Serum Zonulin and Lipopolysaccharide (LPS) Levels in Early Myocardial Infarction: Association with Left Ventricular Ejection Fraction
Abstract
1. Introduction
1.1. Lipopolysaccharide (LPS) and Cardiovascular Health
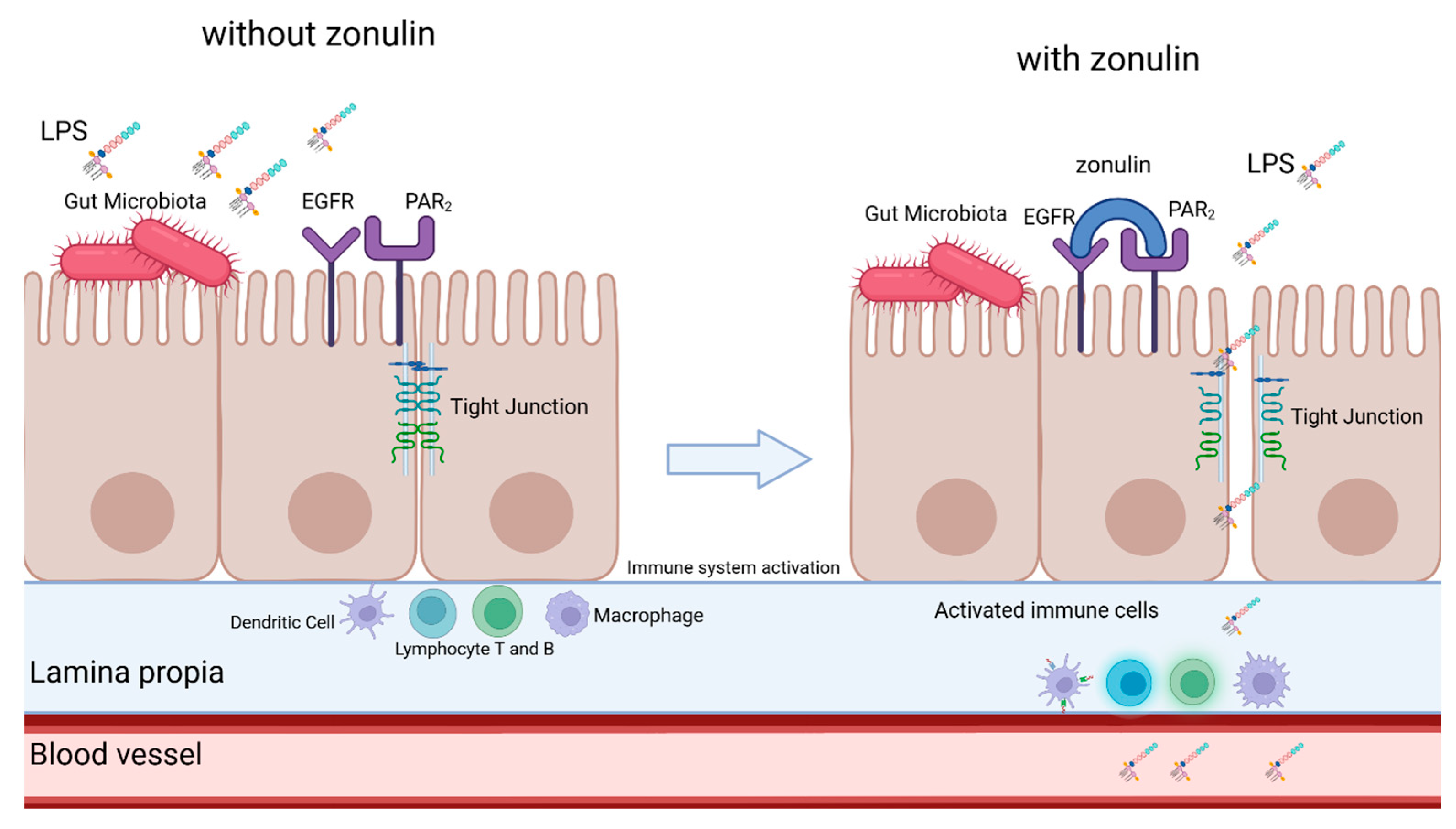
1.2. Zonulin and Gut-Blood Barrier Integrity
1.3. Study Objectives
2. Materials and Methods
2.1. Patients
2.2. Myocardial Infarction Definition
2.3. Serum Collection and Processing
2.4. Biochemical Analysis
2.5. Post-Myocardial Infarction Ejection Fraction
2.6. Statistical Analysis
3. Results
3.1. Zonulin and LPS Status in the Early Phase of MI
3.2. Patient Subgroup Analysis
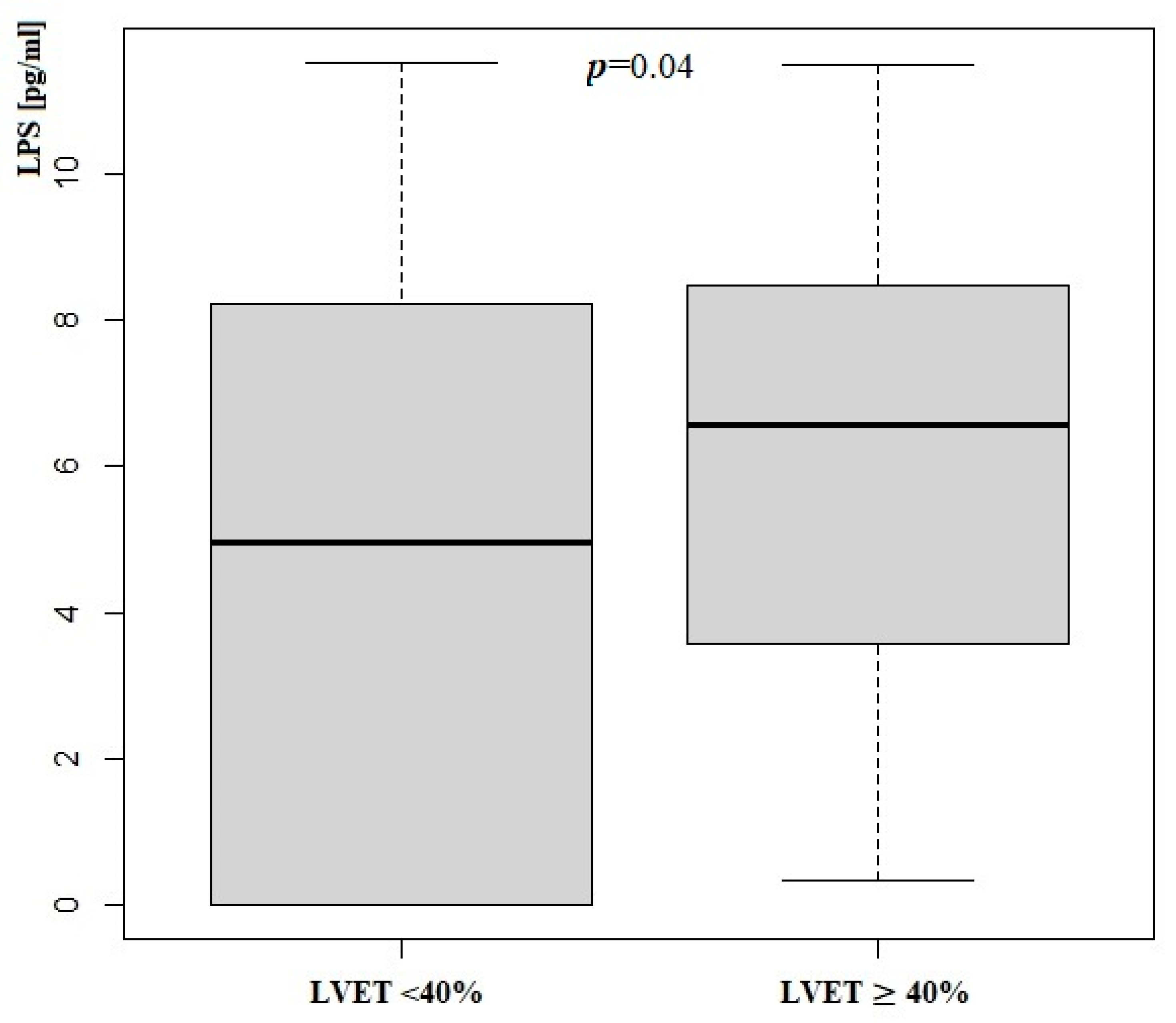
3.3. Zonulin and LPS Status According to LVEF
3.4. Correlation Between LPS or Zonulin and Anthropometric and Biochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Zhao, L.; Song, Y.; Qu, H.; Du, T.; Shi, L.; Cui, Z.; Jiang, Z.; Gao, Z. Trends in gut-heart axis and heart failure research (1993–2023): A bibliometric and visual analysis. Heliyon 2024, 10, e25995. [Google Scholar] [CrossRef] [PubMed]
- León Aguilera, X.E.; Manzano, A.; Pirela, D.; Bermúdez, V. Probiotics and Gut Microbiota in Obesity: Myths and Realities of a New Health Revolution. J. Pers. Med. 2022, 12, 1282. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, M.; Ferro, A.; Gruden, G. Gastrointestinal Microbiota and Type 1 Diabetes Mellitus: The State of Art. J. Clin. Med. 2019, 8, 1843. [Google Scholar] [CrossRef]
- Wu, J.; Yang, K.; Fan, H.; Wei, M.; Xiong, Q. Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1114424. [Google Scholar] [CrossRef]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef]
- Naik, S.S.; Ramphall, S.; Rijal, S.; Prakash, V.; Ekladios, H.; Saju, J.M.; Mandal, N.; I Kham, N.; Shahid, R.; Venugopal, S. Association of Gut Microbial Dysbiosis and Hypertension: A Systematic Review. Cureus 2022, 14, e29927. [Google Scholar] [CrossRef]
- Harikrishnan, S. Diet, the Gut Microbiome and Heart Failure. Card. Fail. Rev. 2019, 5, 119–122. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Sniffen, S.; Percy, K.C.M.; Pallaval, V.B.; Chidipi, B. Gut Dysbiosis and Immune System in Atherosclerotic Cardiovascular Disease (ACVD). Microorganisms 2022, 10, 108. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Stecher, B.; Hardt, W.D. The role of microbiota in infectious disease. Trends Microbiol. 2008, 16, 107–114. [Google Scholar] [CrossRef]
- Surana, N.K.; Kasper, D.L. Deciphering the tête-à-tête between the microbiota and the immune system. J. Clin. Investig. 2014, 124, 4197–4203. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Makwana, R.K.; Shetty, V.; Mukherjee, S.; Narayan, P. Cardiovascular diseases and the heart-gut cross talk. Indian Heart J. 2024, 76, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Akshay, A.; Gasim, R.; E Ali, T.; Kumar, Y.S.; Hassan, A. Unlocking the Gut-Cardiac Axis: A Paradigm Shift in Cardiovascular Health. Cureus 2023, 15, e51039. [Google Scholar] [CrossRef]
- Oktaviono, Y.H.; Lamara, A.D.; Saputra, P.B.T.; Arnindita, J.N.; Pasahari, D.; Saputra, M.E.; Suasti, N.M.A. The roles of trimethylamine-N-oxide in atherosclerosis and its potential therapeutic aspect: A literature review. Biomol. Biomed. 2023, 23, 936–948. [Google Scholar] [CrossRef]
- Lewis, C.V.; Taylor, W.R. Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1227–H1233. [Google Scholar] [CrossRef]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]
- Baldrian, P.; López-Mondéjar, R.; Kohout, P. Forest microbiome and global change. Nat. Rev. Microbiol. 2023, 21, 487–501. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Khosrojerdi, A.; Jamialahmadi, T.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Implications for the role of lipopolysaccharide in the development of atherosclerosis. Trends Cardiovasc. Med. 2022, 32, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.; West, M.A.; Zaru, R. TLR signalling regulated antigen presentation in dendritic cells. Curr. Opin. Immunol. 2010, 22, 124–130. [Google Scholar] [CrossRef]
- Balija, T.M.; Lowry, S.F. Lipopolysaccharide and sepsis-associated myocardial dysfunction. Curr. Opin. Infect. Dis. 2011, 24, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Sawa, Y.; Ueki, T.; Hata, M.; Iwasawa, K.; Tsuruga, E.; Kojima, H.; Ishikawa, H.; Yoshida, S. LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J. Histochem. Cytochem. 2008, 56, 97–109. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M.; Pihlsgård, M.; et al. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight junctions: From molecules to gastrointestinal diseases. Tissue Barriers 2023, 11, 2077620. [Google Scholar] [CrossRef]
- Żak-Gołąb, A.; Kocełak, P.; Aptekorz, M.; Zientara, M.; Juszczyk, Ł.; Martirosian, G.; Chudek, J.; Olszanecka-Glinianowicz, M. Gut microbiota, microinflammation, metabolic profile, and zonulin concentration in obese and normal weight subjects. Int. J. Endocrinol. 2013, 2013, 674106. [Google Scholar] [CrossRef]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 113 Pt 24, 4435–4440. [Google Scholar] [CrossRef]
- Veres-Székely, A.; Szász, C.; Pap, D.; Szebeni, B.; Bokrossy, P.; Vannay, Á. Zonulin as a Potential Therapeutic Target in Microbiota-Gut-Brain Axis Disorders: Encouraging Results and Emerging Questions. Int. J. Mol. Sci. 2023, 24, 7548. [Google Scholar] [CrossRef]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef]
- Fasano, A. Intestinal permeability and its regulation by zonulin: Diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 2012, 10, 1096–1100. [Google Scholar] [CrossRef]
- Jauregi-Miguel, A. The tight junction and the epithelial barrier in coeliac disease. Int. Rev. Cell Mol. Biol. 2021, 358, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.-M.; Niu, Y.; Zhang, X.; Yin, J.-B.; Zhao, C.-J.; Wang, R.-T. Serum Zonulin in HBV-Associated Chronic Hepatitis, Liver Cirrhosis, and Hepatocellular Carcinoma. Dis. Markers 2019, 2019, 5945721. [Google Scholar] [CrossRef]
- A Voulgaris, T.; Karagiannakis, D.; Hadziyannis, E.; Manolakopoulos, S.; Karamanolis, G.P.; Papatheodoridis, G.; Vlachogiannakos, J. Serum zonulin levels in patients with liver cirrhosis: Prognostic implications. World J. Hepatol. 2021, 13, 1394–1404. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Dughera, F.; Ribaldone, D.G.; Rosso, C.; Abate, M.L.; Pellicano, R.; Bresso, F.; Smedile, A.; Saracco, G.M.; Astegiano, M. Serum zonulin in patients with inflammatory bowel disease: A pilot study. Minerva Med. 2019, 110, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B.; Orho-Melander, M.; Nilsson, P.M. Higher Levels of Serum Zonulin May Rather Be Associated with Increased Risk of Obesity and Hyperlipidemia, Than with Gastrointestinal Symptoms or Disease Manifestations. Int. J. Mol. Sci. 2017, 18, 582. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Marta, D.; Dănău, A.; Lefter, A.; Tulbă, D.; Cozma, L.; Manole, E.; Gherghiceanu, M.; Ceafalan, L.C.; Popescu, B.O. Serum and Fecal Markers of Intestinal Inflammation and Intestinal Barrier Permeability Are Elevated in Parkinson’s Disease. Front. Neurosci. 2021, 15, 689723. [Google Scholar] [CrossRef]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl. Acad. Sci. USA 2009, 106, 16799–16804. [Google Scholar] [CrossRef]
- Kamo, T.; Akazawa, H.; Suzuki, J.-I.; Komuro, I. Novel Concept of a Heart-Gut Axis in the Pathophysiology of Heart Failure. Korean Circ. J. 2017, 47, 663–669. [Google Scholar] [CrossRef]
- Ramani, G.V.; Uber, P.A.; Mehra, M.R. Chronic heart failure: Contemporary diagnosis and management. Mayo Clin. Proc. 2010, 85, 180–195. [Google Scholar] [CrossRef]
- Li, C.; Gao, M.; Zhang, W.; Chen, C.; Zhou, F.; Hu, Z.; Zeng, C. Zonulin Regulates Intestinal Permeability and Facilitates Enteric Bacteria Permeation in Coronary Artery Disease. Sci. Rep. 2016, 6, 29142. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Carrera-Bastos, P.; Picazo, Ó.; Fontes-Villalba, M.; Pareja-Galeano, H.; Lindeberg, S.; Martínez-Selles, M.; Lucia, A.; Emanuele, E. Serum Zonulin and Endotoxin Levels in Exceptional Longevity versus Precocious Myocardial Infarction. Aging Dis. 2018, 9, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, N.T.; Foley, J.B. Inflammation in acute coronary syndromes. Heart 2002, 87, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Perticone, M.; Gigliotti, S.; Shehaj, E.; Maio, R.; Suraci, E.; Miceli, S.; Andreozzi, F.; Matera, G.; Perticone, F. Gut Permeability and Immune-Mediated Inflammation in Heart Failure. Biomedicines 2024, 12, 1217. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Wenying, S.; Jing, H.; Ying, L.; Hui, D. The role of TLR4/MyD88/NF-κB in the protective effect of ulinastatin on the intestinal mucosal barrier in mice with sepsis. BMC Anesthesiol. 2023, 23, 414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bui, T.V.A.; Hwangbo, H.; Lai, Y.; Hong, S.B.; Choi, Y.-J.; Park, H.-J.; Ban, K. The Gut-Heart Axis: Updated Review for The Roles of Microbiome in Cardiovascular Health. Korean Circ. J. 2023, 53, 499–518. [Google Scholar] [CrossRef] [PubMed]

| Exclusion Criteria |
|---|
| hematologic diseases |
| liver diseases—AST or ALT > 150 UI/L |
| kidney diseases—GFR < 30 mL/min/1.73 m2 |
| PCI complication |
| hypersensitivity reactions to antiplatelet drugs |
| Parameters | All Patients | |||
|---|---|---|---|---|
| Median | IQR | Mean | SD | |
| Age [years] | 66.3 | 18.21 | 65.2 | 11.7 |
| LVEF [%] | 49 | 13.03 | 52 | 10.4 |
| TNT [µg/L] | 0.11 | 0.51 | 0.34 | 0.45 |
| Hba1c [%] | 5.8 | 0.6 | 5.2 | 1.8 |
| LDL [mg/dL] | 124.5 | 62.7 | 172 | 89 |
| WBC [g/L] | 9.52 | 3.95 | 9.08 | 4.87 |
| PLT [g/L] | 221.5 | 72.5 | 294 | 85 |
| Hgb [mmol/L] | 9.15 | 1.15 | 9.68 | 1.54 |
| RBC [t/L] | 4.6 | 0.67 | 4.8 | 0.82 |
| GFR [mL/min/1.73 m2] | 82 | 32 | 92 | 46 |
| BMI [kg/m2] | 27.9 | 5.59 | 28.3 | 4.4 |
| Parameters | All Patients | |
|---|---|---|
| Median | IQR | |
| Zonulin [µg/mL] | 2.15 | 0.51 |
| LPS [pg/mL] | 6.19 | 5.07 |
| Parametrs | LVEF ≥ 40% | LVEF < 40% | p | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Age [years] | 67 | 17.3 | 57 | 13 | 0.69 |
| LVEF % | 51 | 10 | 34 | 11 | p = 0.001 |
| TNT [µg/L] | 0.116 | 0.52 | 0.213 | 0.83 | 0.42 |
| Hba1c [%] | 5.8 | 0.8 | 5.9 | 0.32 | 0.44 |
| LDL [mg/dL] | 138 | 66.5 | 123.5 | 45 | 0.38 |
| WBC [g/L] | 9.9 | 3.84 | 10.46 | 5.69 | 0.32 |
| PLT [g/L] | 221 | 92 | 221.5 | 37.5 | 0.89 |
| Hgb [mmol/L] | 9 | 1.05 | 9.3 | 0.77 | 0.81 |
| RBC [t/L] | 4.62 | 0.74 | 4.82 | 0.41 | 0.73 |
| GFR [mL/min/1.73 m2] | 81.5 | 28 | 81.5 | 26 | 0.84 |
| BMI [kg/m2] | 28.3 | 5.52 | 26.23 | 4.69 | 0.08 |
| Parameters | LVEF ≥ 40 % | LVEF < 40 % | p | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Zonulin [µg/mL] | 2.17 | 0.53 | 2.2 | 0.57 | 0.47 |
| LPS [pg/mL] | 4.97 | 5.75 | 6.34 | 5.22 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olędzki, S.; Kukowka, A.; Siennicka, A.; Jakubiak, N.; Maciejewska-Markiewicz, D.; Kiedrowicz, R.; Kaźmierczak, J. Serum Zonulin and Lipopolysaccharide (LPS) Levels in Early Myocardial Infarction: Association with Left Ventricular Ejection Fraction. J. Clin. Med. 2025, 14, 6216. https://doi.org/10.3390/jcm14176216
Olędzki S, Kukowka A, Siennicka A, Jakubiak N, Maciejewska-Markiewicz D, Kiedrowicz R, Kaźmierczak J. Serum Zonulin and Lipopolysaccharide (LPS) Levels in Early Myocardial Infarction: Association with Left Ventricular Ejection Fraction. Journal of Clinical Medicine. 2025; 14(17):6216. https://doi.org/10.3390/jcm14176216
Chicago/Turabian StyleOlędzki, Szymon, Arnold Kukowka, Aldona Siennicka, Natalia Jakubiak, Dominika Maciejewska-Markiewicz, Radosław Kiedrowicz, and Jarosław Kaźmierczak. 2025. "Serum Zonulin and Lipopolysaccharide (LPS) Levels in Early Myocardial Infarction: Association with Left Ventricular Ejection Fraction" Journal of Clinical Medicine 14, no. 17: 6216. https://doi.org/10.3390/jcm14176216
APA StyleOlędzki, S., Kukowka, A., Siennicka, A., Jakubiak, N., Maciejewska-Markiewicz, D., Kiedrowicz, R., & Kaźmierczak, J. (2025). Serum Zonulin and Lipopolysaccharide (LPS) Levels in Early Myocardial Infarction: Association with Left Ventricular Ejection Fraction. Journal of Clinical Medicine, 14(17), 6216. https://doi.org/10.3390/jcm14176216









