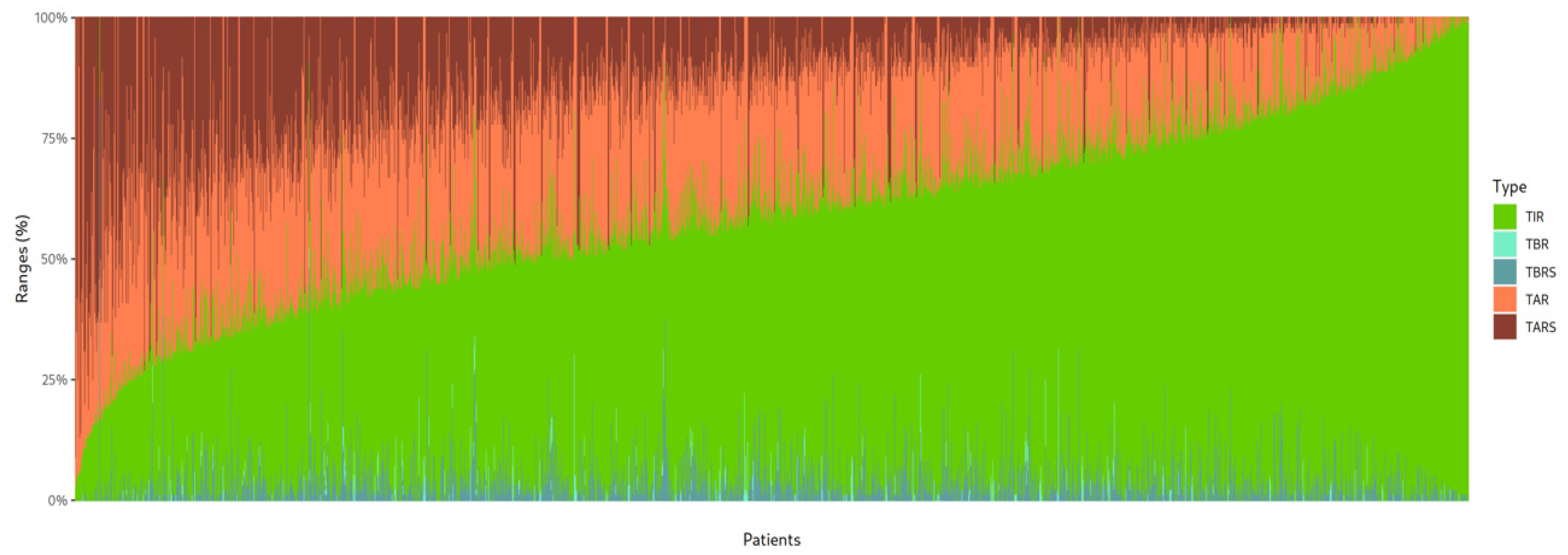
3.2. Glucometric Control Using Andiacare Algorithm
3.3. Correlation Between Glucometric Parameters and Number of Daily Scans
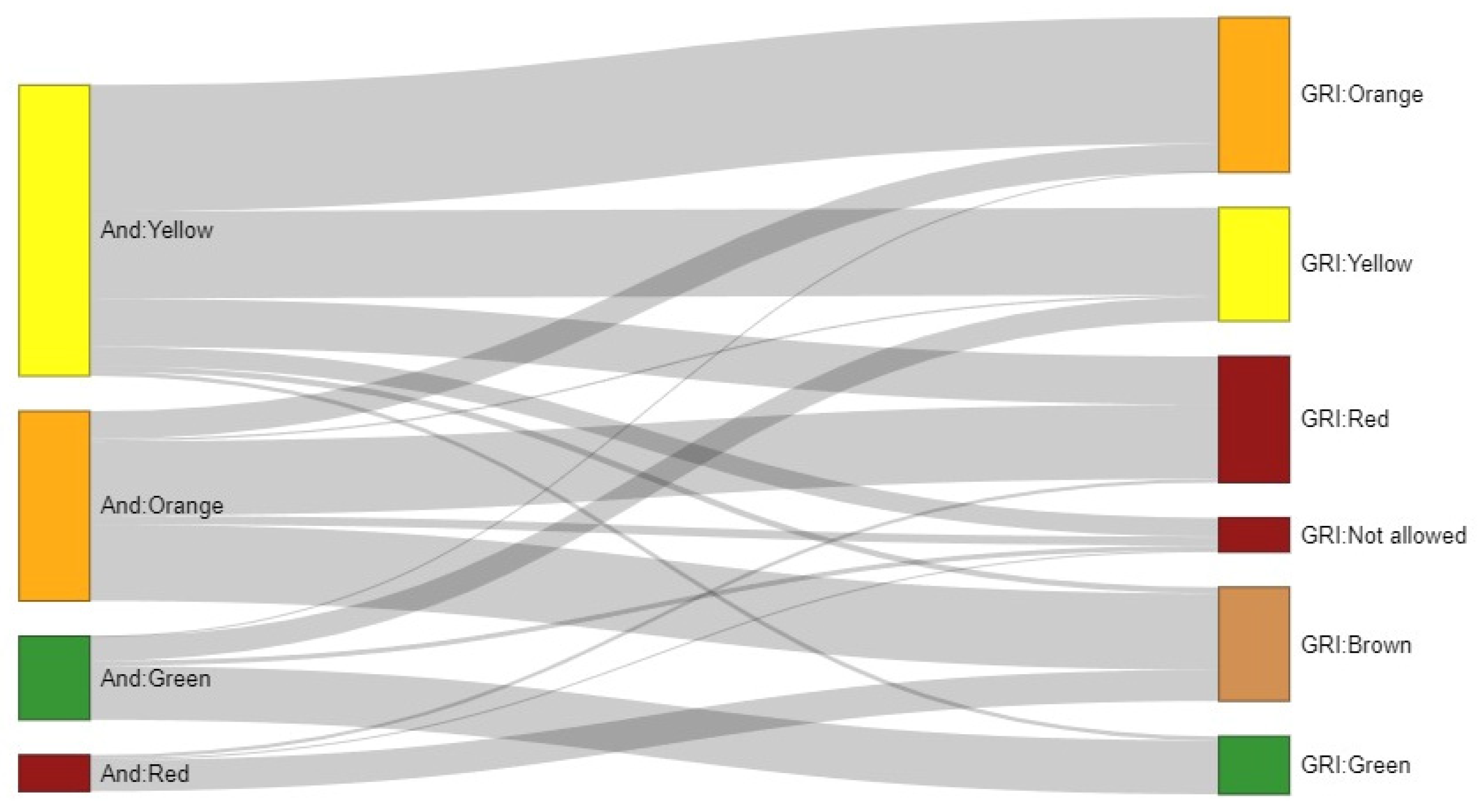
3.4. Comparison Between Andiacare Platform and GRI Algorithms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Prior Presentation
Abbreviations
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| ATTD | Advanced Technologies and Treatments for Diabetes |
| TIR | Time In Range |
| TAR1 | Time Above Range level 1 |
| TAR2 | Time Above Range level 2 |
| TBR1 | Time Below Range level 1 |
| TBR2 | Time Below Range level 2 |
| CV | Coefficient of variation |
| MDI | Multiple daily injections of insulin |
| CSII | Continuous subcutaneous insulin infusion |
| CGM | Continuous glucose monitoring |
| rtCGM | Real-time continuous glucose monitoring |
| isCGM | Intermittently scanned continuous glucose monitoring |
| GRI | Glycemic Risk Index |
| GMI | Glucose Management Indicator |
| SD | Standard deviation |
| IQR | Interquartile range |
References
- David, R.; Weber, J.N. Diabetes Mellitus in Children. In Nelson Textbook of Pediatrics, 21st ed.; Kliegman, R.M., Geme, J.W.S., Blum, N.J., Shah, S.S., Tasker, R.C., Wilson, K.M., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 3019–3052. [Google Scholar]
- Ramírez-Mendoza, F.; González, J.E.; Gasca, E.; Camacho, M.; Cruz, M.V.; Caraveo, D.; Velázquez, A.; Cruz, Z.; Segoviano, M.; Romano, M.; et al. Time in range and HbA1C after 6 months with a multidisciplinary program for children and adolescents with diabetes mellitus, real world data from Mexico City. Pediatr. Diabetes 2020, 21, 61–68. [Google Scholar] [CrossRef]
- Nathan, D.M.; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef]
- Cardona-Hernandez, R.; Schwandt, A.; Alkandari, H.; Bratke, H.; Chobot, A.; Coles, N.; Corathers, S.; Goksen, D.; Goss, P.; Imane, Z.; et al. Glycemic Outcome Associated With Insulin Pump and Glucose Sensor Use in Children and Adolescents With Type 1 Diabetes. Data From the International Pediatric Registry SWEET. Diabetes Care 2021, 44, 1176–1184. [Google Scholar] [CrossRef]
- Urakami, T. Significance of the CGM metric of time in range in children and adolescents with type 1 diabetes. Endocr. J. 2022, 69, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Dovc, K.; Lanzinger, S.; Cardona-Hernandez, R.; Tauschmann, M.; Marigliano, M.; Cherubini, V.; Preikša, R.; Schierloh, U.; Clapin, H.; AlJaser, F.; et al. Association of Achieving Time in Range Clinical Targets with Treatment Modality Among Youths With Type 1 Diabetes. JAMA Netw. Open 2023, 6, e230077. [Google Scholar] [CrossRef]
- Advani, A. Data from: Positioning time in range in diabetes management. Diabetologia 2020, 63, 242–252. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, V.; Bonfanti, R.; Casertano, A.; De Nitto, E.; Iannilli, A.; Lombardo, F.; Maltoni, G.; Marigliano, M.; Bassi, M.; Minuto, N.; et al. Time In Range in Children with Type 1 Diabetes Using Treatment Strategies Based on Nonautomated Insulin Delivery Systems in the Real World. Diabetes Technol. Ther. 2020, 22, 509–515. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Wang, J.; Rodbard, D.; Kohn, M.A.; Li, C.; Liepmann, D.; Kerr, D.; Ahn, D.; Peters, A.L.; Umpierrez, G.E.; et al. A Glycemia Risk Index (GRI) of Hypoglycemia and Hyperglycemia for Continuous Glucose Monitoring Validated by Clinician Ratings. J. Diabetes Sci. Technol. 2023, 17, 1226–1242. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Soto, G.; Pérez-López, P.; Férnandez-Velasco, P.; Marca, M.d.l.O.N.d.l.; Delgado, E.; del Amo, S.; de Luis, D.; Bahillo-Curieses, P. Glycemia Risk Index Assessment in a Pediatric and Adult Patient Cohort With Type 1 Diabetes Mellitus. J. Diabetes Sci. Technol. 2024, 18, 1063–1069. [Google Scholar] [CrossRef]
- Panfil, K.; Vandervelden, C.A.; Lockee, B.; Tallon, E.M.; Williams, D.D.; Lee, J.M. The Glycemia Risk Index Predicts Performance of Diabetes Self-Management Habits in Youth With Type 1 Diabetes Mellitus. J. Diabetes Sci. Technol. 2024, 18, 779–786. [Google Scholar] [CrossRef]
- Panfil, K.; Redel, J.M.; Vandervelden, C.A.; Lockee, B.; Kahkoska, A.R.; Tallon, E.M.; Williams, D.D.; Clements, M.A. Data from: Correlation Between the Glycemia Risk Index and Longitudinal Hemoglobin A1c in Children and Young Adults with Type 1 Diabetes. J. Diabetes Sci. Technol. 2024, 18, 771–778. [Google Scholar] [CrossRef] [PubMed]
- López Siguero, J.P.; Pérez González, O.; Gómez Gila, A.L.; Leiva Gea, I. Situación de la diabetes mellitus tipo 1 en Andalucía. Datos asistenciales, uso de terapias avanzadas y recursos humanos. An. Pediatr. 2018, 89, 111–116. [Google Scholar] [CrossRef]
- Díaz-Soto, G.; Bahíllo-Curieses, M.P.; Jimenez, R.; Nieto, M.d.l.O.; Gomez, E.; Torres, B.; Gomez, J.J.L.; de Luis, D. Relación entre hemoglobina glucosilada, tiempo en rango y variabilidad glucémica en una cohorte de pacientes pediátricos y adultos con diabetes tipo 1 con monitorización flash de glucosa. Endocrinol. Diabetes Nutr. 2021, 68, 465–471. [Google Scholar] [CrossRef]
- Evans, M.; Welsh, Z.; Seibold, A. Reductions in HbA1c with Flash Glucose Monitoring Are Sustained for up to 24 Months: A Meta-Analysis of 75 Real-World Observational Studies. Diabetes Ther. 2022, 13, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.M.; Murphy, N.P.; Stewart, C.; Biester, T.; Kordonouri, O. Outcomes of using flash glucose monitoring technology by children and young people with type 1 diabetes in a single arm study. Pediatr. Diabetes 2018, 19, 1294–1301. [Google Scholar] [CrossRef]
- Suzuki, J.; Urakami, T.; Yoshida, K.; Kuwabara, R.; Mine, Y.; Aoki, M.; Morioka, I. Association between scanning frequency of flash glucose monitoring and continuous glucose monitoring-derived glycemic makers in children and adolescents with type 1 diabetes. Pediatr. Int. 2021, 63, 154–159. [Google Scholar] [CrossRef]
- Gomez-Peralta, F.; Dunn, T.; Landuyt, K.; Xu, Y.; Merino-Torres, J.F. Flash glucose monitoring reduces glycemic variability and hypoglycemia: Real-world data from Spain. BMJ Open Diabetes Res. Care 2020, 8, e001052. [Google Scholar] [CrossRef]
- Bahíllo-Curieses, M.P.; Díaz-Soto, G.; Vidueira-Martínez, A.M.; Torres-Ballester, I.; Gómez-Hoyos, E.; de Luis-Román, D. Assessment of metabolic control and use of flash glucose monitoring systems in a cohort of pediatric, adolescents, and adults patients with Type 1 diabetes. Endocrine 2021, 73, 47–51. [Google Scholar] [CrossRef]
- Edge, J.; Acerini, C.; Campbell, F.; Hamilton-Shield, J.; Moudiotis, C.; Rahman, S.; Randell, T.; Smith, A.; Trevelyan, N. An alternative sensor-based method for glucose monitoring in children and young people with diabetes. Arch. Dis. Child. 2017, 102, 543–549. [Google Scholar] [CrossRef]
- Wake, D.J.; He, J.; Czesak, A.M.; Mughal, F.; Cunningham, S.G. MyDiabetesMyWay: An Evolving National Data Driven Diabetes Self-Management Platform. J. Diabetes Sci. Technol. 2016, 10, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Casaño, M.; Alonso Montejo, M.D.M.; Leiva Gea, I.; Hinojosa, J.M.J.; Mata, M.Á.S.; Macías, F.; Pérez, M.d.M.R.; de Toro, M.; Martínez, G.; Munguira, P.; et al. Study of direct costs of type 1 diabetes mellitus in Andalusian patients aged 2–16 years. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2019, 66, 480–486. [Google Scholar] [CrossRef]
- Leiva-Gea, I.; Porcel Chacón, R.; Ariza Jiménez, A.B.; Loro, M.M.; Tapia-Ceballos, L.; Jiménez-Hinojosa, J.; Perea, A.G.; Siguero, J.P.L. Impact on variables of severe hypoglycaemia and healthcare costs of the use of the FreeStyle system in paediatric population with type 1 diabetes mellitus. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2022, 69, 561–565. [Google Scholar] [CrossRef]
- Leiva-Gea, I.; Martos-Lirio, M.F.; Gómez-Perea, A.; Ariza-Jiménez, A.-B.; Tapia-Ceballos, L.; Jiménez-Hinojosa, J.M.; Lopez-Siguero, J.P. Metabolic Control of the FreeStyle Libre System in the Pediatric Population with Type 1 Diabetes Dependent on Sensor Adherence. J. Clin. Med. 2022, 11, 286. [Google Scholar] [CrossRef]
- Urakami, T.; Terada, H.; Tanabe, S.; Mine, Y.; Aoki, M.; Aoki, R.; Suzuki, J.; Morioka, I. Clinical significance of coefficient of variation in continuous glucose monitoring for glycemic management in children and adolescents with type 1 diabetes. J. Diabetes Investig. 2024, 15, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, L.A.; Kanapka, L.G.; DeSalvo, D.J.; Anderson, B.J.; Harrington, K.R.; Hilliard, M.E.; Laffel, L.M.; Tamborlane, W.V.; Van Name, M.A.; Wadwa, R.P.; et al. Time spent outside of target glucose range for young children with type 1 diabetes: A continuous glucose monitor study. Diabet. Med. 2020, 37, 1308–1315. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Hybrid Closed Loop Systems for Managing Blood Glucose Levels in Type 1 Diabetes. Published online 19 December 2023. Available online: https://www.nice.org.uk/guidance/ta943 (accessed on 8 April 2024).
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Diabetes Technology: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. 1), S111–S127. [Google Scholar] [CrossRef]
- Phillip, M.; Nimri, R.; Bergenstal, R.M.; Barnard-Kelly, K.; Danne, T.; Hovorka, R.; Kovatchev, B.P.; Messer, L.H.; Parkin, C.G.; Ambler-Osborn, L.; et al. Consensus Recommendations for the Use of Automated Insulin Delivery Technologies in Clinical Practice. Endocr. Rev. 2023, 44, 254–280. [Google Scholar] [CrossRef] [PubMed]
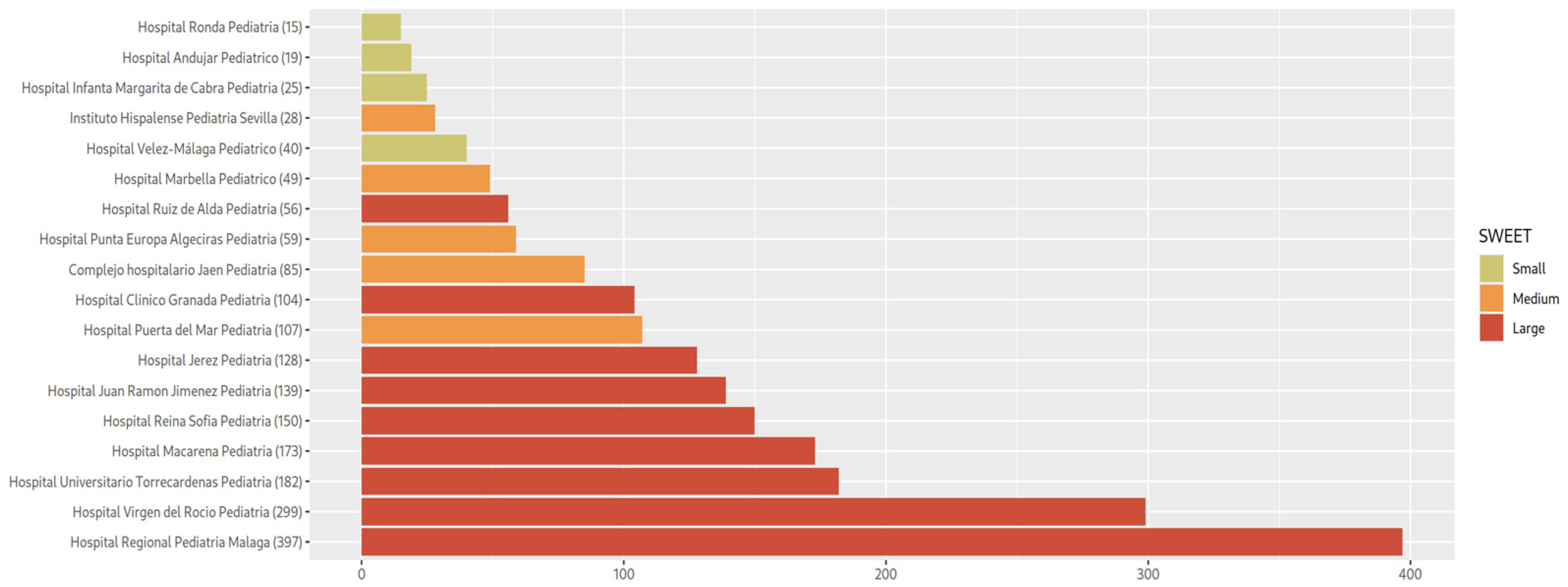
| Age Group (Size) | Mean ± SD | Meet ATTD Targets (Size) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TAR2 | TAR1 | TIR | TBR1 | TBR2 | Daily Scan Reads | TAR2 | TAR1 | TIR | TBR1 | TBR2 | |
| Age 12–18 (1315) | 17.27 ± 16.31 | 40.43 ± 20.35 | 54.2 ± 19.48 | 4.65 ± 5.83 | 4.34 ± 5.58 | 9.12 ± 8.04 | 23.57% (310) | 23.04% (303) | 21.29% (280) | 55.36% (728) | 34.29% (451) |
| Age 6–12 (776) | 12.14 ± 11.68 | 34.49 ± 18.21 | 62.07 ± 17.88 | 3.45 ± 4.29 | 3.23 ± 4.05 | 13.75 ± 11.76 | 30.67% (238) | 31.06% (241) | 32.99% (256) | 68.43% (531) | 40.72% (316) |
| Age 4–6 (124) | 14.04 ± 13.59 | 35.82 ± 18.76 | 60.46 ± 17.39 | 3.72 ± 4.78 | 3.63 ± 4.72 | 20.38 ± 18.13 | 28.23% (35) | 27.42% (34) | 29.03% (36) | 66.94% (83) | 43.55% (54) |
| TOTAL (2215) | 15.29 ± 14.89 | 38.09 ± 19.74 | 57.73 ± 19.12 | 4.18 ± 5.31 | 3.91 ± 4.07 | 11.42 ± 10.76 | 26.32% (583) | 26.09% (578) | 25.82% (572) | 60.59% (1342) | 37.07% (821) |
| Age Group | p Value | |||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 1–2 | Group 1–3 | Group 2–3 | |
| TAR2 (%) | 14.04 ± 13.59 | 12.14 ± 11.68 | 17.27 ± 16.31 | 0.279 | 0.056 | 2.58 × 10−7 |
| TAR1 (%) | 35.82 ± 18.76 | 34.49 ± 18.21 | 40.43 ± 20.35 | 0.637 | 0.036 | 8.06 × 10−7 |
| TIR (%) | 60.46 ± 17.39 | 62.07 ± 17.88 | 54.2 ± 19.48 | 0.508 | 0.004 | 1.56 × 10−9 |
| TBR1 (%) | 3.72 ± 4.78 | 3.45 ± 4.29 | 4.65 ± 5.83 | 0.859 | 0.859 | 2.66 × 10−4 |
| TBR2 (%) | 3.63 ± 4.72 | 3.23 ± 4.05 | 4.34 ± 5.58 | 0.703 | 0.278 | 5.96 × 10−4 |
| Mean Reads | 20.38 ± 18.13 | 13.75 ± 11.76 | 9.12 ± 8.04 | 0.004 | 5.43 × 10−6 | 1.05 × 10−12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiva-Gea, I.; Moreno-Jabato, F.; Ariza-Jiménez, A.B.; Lendínez-Jurado, A.; Gómez-Perea, A.; Romero-Pérez, M.d.M.; García-García, E.; Santos Mata, M.Á.; Martínez-Moya, G.; Momblan, J.; et al. Glycemic Analysis and Stratification of Pediatric Patients with Type 1 Diabetes Using isCGM in Southern Spain: Insights from the Andiacare Digital Platform. J. Clin. Med. 2025, 14, 6243. https://doi.org/10.3390/jcm14176243
Leiva-Gea I, Moreno-Jabato F, Ariza-Jiménez AB, Lendínez-Jurado A, Gómez-Perea A, Romero-Pérez MdM, García-García E, Santos Mata MÁ, Martínez-Moya G, Momblan J, et al. Glycemic Analysis and Stratification of Pediatric Patients with Type 1 Diabetes Using isCGM in Southern Spain: Insights from the Andiacare Digital Platform. Journal of Clinical Medicine. 2025; 14(17):6243. https://doi.org/10.3390/jcm14176243
Chicago/Turabian StyleLeiva-Gea, Isabel, Fernando Moreno-Jabato, Ana Belén Ariza-Jiménez, Alfonso Lendínez-Jurado, Ana Gómez-Perea, María del Mar Romero-Pérez, Emilio García-García, María Ángeles Santos Mata, Gabriela Martínez-Moya, Jerónimo Momblan, and et al. 2025. "Glycemic Analysis and Stratification of Pediatric Patients with Type 1 Diabetes Using isCGM in Southern Spain: Insights from the Andiacare Digital Platform" Journal of Clinical Medicine 14, no. 17: 6243. https://doi.org/10.3390/jcm14176243
APA StyleLeiva-Gea, I., Moreno-Jabato, F., Ariza-Jiménez, A. B., Lendínez-Jurado, A., Gómez-Perea, A., Romero-Pérez, M. d. M., García-García, E., Santos Mata, M. Á., Martínez-Moya, G., Momblan, J., Lechuga-Sancho, A. M., Gómez-Vida, J. M., Mier-Palacios, M., Ranchal-Pérez, M. d. P., Vivas-González, G., Calleja Cabeza, P., Fernández-Hernández, E., Jiménez-Martín, A. P., Guarino-Narváez, J., ... Martínez-Brocca, M. A. (2025). Glycemic Analysis and Stratification of Pediatric Patients with Type 1 Diabetes Using isCGM in Southern Spain: Insights from the Andiacare Digital Platform. Journal of Clinical Medicine, 14(17), 6243. https://doi.org/10.3390/jcm14176243






