Clinical and Imaging Features of Chronic Occult Infectious Arthritis and Undifferentiated Oligoarthritis: A Comparative Analysis
Abstract
1. Introduction
2. Materials and Methods
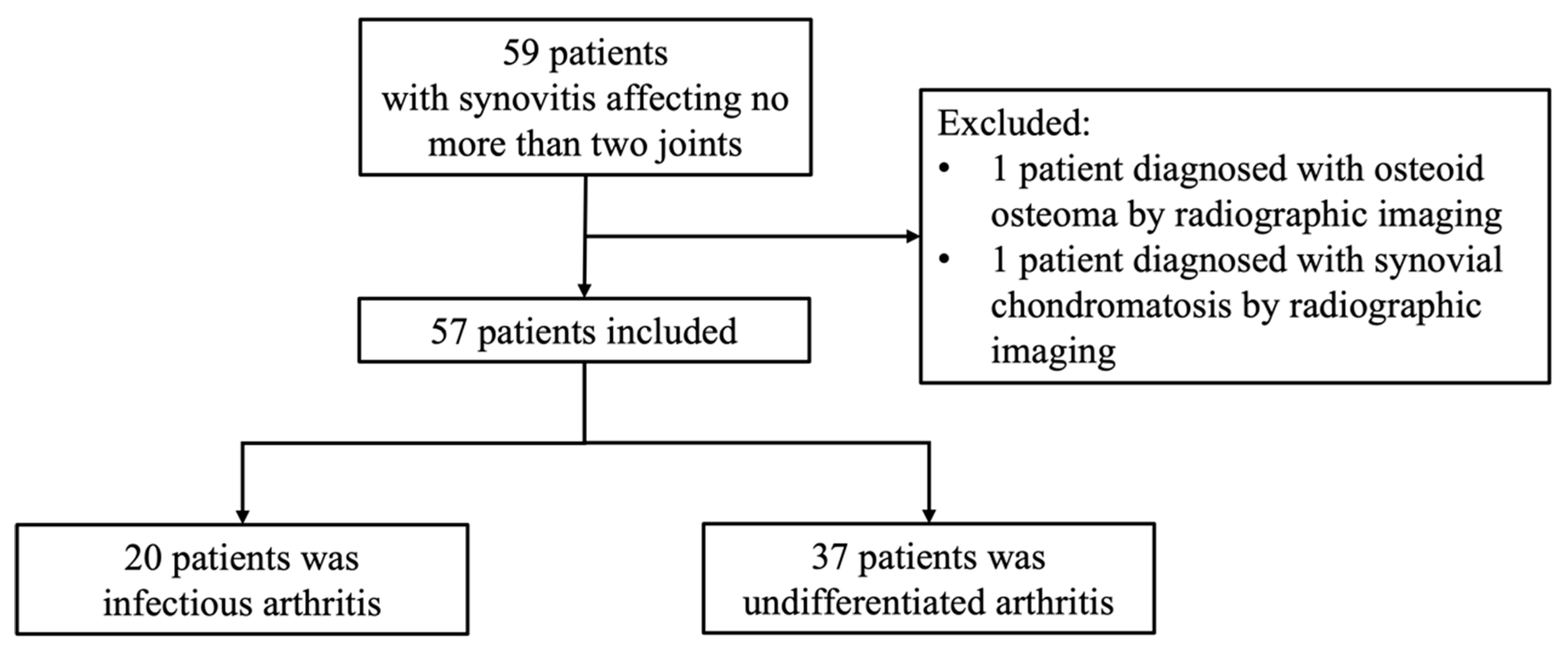
2.1. Patients
2.2. Clinical Data
2.3. NGS Workflow and Quality Control
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics Between Pathogen-Positive and Pathogen-Negative Group
3.2. Imaging Features in Pathogen-Negative and -Positive Patients
3.3. Factors for Positive Pathogen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hazes, J.M.; Luime, J.J. The epidemiology of early inflammatory arthritis. Nat. Rev. Rheumatol. 2011, 7, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of rheumatoid arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- van der Helm-van Mil, A.H.M.; Huizinga, T.W.J. Advances in the genetics of rheumatoid arthritis point to subclassification into distinct disease subsets. Arthritis Res. Ther. 2008, 10, 205. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Mader, J.T. Acute septic arthritis. Clin. Microbiol. Rev. 2002, 15, 527–544. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Bloom, K.A.; Chung, D.; Cunningham-Rundles, C. Osteoarticular infectious complications in patients with primary immunodeficiencies. Curr. Opin. Rheumatol. 2008, 20, 480–485. [Google Scholar] [CrossRef]
- Al-Matar, M.J.; Cabral, D.A.; Petty, R.E. Isolated tuberculous monoarthritis mimicking oligoarticular juvenile rheumatoid arthritis. J. Rheumatol. 2001, 28, 204–206. [Google Scholar]
- Zimmerli, W.; Sendi, P. Pathogenesis of implant-associated infection: The role of the host. Semin. Immunopathol. 2011, 33, 295–306. [Google Scholar] [CrossRef]
- Machado, P.; Castrejon, I.; Katchamart, W.; Koevoets, R.; Kuriya, B.; Schoels, M.; Silva-Fernández, L.; Thevissen, K.; Vercoutere, W.; Villeneuve, E.; et al. Multinational evidence-based recommendations on how to investigate and follow-up undifferentiated peripheral inflammatory arthritis: Integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann. Rheum. Dis. 2011, 70, 15–24. [Google Scholar]
- Upfill-Brown, A.; Bruins, M.F.; Dix-Peek, S.; Laubscher, M.; Bernthal, N.M.; Held, M. A clinical decision tool for septic arthritis in children based on epidemiologic data of atraumatic swollen painful joints in South Africa. Int. Orthop. 2020, 44, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Terslev, L.; Naredo, E.; Aegerter, P.; Wakefield, R.J.; Backhaus, M.; Balint, P.; Bruyn, G.A.; Iagnocco, A.; Jousse-Joulin, S.; Schmidt, W.A.; et al. Scoring ultrasound synovitis in rheumatoid arthritis: A EULAR-OMERACT ultrasound taskforce-Part 2, reliability and application to multiple joints of a standardised consensus-based scoring system. RMD Open 2017, 3, e000427. [Google Scholar] [CrossRef] [PubMed]
- Szkudlarek, M.; Court-Payen, M.; Jacobsen, S.; Klarlund, M.; Thomsen, H.S.; Østergaard, M. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 955–962. [Google Scholar] [CrossRef]
- Østergaard, M.; Peterfy, C.; Conaghan, P.; McQueen, F.; Bird, P.; Ejbjerg, B.; Shnier, R.; O’connor, P.; Klarlund, M.; Emery, P.; et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J. Rheumatol. 2003, 30, 1385–1386. [Google Scholar]
- Gupta, N.; Chaudhry, R.; Soneja, M.; Valappil, V.E.; Malla, S.; Razik, A.; Vyas, S.; Ray, A.; Khan, M.A.; Kumar, U.; et al. Infectious versus non-infectious causes of oligoarticular inflammatory arthritis: A prospective study from a tertiary care hospital in north India. Drug Discov. Ther. 2019, 13, 96–100. [Google Scholar] [CrossRef]
- Guo, Y.-P.; Wang, C.-G.; Liu, X.; Huang, Y.-Q.; Guo, D.-L.; Jing, X.-Z.; Yuan, C.-G.; Yang, S.; Liu, J.-M.; Han, M.-S.; et al. The prevalence of antinuclear antibodies in the general population of china: A cross-sectional study. Curr. Ther. Res. Clin. Exp. 2014, 76, 116–119. [Google Scholar] [CrossRef]
- Long, B.; Koyfman, A.; Gottlieb, M. Evaluation and management of septic arthritis and its mimics in the emergency department. West. J. Emerg. Med. 2019, 20, 331–341. [Google Scholar] [CrossRef]
- Ballantyne, F.C.; Fleck, A.; Dick, W.C. Albumin metabolism in rheumatoid arthritis. Ann. Rheum. Dis. 1971, 30, 265–270. [Google Scholar] [CrossRef]
- Graif, M.; Schweitzer, M.E.; Deely, D.; Matteucci, T. The septic versus nonseptic inflamed joint: MRI characteristics. Skelet. Radiol. 1999, 28, 616–620. [Google Scholar] [CrossRef]
- Kaeley, G.S.; Bakewell, C.; Deodhar, A. The importance of ultrasound in identifying and differentiating patients with early inflammatory arthritis: A narrative review. Arthritis Res. Ther. 2020, 22, 1. [Google Scholar] [CrossRef]
- Ravn, C.; Neyt, J.; Benito, N.; Abreu, M.A.; Achermann, Y.; Bozhkova, S.; Coorevits, L.; Ferrari, M.C.; Gammelsrud, K.W.; Gerlach, U.-J.; et al. Guideline for management of septic arthritis in native joints (SANJO). J. Bone Jt. Infect. 2023, 8, 29–37. [Google Scholar] [CrossRef]
- Mack, D.; Rohde, H.; Harris, L.G.; Davies, A.P.; Horstkotte, M.A.; Knobloch, J.K. Biofilm formation in medical device-related infection. Int. J. Artif. Organs 2006, 29, 343–359. [Google Scholar] [CrossRef]
- Williams, D.L.; Haymond, B.S.; Woodbury, K.L.; Beck, J.P.; Moore, D.E.; Epperson, R.T.; Bloebaum, R.D. Experimental model of biofilm implant-related osteomyelitis to test combination biomaterials using biofilms as initial inocula. J. Biomed. Mater. Res. A 2012, 100, 1888–1900. [Google Scholar] [CrossRef]
- Mathews, C.J.; Weston, V.C.; Jones, A.; Field, M.; Coakley, G. Bacterial septic arthritis in adults. Lancet 2010, 375, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Linke, S.; Thürmer, A.; Bienger, K.; Kleber, C.; Bellova, P.; Lützner, J.; Stiehler, M. Microbiological pathogen analysis in native versus periprosthetic joint infections: A retrospective study. J. Orthop. Surg. Res. 2022, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, F.; Li, Y.; Mao, L.; He, W.; Wu, S.; Xia, H.; He, P.; Zheng, H.; Zhou, Y.; et al. Transmission characteristics in Tuberculosis by WGS: Nationwide cross-sectional surveillance in China. Emerg. Microbes Infect. 2024, 13, 2348505. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.M.; Shin, C.P. Native joint infections caused by Parvimonas micra. Anaerobe 2021, 71, 102412. [Google Scholar] [CrossRef]

| Pathogen Negative (n = 37) | Pathogen Positive (n = 20) | p Value | |
|---|---|---|---|
| Female (n, %) | 24 (64.9) | 11 (55.0) | 0.66 |
| Age (years, median [IQR]) | 39 [30, 47] | 46 [35, 59] | 0.23 |
| BMI (kg/m2, median [IQR]) | 23.5 [21.8, 26.8] | 22.3 [20.8, 25.4] | 0.23 |
| Course of disease (months, median [IQR]) | 12 [6, 36] | 17 [3, 30] | 0.49 |
| VAS (median [IQR]) | 6 [5, 8] | 6 [5, 8] | 0.65 |
| History of infection, diarrhea or stomatology operations (n, %) | 5 (17.2) | 6 (37.5) | 0.25 |
| History of fever (n, %) | 6 (18.2) | 5 (26.3) | 0.74 |
| Number of affected joints | |||
| One joint | 25 (67.6) | 19 (95.0) | 0.02 |
| Two joints | 12 (32.4) | 1 (5.0) | |
| Morning stiffness (n, %) | 8 (26.7) | 7 (43.8) | 0.40 |
| Location of Affected Joints | |||
| Wrist joint (n, %) | 15 (40.5) | 5 (25.0) | 0.63 |
| Elbow joint (n, %) | 4 (10.8) | 1 (5.0) | |
| Shoulder joint (n, %) | 0 (0) | 1 (5.0) | |
| Knee joint (n, %) | 16 (43.2) | 5 (25.0) | |
| Hip joint (n, %) | 2 (5.4) | 1 (5.0) | |
| Ankle joint (n, %) | 2 (5.4) | 6 (30.0) | |
| Sacroiliac joint (n, %) | 0 (0) | 2 (10.0) | |
| Joint examination | |||
| Swelling (n, %) | 32 (86.5) | 18 (94.7) | 0.63 |
| Tenderness (n, %) | 27 (75.0) | 19 (95.0) | 0.13 |
| Decreased ROM (n, %) | 30 (81.1) | 20 (100.0) | 0.10 |
| Warmth (n, %) | 20 (60.6) | 13 (72.2) | 0.60 |
| ESR (mm/h, mean ± SD) | 25.14 ± 27.36 | 40.1 ± 35.1 | 0.09 |
| CRP (mg/L, mean ± SD) | 10.67 ± 20.40 | 21.3 ± 37.3 | 0.18 |
| Routine bloods | |||
| White blood cell (×109/L, mean ± SD) | 6.4 ± 2.3 | 6.4 ± 1.7 | 0.98 |
| Neutrophil (×109/L, mean ± SD) | 5.9 ± 11.0 | 3.8 ± 1.1 | 0.42 |
| Lymphocyte (×109/L, mean ± SD) | 1.94 ± 0.72 | 1.96 ± 0.66 | 0.94 |
| Monocyte (×109/L, mean ± SD) | 2.6 ± 11.0 | 0.4 ± 0.2 | 0.39 |
| Platelet (×109/L, mean ± SD) | 270.5 ± 78.5 | 290.3 ± 94.6 | 0.42 |
| Globulin | |||
| Immune globulin G (g/L, mean ± SD) | 14.0 ± 3.9 | 12.2 ± 3.2 | 0.15 |
| Immune globulin A (g/L, mean ± SD) | 2.8 ± 1.4 | 2.6 ± 1.2 | 0.73 |
| Immune globulin M (g/L, mean ± SD) | 1.3 ± 1.0 | 1.0 ± 0.6 | 0.39 |
| Serum calcium (mmol/L, mean ± SD) | 2.3 ± 0.1 | 2.3 ± 0.1 | 0.85 |
| Serum phosphate (mmol/L, mean ± SD) | 1.1 ± 0.2 | 1.2 ± 0.2 | 0.77 |
| Alb (g/L, mean ± SD) | 20.7 ± 8.5 | 41.1 ± 3.9 | <0.001 |
| Alkaline phosphatase (U/L, mean ± SD) | 86.7 ± 35.0 | 92.6 ± 30.7 | 0.54 |
| Total procollagen type 1 N-terminal propeptide (ng/mL, mean ± SD) | 57.7 ± 32.3 | 62.9 ± 25.7 | 0.61 |
| β-C-terminal telopeptide of type-I collagen (ng/mL, mean ± SD) | 0.5 ± 0.3 | 0.7 ± 0.5 | 0.16 |
| Parathyroid hormone (pg/mL, mean ± SD) | 31.8 ± 13.4 | 31.3 ± 13.9 | 0.91 |
| 25-hydroxyvitamin D3 (ng/mL, mean ± SD) | 19.8 ± 6.5 | 19.1 ± 7.1 | 0.71 |
| ANA positive (n, %) | 13 (35.1) | 6 (33.3) | >0.99 |
| Ultrasound | |||
| Synovial thickness (cm, mean ± SD) | 0.6 ± 0.5 | 0.8 ± 0.7 | 0.18 |
| Doppler blood flow | |||
| Level 0 (n, %) | 8 (21.6) | 9 (52.9) | 0.12 |
| Level 1 (n, %) | 8 (21.6) | 3 (17.6) | |
| Level 2 (n, %) | 16 (43.2) | 3 (17.6) | |
| Level 3 (n, %) | 1 (2.7) | 2 (11.8) | |
| Bone erosion | |||
| Level 0 (n, %) | 20 (54.1) | 2 (11.8) | 0.02 |
| Level 1 (n, %) | 7 (18.9) | 9 (52.9) | |
| Level 2 (n, %) | 2 (6.2) | 1 (5.9) | |
| Level 3 (n, %) | 3 (8.1) | 5 (29.4) | |
| Effusion (n, %) | 29 (78.4) | 14 (70.0) | 0.71 |
| MRI | |||
| Bone marrow edema | |||
| 0 (n, %) | 7 (18.9) | 1 (5.0) | 0.36 |
| 1 (n, %) | 3 (8.1) | 2 (11.8) | |
| 2 (n, %) | 6 (16.2) | 3 (17.6) | |
| 3 (n, %) | 7 (18.9) | 4 (10.8) | |
| Bone erosion | |||
| 0 (n, %) | 12 (32.4) | 4 (20.0) | 0.25 |
| 1 (n, %) | 7 (18.9) | 3 (17.6) | |
| 2 (n, %) | 3 (8.1) | 1 (5.9) | |
| 3 (n, %) | 0 (0) | 2 (11.8) | |
| 4 (n, %) | 1 (2.7) | 0 (0) | |
| CT or X ray | |||
| Joint space narrowing (n, %) | 12 (35.3) | 9 (65.0) | 0.07 |
| Joint surface destruction (n, %) | 2 (6.2) | 10 (58.8) | <0.001 |
| Pathogen Spectrum | Count |
|---|---|
| Mycobacteria | |
| Mycobacterium tuberculosis | 4 |
| Gram-positive bacteria | |
| Staphylococcus aureus | 3 |
| Staphylococcus capitis | 2 |
| Staphylococcus cohnii | 1 |
| Staphylococcus epidermidis | 1 |
| Corynebacterium jeikeium | 1 |
| Peptostreptococcus anaerobius | 1 |
| Gram-negative bacteria | |
| Brucella spp. | 2 |
| Escherichia coli | 1 |
| Fusobacterium nucleatum | 1 |
| Pseudomonas aeruginosa | 1 |
| Xylophilus ampelinus | 1 |
| Virus | |
| Hepatitis B Virus | 1 |
| Facror | Pathogen-Positive OR (95%CI) | p Value |
|---|---|---|
| Alb (g/L) | 0.64 (0.45, 0.90) | 0.002 |
| Bone erosion in ultrasound | ||
| Level 1 | 1.22 (0.93, 1.59) | 0.16 |
| Level 2 | 0.95 (0.55, 1.62) | 0.84 |
| Level 3 | 1.38 (1.02, 1.87) | 0.045 |
| Joint surface destruction in CT or X ray | 1.61 (1.20, 2.15) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Chen, T.; Wang, Y.; Guo, Z.; Tang, W.; Zhao, H.; Lv, X.; Deng, X. Clinical and Imaging Features of Chronic Occult Infectious Arthritis and Undifferentiated Oligoarthritis: A Comparative Analysis. J. Clin. Med. 2025, 14, 6213. https://doi.org/10.3390/jcm14176213
Wu L, Chen T, Wang Y, Guo Z, Tang W, Zhao H, Lv X, Deng X. Clinical and Imaging Features of Chronic Occult Infectious Arthritis and Undifferentiated Oligoarthritis: A Comparative Analysis. Journal of Clinical Medicine. 2025; 14(17):6213. https://doi.org/10.3390/jcm14176213
Chicago/Turabian StyleWu, Lingge, Tao Chen, Yan Wang, Zhe Guo, Wangna Tang, Hong Zhao, Xueya Lv, and Xiaoli Deng. 2025. "Clinical and Imaging Features of Chronic Occult Infectious Arthritis and Undifferentiated Oligoarthritis: A Comparative Analysis" Journal of Clinical Medicine 14, no. 17: 6213. https://doi.org/10.3390/jcm14176213
APA StyleWu, L., Chen, T., Wang, Y., Guo, Z., Tang, W., Zhao, H., Lv, X., & Deng, X. (2025). Clinical and Imaging Features of Chronic Occult Infectious Arthritis and Undifferentiated Oligoarthritis: A Comparative Analysis. Journal of Clinical Medicine, 14(17), 6213. https://doi.org/10.3390/jcm14176213







