Prognostic Significance of Albumin in Modern Left Ventricular Assist Device Therapy: Relevance in the HeartMate 3 Era?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Evaluation of Albumin Status
2.3. Outcome Definition and Definition of Hemocompatibility-Related Adverse Events (HRAEs)
2.4. Risk Factor Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics and Baseline Characteristics
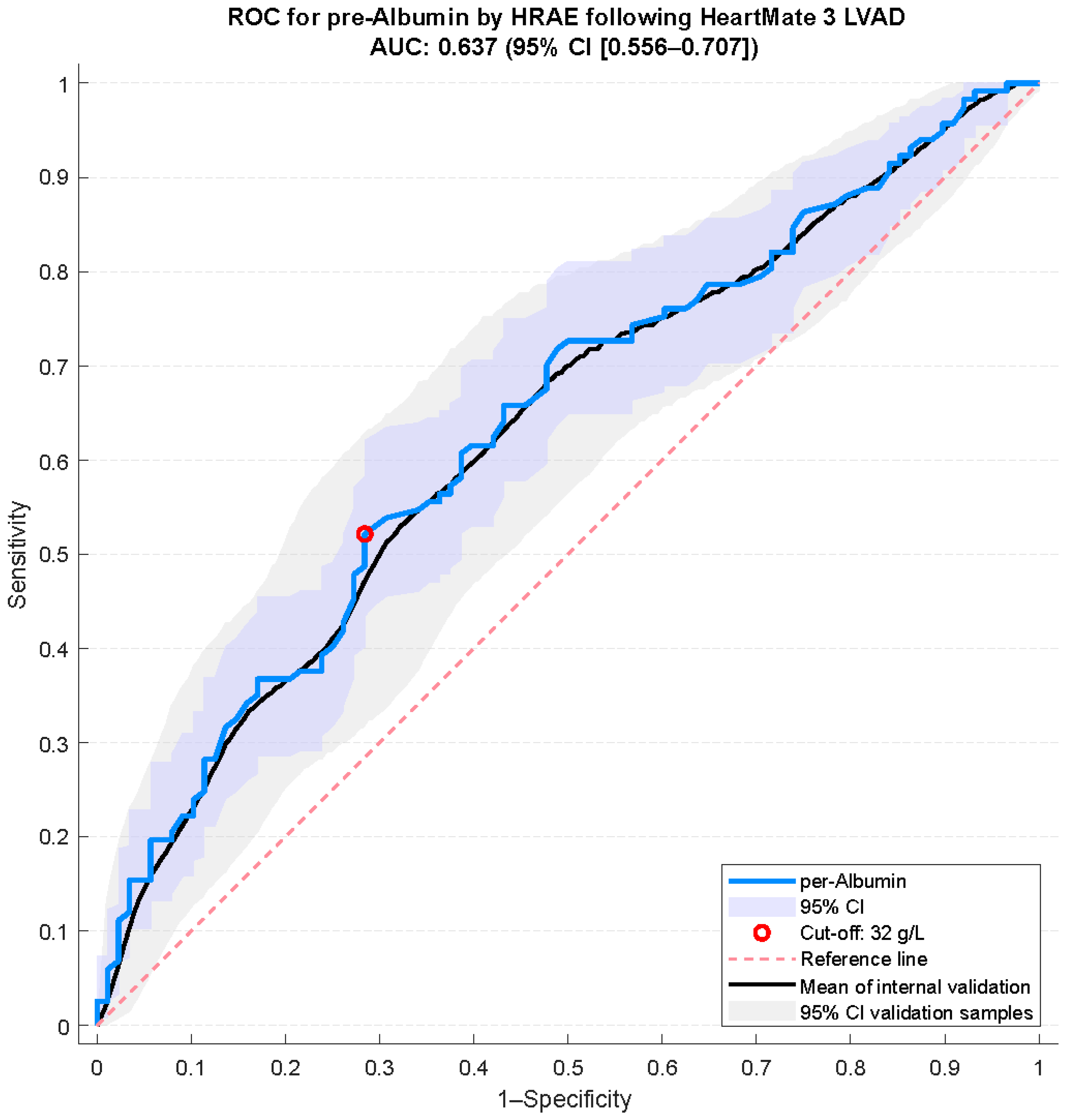
3.2. Albumin Cut-Off Determination
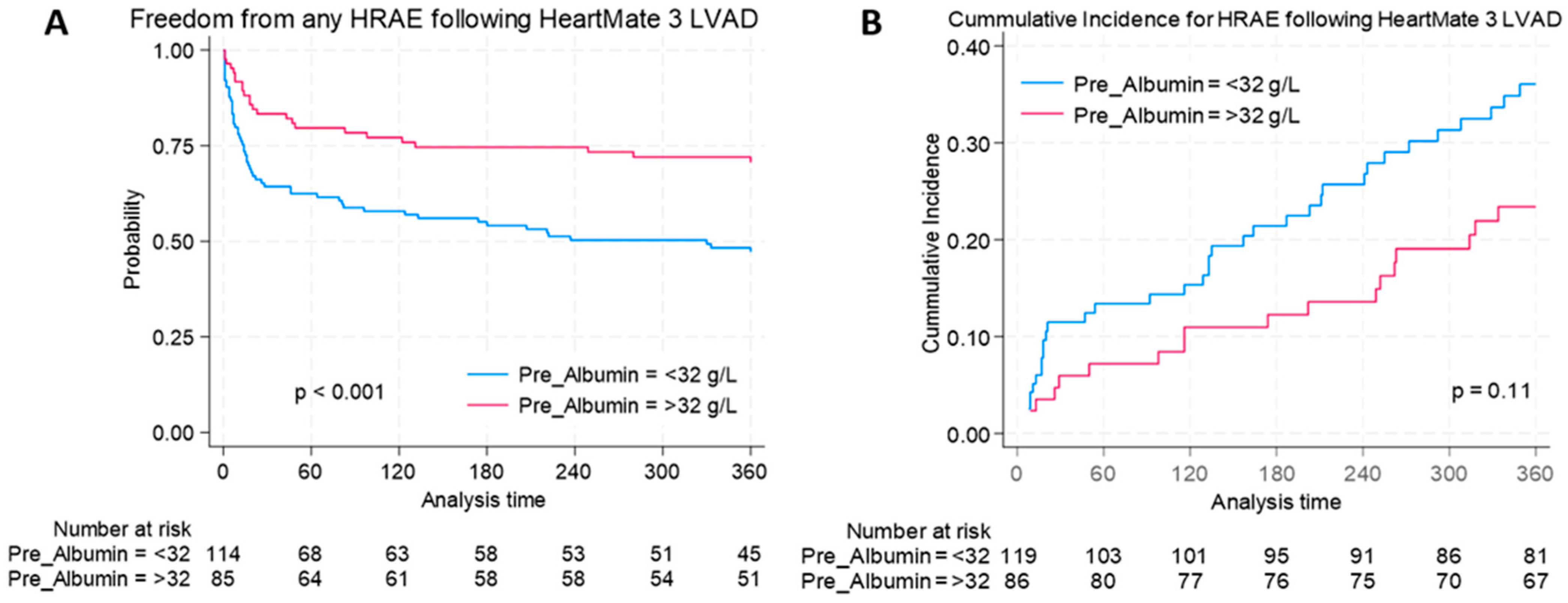
3.3. Hemocompatibility-Related Adverse Events
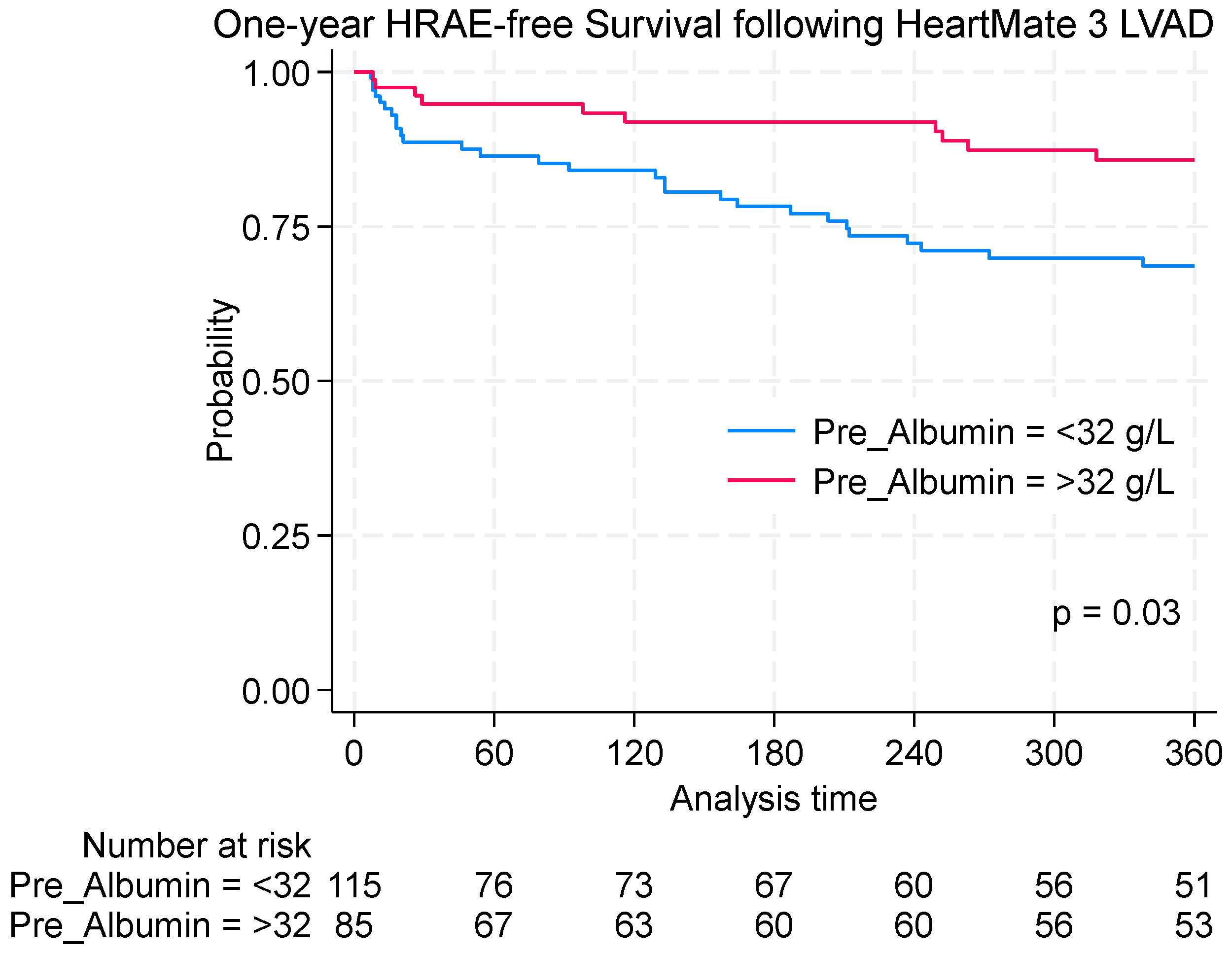
3.4. Survival Outcomes
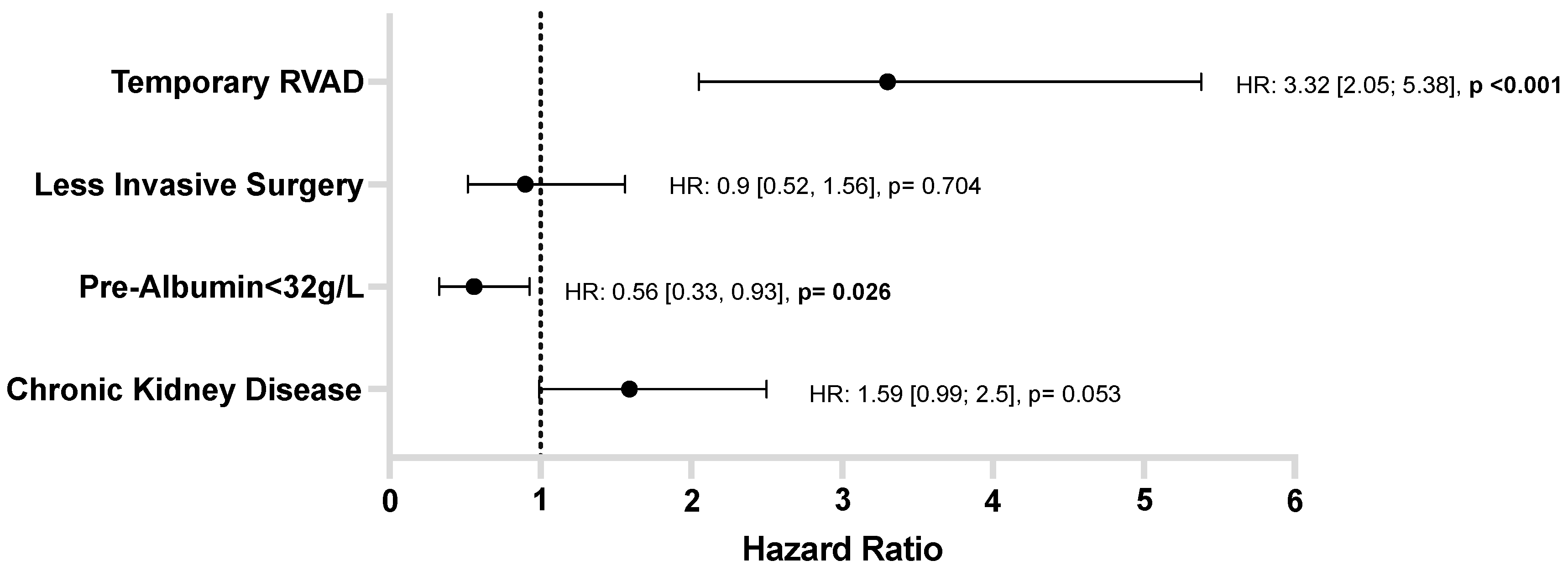
3.5. HRAEs: Risk Factor Analysis
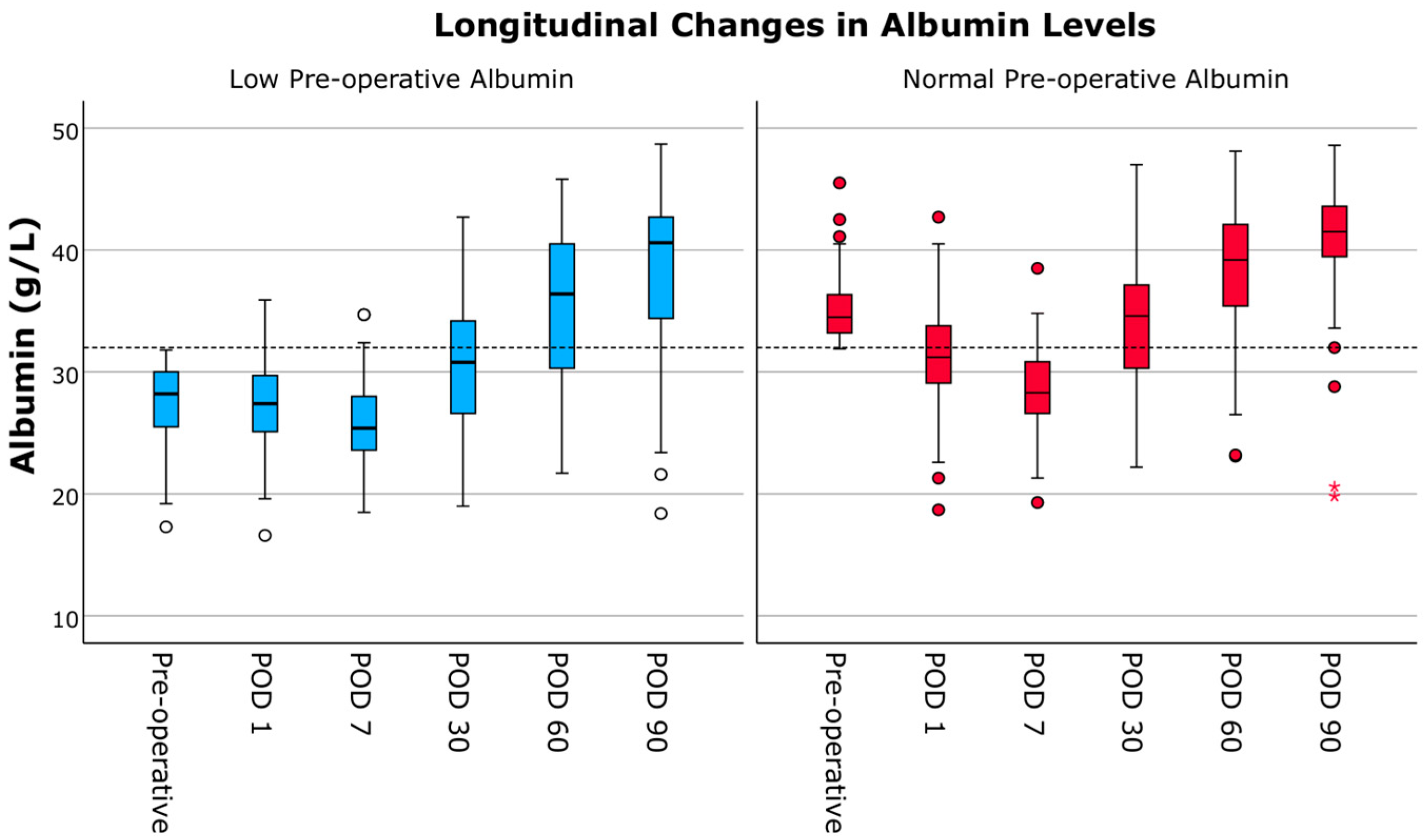
3.6. Longitudinal Changes in Albumin
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| ALAT | Alanin-aminotransferase |
| AUC | Area under the curve |
| BIVAD | Biventricular assist device |
| BMI | Body mass index |
| CABG | Coronary artery bypass grafting |
| CI | Confidence interval |
| CONUT | Controlling nutritional status |
| CRP | C-reactive protein |
| HM3 | HeartMate 3 |
| HR | Hazard ratio |
| HRAE | Hemocompatibility-related adverse events |
| ICU | Intensive care unit |
| LVAD | Left ventricular assist device |
| MDRD-GFR | Modification of diet in renal disease glomerular filtration rate |
| NRI | Nutritional risk index |
| OPCB | Off-pump coronary artery bypass |
| PNI | Prognostic nutritional index |
| ROC | Receiver operating characteristic |
| RVAD | Right ventricular assist device |
| VAD | Ventricular assist device |
| WBC | White blood count |
Appendix A
| Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| Age (years) | 1.00 | [0.98; 1.03] | 0.930 |
| Gender (female vs. male) | 1.03 | [0.47; 2.23] | 0.946 |
| BMI | 0.97 | [0.93; 1.02] | 0.266 |
| INTERMACS | |||
| Level 1 (Reference) | 1.00 | - | - |
| Level 2 | 0.82 | [0.43; 1.57] | 0.547 |
| Level 3 | 0.58 | [0.29; 1.15] | 0.120 |
| Level 4–7 | 0.56 | [0.32; 1.01] | 0.055 |
| Hypertension | 0.66 | [0.40; 1.10] | 0.111 |
| Peripheral artery disease | 0.92 | [0.34; 2.51] | 0.868 |
| Chronic kidney disease | 1.63 | [1.02; 2.59] | 0.04 |
| Diabetes type 2 | 1.00 | [1.00; 1.00] | 0.225 |
| Cardiac infarction | 1.01 | [0.64; 1.59] | 0.954 |
| Atrial fibrillation | 1.43 | [0.93; 2.21] | 0.103 |
| Peripheral edema | 1.55 | [0.95; 2.54] | 0.080 |
| Stroke history | 1.72 | [0.89; 3.33] | 0.107 |
| Serum-albumin (pre-operative) | |||
| <32 g/L | 1.00 | - | - |
| ≥32 g/L | 0.43 | [0.26; 0.71] | <0.001 |
| Previous cardiac surgery | 1.17 | [0.60; 2.28] | 0.638 |
| Concomitant procedures | 0.92 | [0.59; 1.41] | 0.692 |
| Bypass support type | |||
| Off-pump vs. CPB (Reference) | 0.89 | [0.36; 2.20] | 0.792 |
| ECMO vs. CPB (Reference) | 1.43 | [0.62; 3.31] | 0.398 |
| Less invasive implant method | 0.58 | [0.34; 0.97] | 0.038 |
| LVAD anastomosis site | |||
| Ascending Aorta (Reference) | 1.00 | - | - |
| Other (Subclavian/Descending Aorta) | 2.03 | [0.93; 4.42] | 0.074 |
| Temporary RVAD support | 3.91 | [2.47; 6.16] | <0.001 |
| PNI | 1.00 | [1.00; 1.00] | 0.186 |
| CONUT Score | 0.96 | [0.84; 1.09] | 0.522 |
| CONUT categorial | |||
| Normal (Reference) vs. Mild | 1.04 | [0.14; 7.86] | 0.972 |
| Normal (Reference) vs. Moderate | 0.59 | [0.08; 4.50] | 0.614 |
| Normal (Reference) vs. Severe | 0.91 | [0.11; 7.30] | 0.931 |
| Central venous pressure (mmHg) | 1.01 | [0.97; 1.04] | 0.735 |
| Mean pulmonary artery pressure (mmHg) | 0.99 | [0.97; 1.02] | 0.553 |
| Bilirubin (mg/dL) | 0.98 | [0.86; 1.12] | 0.742 |
| Creatine (mg/dL) | 1.07 | [0.84; 1.35] | 0.586 |
| MDRD-GFR (mL/min) | 0.99 | [0.99; 1.00] | 0.066 |
| WBC (G/L) | 1.02 | [0.98; 1.07] | 0.356 |
| CRP (mg/dL) | 1.00 | [0.97; 1.04] | 0.878 |
| Cholesterol (mg/dL) | 1.00 | [0.99; 1.01] | 0.780 |
| Creatine kinase (U/L) | 1.00 | [1.00; 1.00] | 0.590 |
| ALAT (U/L) | 1.00 | [1.00; 1.00] | 0.304 |
| Sodium (mmol/L) | 1.00 | [0.96; 1.05] | 0.902 |
References
- Jorde, U.P.; Saeed, O.; Koehl, D.; Morris, A.A.; Wood, K.L.; Meyer, D.M.; Cantor, R.; Jacobs, J.P.; Kirklin, J.K.; Pagani, F.D.; et al. The Society of Thoracic Surgeons Intermacs 2023 Annual Report: Focus on Magnetically Levitated Devices. Ann. Thorac. Surg. 2024, 117, 33–44. [Google Scholar] [CrossRef]
- Kato, T.S.; Kitada, S.; Yang, J.; Wu, C.; Takayama, H.; Naka, Y.; Farr, M.; Mancini, D.M.; Schulze, P.C. Relation of preoperative serum albumin levels to survival in patients undergoing left ventricular assist device implantation. Am. J. Cardiol. 2013, 112, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Go, P.H.; Hodari, A.; Nemeh, H.W.; Borgi, J.; Lanfear, D.E.; Williams, C.T.; Paone, G.; Morgan, J.A. Effect of Preoperative Albumin Levels on Outcomes in Patients Undergoing Left Ventricular Device Implantation. Asaio J. 2015, 61, 734–737. [Google Scholar] [CrossRef]
- Mehra, M.R.; Goldstein, D.J.; Cleveland, J.C.; Cowger, J.A.; Hall, S.; Salerno, C.T.; Naka, Y.; Horstmanshof, D.; Chuang, J.; Wang, A.; et al. Five-Year Outcomes in Patients with Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices in the MOMENTUM 3 Randomized Trial. JAMA 2022, 328, 1233–1242. [Google Scholar] [CrossRef]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef]
- Kato, T.S.; Cheema, F.H.; Yang, J.; Kawano, Y.; Takayama, H.; Naka, Y.; Farr, M.; Lederer, D.J.; Baldwin, M.R.; Jin, Z.; et al. Preoperative serum albumin levels predict 1-year postoperative survival of patients undergoing heart transplantation. Circ. Heart Fail. 2013, 6, 785–791. [Google Scholar] [CrossRef]
- de la Cruz, K.I.; Bakaeen, F.G.; Wang, X.L.; Huh, J.; LeMaire, S.A.; Coselli, J.S.; Chu, D. Hypoalbuminemia and long-term survival after coronary artery bypass: A propensity score analysis. Ann. Thorac. Surg. 2011, 91, 671–675. [Google Scholar] [CrossRef]
- Engelman, D.T.; Adams, D.H.; Byrne, J.G.; Aranki, S.F.; Collins, J.J., Jr.; Couper, G.S.; Allred, E.N.; Cohn, L.H.; Rizzo, R.J. Impact of body mass index and albumin on morbidity and mortality after cardiac surgery. J. Thorac. Cardiovasc. Surg. 1999, 118, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Critsinelis, A.C.; Kurihara, C.; Kawabori, M.; Sugiura, T.; Lee, V.-V.; Civitello, A.B.; Morgan, J.A. Predictive value of preoperative serum albumin levels on outcomes in patients undergoing LVAD implantation. J. Card. Surg. 2018, 33, 469–478. [Google Scholar] [CrossRef]
- Mehra, M.R.; Goldstein, D.J.; Uriel, N.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Ewald, G.A.; et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N. Engl. J. Med. 2018, 378, 1386–1395. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Koertzen, M.; Punjabi, P.; Lockwood, G. Pre-operative serum albumin concentration as a predictor of mortality and morbidity following cardiac surgery. Perfusion 2013, 28, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Padkins, M.; Breen, T.; Anavekar, N.; Barsness, G.; Kashani, K.; Jentzer, J.C. Association Between Albumin Level and Mortality Among Cardiac Intensive Care Unit Patients. J. Intensiv. Care Med. 2021, 36, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Hao, M.; Zhou, W.; Liu, M.; Wei, Y.; Xu, J.; Zhang, W. Preoperative hypoalbuminemia in patients undergoing cardiac surgery: A meta-analysis. Surg. Today 2023, 53, 861–872. [Google Scholar] [CrossRef]
- Lee, E.H.; Chin, J.H.; Choi, D.K.; Hwang, B.-Y.; Choo, S.-J.; Song, J.-G.; Kim, T.-Y.; Choi, I.-C. Postoperative hypoalbuminemia is associated with outcome in patients undergoing off-pump coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2011, 25, 462–468. [Google Scholar] [CrossRef]
- Critsinelis, A.C.; Kurihara, C.; Kawabori, M.; Sugiura, T.; Civitello, A.B.; Morgan, J.A. Preoperative Prealbumin Level as a Predictor of Outcomes in Patients Who Underwent Left Ventricular Assist Device Implantation. Am. J. Cardiol. 2017, 120, 1998–2002. [Google Scholar] [CrossRef]
- Imamura, T.; Combs, P.; Siddiqi, U.; Mirzai, S.; Stonebraker, C.; Bullard, H.; Simone, P.; Jeevanandam, V. Perioperative improvement in serum albumin level in patients with left ventricular assist device. J. Card. Surg. 2020, 35, 3070–3077. [Google Scholar] [CrossRef]
- George, T.J.; Van Dinter, T.; Rawitscher, D.; DiMaio, J.M.; Kabra, N.; Afzal, A. Impact of Preoperative Liver Function on Short-Term HeartMate 3 Outcomes. Am. J. Cardiol. 2022, 183, 62–69. [Google Scholar] [CrossRef]
- Gopal, D.J.; Hanff, T.C.; Mazurek, J.A.; Grandin, W.E.; Howard, J.; Forde-McLean, R.; Wald, J.; King, K.; Acker, M.A.; Goldberg, L.R.; et al. Prognostic Implications of Changes in Albumin Following Left Ventricular Assist Device Implantation in Patients with Severe Heart Failure. Am. J. Cardiol. 2017, 120, 2003–2007. [Google Scholar] [CrossRef]
- Cowger, J.A.; Castle, L.; Aaronson, K.D.; Slaughter, M.S.; Moainie, S.; Walsh, M.; Salerno, C. The HeartMate II Risk Score: An Adjusted Score for Evaluation of All Continuous-Flow Left Ventricular Assist Devices. Asaio J. 2016, 62, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Nayak, A.; Morris, A.A.; Lanfear, D.E.; Nemeh, H.; Desai, S.; Bansal, A.; Guerrero-Miranda, C.; Hall, S.; Cleveland, J.C.; et al. Prediction of Survival After Implantation of a Fully Magnetically Levitated Left Ventricular Assist Device. JACC Heart Fail. 2022, 10, 948–959. [Google Scholar] [CrossRef]
- Yost, G.; Tatooles, A.; Bhat, G. Preoperative Nutritional Assessment with the Prognostic Nutrition Index in Patients Undergoing Left Ventricular Assist Device Implantation. Asaio J. 2018, 64, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Milaniak, I.; Tomaszek, L.; Wiśniowska-Śmiałek, S.; Górkiewicz-Kot, I.; Wasilewski, G.; Kurleto, P.; Kaleta, M.; Sobczyk, D.; Wierzbicki, K. Nutritional Risk Assessment and Adverse Events in Patients Undergoing Left Ventricular Assist Device Implantation-A Retrospective Cohort Study Using Hospital Information System. J. Clin. Med. 2023, 12, 7181. [Google Scholar] [CrossRef]
- Contreras, F.J.; Pinsker, B.L.; Katz, J.N.; Russell, S.D.; Schroder, J.; Bryner, B.; Gunn, A.H.; Amin, K.; Milano, C. Value of nutritional indices in predicting survival free from pump replacement and driveline infections in centrifugal left ventricular assist devices. JTCVS Open 2024, 19, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, A.; Rojas, S.V.; Hanke, J.S.; Dogan, G.; Siemeni, T.; Kaufeld, T.; Ius, F.; Goecke, T.; Rojas-Hernandez, S.; Warnecke, G.; et al. Prognostic Value of the Nutritional Risk Index in Candidates for Continuous Flow Left Ventricular Assist Device Therapy. Rev. Esp. Cardiol. (Engl. Ed.) 2019, 72, 608–615. [Google Scholar] [CrossRef]
- Saito, A.; Amiya, E.; Hatano, M.; Shiraishi, Y.; Nitta, D.; Minatsuki, S.; Maki, H.; Hosoya, Y.; Tsuji, M.; Bujo, C.; et al. Controlling Nutritional Status Score as a Predictive Marker for Patients with Implantable Left Ventricular Assist Device. Asaio J. 2020, 66, 166–172. [Google Scholar] [CrossRef]
- Felpel, K.; Palmese, L.; Urrutia, L.; Zhang, Z.; Shapero, M.; Esbenshade, J.; Hamid, S.; Vest, M.T. Nutritional assessment and comparison of nutritional indices in predicting adverse outcomes in patients undergoing left ventricular assist device implantation. Nutrition 2021, 89, 111287. [Google Scholar] [CrossRef]
- Holdy, K.; Dembitsky, W.; Eaton, L.L.; Chillcott, S.; Stahovich, M.; Rasmusson, B.; Pagani, F. Nutrition assessment and management of left ventricular assist device patients. J. Heart Lung Transplant. 2005, 24, 1690–1696. [Google Scholar] [CrossRef]

| Variable n (%), Mean ± SD or Median (IQR) | Albumin < 32 g/L N = 119 | Albumin ≥ 32 g/L N = 86 | p-Value |
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | 63 ± 10.6 | 61 ± 8.8 | 0.905 |
| Gender | 0.465 | ||
| male | 106 (89.1) | 80 (93.0) | |
| female | 13 (10.9) | 6 (7.0) | |
| BMI (kg/m2) | 26.8 ± 4.8 | 27.8 ± 4.6 | 0.365 |
| Cardiomyopathy | 0.269 | ||
| Dilated | 47 (39.5) | 27 (31.4) | |
| Ischemic | 70 (58.8) | 55 (64.0) | |
| Other | 2 (1.6) | 4 (4.6) | |
| INTERMACS | <0.001 | ||
| Level 1 | 38 (31.9) | 6 (7.0) | |
| Level 2 | 25 (21.0) | 15 (17.4) | |
| Level 3 | 19 (16.0) | 23 (26.7) | |
| Level 4–7 | 37 (31.1) | 42 (48.8) | |
| Hypertension | 38 (32.5) | 29 (33.7) | 0.881 |
| Peripheral artery disease | 7 (6.0) | 4 (4.7) | 0.763 |
| Chronic kidney disease | 35 (30.2) | 24 (27.9) | 0.756 |
| Diabetes Type 2 | 38 (32.8) | 32 (38.1) | 0.605 |
| Atrial fibrillation | 53 (49.1) | 34 (40.0) | 0.244 |
| Stroke history | 12 (10.1) | 5 (5.8) | 0.320 |
| Peripheral edema | 22 (19.1) | 17 (20.2) | 0.860 |
| Cardiac infarction | 36 (30.3) | 27 (31.4) | 0.880 |
| Surgical implant data | |||
| Previous cardiac surgery | 14 (11.8) | 10 (11.6) | 1.000 |
| Less invasive implant method | 31 (26.1) | 37 (43.0) | 0.016 |
| Bypass support | 0.130 | ||
| CPB | 100 (84.9) | 77 (89.5) | |
| ECMO | 11 (9.2) | 1 (1.2) | |
| Off-pump | 6 (6.7) | 7 (8.1) | |
| LVAD anastomosis site | 0.845 | ||
| Ascending aorta | 112 (94.1) | 82 (95.3) | |
| Descending aorta | 1 (0.8) | 1 (1.2) | |
| Left subclavian artery | 6 (5.0) | 3 (3.5) | |
| Temporary RVAD | 52 (43.7) | 20 (23.3) | 0.003 |
| Concomitant procedures | 48 (40.3) | 30 (34.9) | 0.468 |
| Pre-operative laboratory parameters | |||
| Creatine kinase (U/L) | 62.0 (59.0) | 66.0 (111) | 0.921 |
| Creatine (mg/dL) | 1.35 (0.74) | 1.29 (0.96) | 0.994 |
| MDRD-GFR (ml/min) | 57.8 (35.3) | 56.4 (44.9) | 0.941 |
| Cholesterol (mg/dL) | 122.4 ± 36.8 | 116.7 ± 49.5 | 0.941 |
| Sodium (mmol/L) | 138.9 ± 4.5 | 138.5 ± 5.4 | 0.618 |
| ALAT (U/L) | 29.0 (31.0) | 40.5 (103.5) | 0.002 |
| Bilirubin (mg/dL) | 0.92 (0.86) | 1.09 (1.33) | 0.286 |
| WBC (G/L) | 8.0 (4.0) | 8.4 (4.5) | 0.402 |
| CRP (mg/dL) | 1.3 (4.2) | 2.8 (5.0) | 0.197 |
| Nutritional Scores | |||
| PNI | 39.1 (7.6) | 39.2 (11.0) | 0.598 |
| CONUT | 5 (4) | 6 (4) | 0.377 |
| Pre-operative hemodynamics | |||
| Central venous pressure (mmHg) | 14.5 (9.0) | 15.0 (7.0) | 0.377 |
| Mean pulmonary artery pressure (mmHg) | 32.9 ± 11.4 | 33.1 ± 10.5 | 0.922 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moayedifar, R.; Celik, M.; Karner, B.; Schaefer, A.-K.; Al Asadi, H.; Marko, C.; Ruoff, L.; Zimpfer, D.; Riebandt, J.; Schlöglhofer, T. Prognostic Significance of Albumin in Modern Left Ventricular Assist Device Therapy: Relevance in the HeartMate 3 Era? J. Clin. Med. 2025, 14, 6193. https://doi.org/10.3390/jcm14176193
Moayedifar R, Celik M, Karner B, Schaefer A-K, Al Asadi H, Marko C, Ruoff L, Zimpfer D, Riebandt J, Schlöglhofer T. Prognostic Significance of Albumin in Modern Left Ventricular Assist Device Therapy: Relevance in the HeartMate 3 Era? Journal of Clinical Medicine. 2025; 14(17):6193. https://doi.org/10.3390/jcm14176193
Chicago/Turabian StyleMoayedifar, Roxana, Muhammed Celik, Barbara Karner, Anne-Kristin Schaefer, Hebe Al Asadi, Christiane Marko, Lukas Ruoff, Daniel Zimpfer, Julia Riebandt, and Thomas Schlöglhofer. 2025. "Prognostic Significance of Albumin in Modern Left Ventricular Assist Device Therapy: Relevance in the HeartMate 3 Era?" Journal of Clinical Medicine 14, no. 17: 6193. https://doi.org/10.3390/jcm14176193
APA StyleMoayedifar, R., Celik, M., Karner, B., Schaefer, A.-K., Al Asadi, H., Marko, C., Ruoff, L., Zimpfer, D., Riebandt, J., & Schlöglhofer, T. (2025). Prognostic Significance of Albumin in Modern Left Ventricular Assist Device Therapy: Relevance in the HeartMate 3 Era? Journal of Clinical Medicine, 14(17), 6193. https://doi.org/10.3390/jcm14176193








