1. Introduction
Laparoscopic surgery is one of the most performed procedures in gynecological practice due to its minimally invasive nature. It offers advantages such as smaller incisions, reduced postoperative pain, and shorter hospital stays compared to traditional laparotomy [
1]. The creation of a surgical workspace in laparoscopy requires the insufflation of carbon dioxide (CO
2) into the abdominal cavity, with intra-abdominal pressure (IAP) typically maintained at 12–15 mmHg (standard pressure) or reduced to 6–10 mmHg in low-pressure pneumoperitoneum [
2,
3,
4].
Despite its benefits, up to 70% of gynecological patients experience postoperative pain. This discomfort originates from three primary components: deep intra-abdominal pain, superficial or incisional pain, and referred shoulder pain [
3,
4]. Pneumoperitoneum is believed to be the leading cause of shoulder pain, as CO
2 insufflation results in diaphragmatic and phrenic nerve irritation and stretching. Additional contributing factors include residual intra-abdominal CO
2, hemoperitoneum, and intraperitoneal acidosis [
5,
6].
Several studies have shown that low-pressure pneumoperitoneum reduces postoperative pain compared to standard-pressure pneumoperitoneum [
3,
7]. However, Madsen et al. found that the combination of low-pressure pneumoperitoneum and deep neuromuscular blockade (NMB) significantly reduced postoperative pain incidence following laparoscopic hysterectomy [
6]. Nevertheless, it remains unclear whether this benefit arises from the lower IAP itself or the deep NMB [
8,
9]. A randomized trial by Park et al. suggested that deep NMB improves surgical conditions and may also have oncologic benefits by enhancing lymph node retrieval [
9].
Emerging evidence indicates that deep NMB may improve surgical conditions by enhancing abdominal wall compliance, reducing intraoperative muscle contractions, and optimizing the surgical workspace. Additionally, it has been associated with reduced postoperative pain scores in bariatric surgery [
5,
6,
10,
11].
This double-blind, randomized controlled trial aims to evaluate the effect of deep NMB reversed with sugammadex compared to moderate NMB reversed with neostigmine on postoperative pain in patients undergoing laparoscopic hysterectomy, maintaining a standardized pneumoperitoneum pressure of 12 mmHg. Additionally, this study seeks to confirm the superiority of sugammadex over neostigmine in facilitating faster recovery from NMB. The findings of this research may contribute to optimizing anesthetic protocols and improving postoperative outcomes in gynecologic laparoscopy.
2. Materials and Methods
This randomized, controlled, double-blind trial was conducted by the Department of Gynecology at Campus Bio-Medico of Rome from April 2018 to December 2024 (No. NCT03519633;
https://clinicaltrials.gov/ct2/show/NCT03519633, accessed on 26 April 2018), following the CONSORT (CONsolidated Standards of Reporting Trials) guidelines. The study adhered to the regulatory standards of Good Clinical Practice and the Declaration of Helsinki (1996) and was approved by the Internal Review Board of Campus Bio-Medico of Rome (No. 39/17 INT ComEtCBM, date 12 July 2017). Written informed consent was obtained from each patient included in the study.
We included women scheduled to undergo laparoscopic subtotal or total hysterectomy under general anesthesia requiring tracheal intubation.
Inclusion criteria: Age between 18 and 75 years, BMI between 16 and 40 kg/m2, ECOG Performance Status 0–1, American Society of Anesthesiologists (ASA) classification 1–3, and ability to provide informed consent.
Exclusion criteria: Pregnancy; active or recent pelvic inflammation; anticipated airway difficulty; history of allergy to rocuronium, neostigmine, or sugammadex; allergy to NSAIDs (Non-Steroidal Anti-Inflammatory Drugs); previous opioid use for chronic pain; patients receiving medications that may alter rocuronium’s duration of action (e.g., aminoglycosides and magnesium); hepatic or renal failure; persistent coagulopathy; neurological or cognitive disorders; conversion to laparotomy; and onset of intraoperative complications.
No protocol deviations occurred during the study. All randomized patients received the allocated intervention and were included in the final analysis (intention-to-treat population).
Patients were randomly assigned to either deep NMB with sugammadex for reversal (SUG group) or moderate NMB with neostigmine plus atropine for reversal (NEO group). Randomization was performed using a computer-generated random number of series via Microsoft® Excel 2016 (version 16.0). Patients were randomized using a computer-generated sequence in blocks of six with a 1:1 allocation ratio. Stratification was not performed. Allocation concealment was ensured using sealed, opaque envelopes prepared and opened by an independent staff member not involved in patient care or data collection. Group allocation was recorded on an Excel sheet, stored in an opaque envelope, and placed in a locked drawer accessible only to the investigator. Anesthesiologists were aware of the treatment assignment, while the surgeon and surgical staff were blinded. Patients and postoperative care providers, including nurses and physicians involved in pain assessment and analgesic administration, were blinded to treatment allocation, and the statistician was blinded. Postoperative pain scores were recorded by trained nurses blinded to treatment allocation, using a standardized numerical rating scale (NRS), minimizing inter-observer variability.
Primary outcomes included postoperative pain within the first 48 h after surgery and reversal time from NMB (train-of-four (TOF) ratio ≥ 0.9). Secondary outcomes included surgical conditions, surgeon satisfaction, anesthesia duration, operation time, extubation time, awakening time, operating room discharge time, incidence of dry mouth and postoperative nausea and vomiting (PONV), need for rescue analgesics (paracetamol, ketorolac and morphine) and antiemetics (metoclopramide), and resolution of postoperative ileus.
Anesthesia was managed with intraoperative monitoring, including a three-lead electrocardiogram, blood pressure, heart rate, pulse oximetry, bispectral index monitoring, and intra-abdominal pressure (IAP). Neuromuscular function was monitored using acceleromyography, measuring the adductor pollicis muscle response via a thumb-placed sensor. The TOF was assessed, and if no thumb twitches were detected, the post-tetanic count (PTC) was measured.
Anesthesia induction included fentanyl (3 mcg/kg), propofol (2–2.5 mg/kg), and rocuronium (0.6 mg/kg for the NEO group, or 1 mg/kg for the SUG group). Before rocuronium administration, the acceleromyography device was calibrated to obtain a baseline TOF ratio. Tracheal intubation was performed two minutes after rocuronium administration. Anesthesia maintenance included desflurane (6%) and remifentanil TCI (2–4 ng/mL) as required. Rocuronium was continuously infused and adjusted to maintain TOF response at 1–2 in the NEO group, or PTC at 1–2 in the SUG group. Preventive analgesia included paracetamol (1 g), dexamethasone (0.1 mg/kg), clonidine (2 mcg/kg), and granisetron (1 mg).
After intubation, patients were placed in the lithotomy position, and hemodynamic stability was assessed before surgery. During laparoscopy, patients were positioned in Trendelenburg to facilitate bowel displacement from the pelvis. Surgical conditions and surgeon satisfaction were evaluated by the same lead surgeon throughout the study every 20 min, using a five-point Surgical Rating Scale (SRS) adapted from the literature [
8,
10,
12], ranging from 1 (extremely poor) to 5 (optimal). Surgeon satisfaction was assessed regarding neck strain, back strain, visual acuity, and overall satisfaction using a five-point scale (1 = very dissatisfied to 5 = very satisfied).
At the end of surgery, hemostasis was achieved, CO2 was evacuated, and trocars were removed. The patient was repositioned horizontally, and incisions were sutured. NMB reversal was initiated during hemostasis using either sugammadex (SUG group) or neostigmine plus atropine (NEO group), titrated according to TOF results. Once TOF ≥ 0.9 was achieved, desflurane was discontinued, the patient was awakened, and extubation was performed.
Postoperative analgesia began 20 min before surgery ended, including ketorolac (90 mg i.v. for the first 24 h). PONV prophylaxis consisted of granisetron (3 mg i.v. for 24 h) and ranitidine (50 mg i.v. daily for 48 h). In the post-anesthesia care unit (PACU), blinded personnel recorded PONV incidence and rescue analgesic/antiemetic use. Pain levels were recorded for the first 48 h at multiple time points (30 min, 60 min, 120 min, 6 h, 12 h, 24 h, 36 h, and 48 h), using a 0–10 numerical rating scale (NRS). Rescue medications included paracetamol (1 g, NRS > 4 or T > 38 °C, max 3 g/day), ketorolac (30 mg, after 24 h, NRS > 6, max 90 mg/day), morphine (2 mg/hourly, NRS > 8, max 6 mg/day), and metoclopramide (10 mg i.v. for nausea).
The trial was supervised by a senior anesthesiologist and a clinical research coordinator, who monitored adherence to the study protocol and adverse-event reporting.
Sample size calculation and statistical analysis: Based on Barron’s study, which reported a reduction in pain from 4.07 to 2.79 (with a common standard deviation of 2.3), the required sample size was 107 patients per group (α = 0.01; β = 0.10) [
13]. Statistical differences between groups were tested using Student’s t-test for normally distributed data or the Mann–Whitney test for non-normally distributed data. Nominal data were presented as absolute numbers with percentages and compared using the χ
2 test or Fisher’s exact test. A
p-value < 0.05 was considered statistically significant. Analyses were performed using SigmaPlot
® software version 14.0. Post hoc power analyses were performed for key secondary outcomes based on the observed effect sizes (Cohen’s d and h). The achieved power was ≥ 0.84 for all tested comparisons, including analgesic consumption, PONV incidence, and extubation time, confirming the adequacy of the sample size in detecting clinically relevant between-group differences across both primary and secondary endpoints. Effect sizes were calculated to estimate the magnitude of between-group differences. Cohen’s d was used for continuous variables, and Cohen’s h for binary outcomes. Given the multiple pain assessments at different time points and conditions, a Bonferroni correction was applied to account for multiplicity.
3. Results
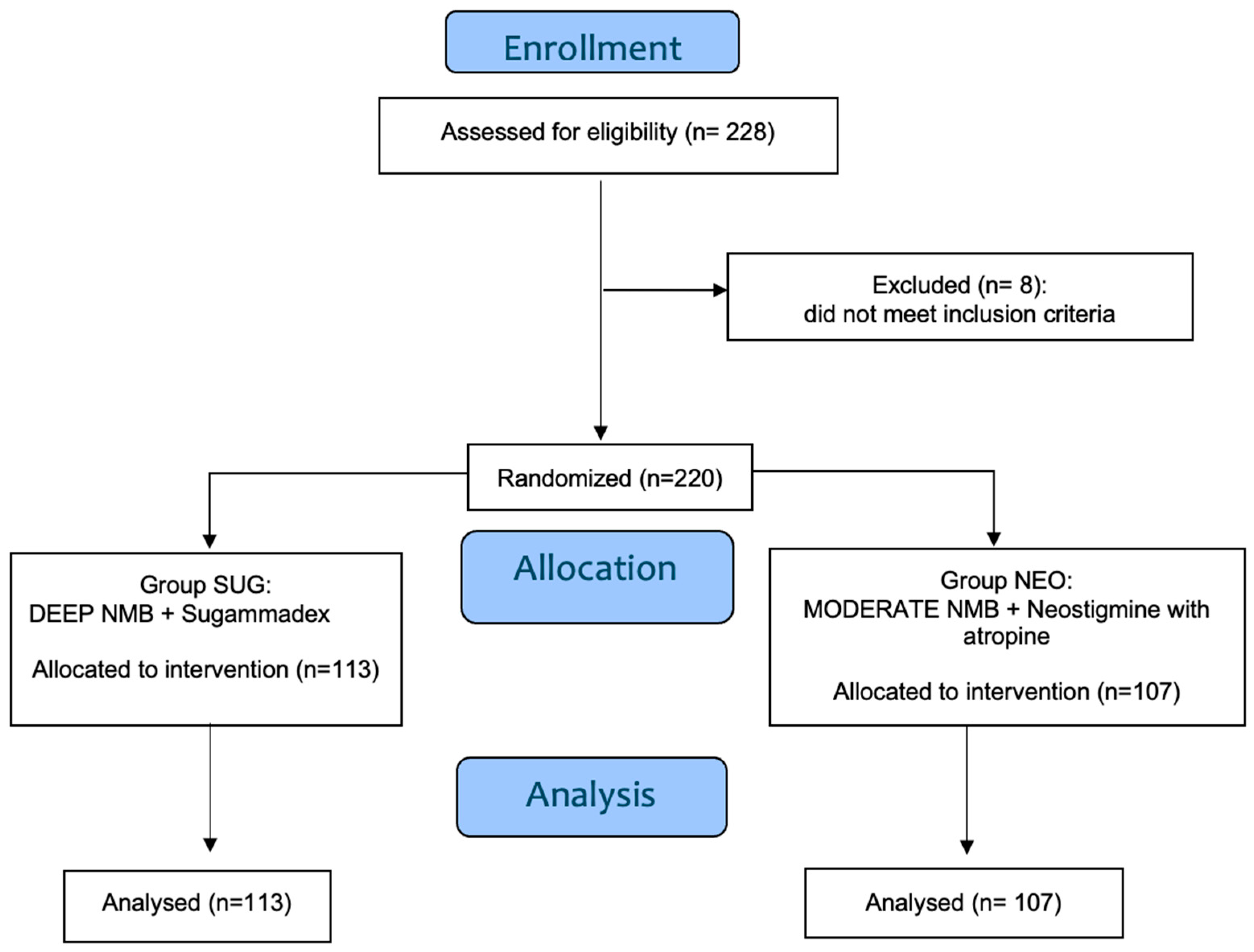
We initially enrolled in this study 228 women scheduled for laparoscopic hysterectomy under general anesthesia, requiring tracheal intubation. Eight patients were excluded before randomization due to meeting one or more exclusion criteria. In total, 220 patients were included in the final analysis, with 113 assigned to the SUG group and 107 to the NEO group.
Figure 1 shows the flowchart of the study.
Table 1 presents patients’ characteristics, demonstrating no significant differences between the two groups in terms of age and preoperative measures.
Postoperative measures of Hb, WBC, PCR, and body temperature were similar in the first two days following surgery.
Intraoperative data (
Table 2) showed no significant differences between the groups in terms of total CO
2 insufflated, insufflation flow, and insufflation time. However, we found that reversal time was significantly lower in the SUG group (147.58 ± 82.26 vs. 488.02 ± 223.07; 95% CI: 132.3–162.9 vs. 445.3–530.8), with a large effect size (Cohen’s d = –2.05).
There were no significant differences in anesthesia and surgery times between the two groups, but extubation time (ΔT1), awakening time (ΔT2), and transfer to the recovery room (ΔT3) were significantly shorter in the SUG group (ΔT1: 7.98 ± 3.87 vs. 13.40 ± 8.27, 95% CI: 7.26–8.70 vs. 11.81–14.99, d = –0.85; ΔT2: 7.51 ± 3.75 vs. 12.73 ± 7.81, 95% CI: 6.81–8.21 vs. 11.23–14.23, d = –0.86; ΔT3: 12.37 ± 5.21 vs. 17.78 ± 7.74, 95% CI: 11.40–13.34 vs. 16.30–19.26, d = –0.82).
Finally, postoperative ileus resolution occurred faster in the SUG group (14.03 ± 6.3 h vs. 19.56 ± 8.2 h, 95% CI: 12.86–15.20 vs. 17.99–21.13), with a moderate-to-large effect size (d = –0.76) (
Table 2).
Pain scores, measured using the NRS scale, are detailed in
Table 3. The SUG group reported significant lower pain at each time point until 6 h (30 min, 1 h, 2 h, and 6 h). The between-group difference in pain at 30 min at rest showed a large effect size (Cohen’s d = –1.30). Across the other conditions (rest, palpation, movement, and cough), pain was significantly lower in the SUG group until 24 h. Data regarding pain after 24 h did not significantly differ between the two groups. After applying Bonferroni correction for 24 comparisons (adjusted α = 0.0021), the between-group differences in postoperative pain scores remained statistically significant across all conditions (rest, palpation, movement, and cough) up to 6 h after surgery (all
p < 0.001). In contrast, differences observed at 12 and 24 h (uncorrected
p-values ranging from 0.01 to 0.04) did not retain statistical significance after correction and should be considered exploratory.
The second primary outcome, the time to reversal of NMB (TOF ratio ≥0.9), was significantly shorter in the SUG group compared to the NEO group (147.58 ± 82.26 vs. 488.02 ± 223.07, 95% CI: 132.3–162.9 vs. 445.3–530.8, p < 0.05).
Surgeon satisfaction was not significantly influenced in our study. Intraoperative surgeon satisfaction assessed through the SRS (1–5 scale) showed similar scores in the SUG group compared to the NEO group at all time points (i.e., overall satisfaction, 60 min (4.6 ± 0.6 vs. 3.8 ± 0.8, Cohen’s d = 1.14)).
Analgesic consumption during PACU stay was significantly lower in the SUG group (16%, 95% CI: 9.2–22.7) compared to the NEO group (38%, 95% CI: 29.1–47.5, p < 0.05, Cohen’s h = –0.51). In addition, rescue analgesic use during the first 48 h was reduced in the SUG group, with significantly less morphine used on day 0 (2%, 95% CI: –0.7–4.2 in SUG vs. 13%, 95% CI: 6.7–19.5 in NEO; p < 0.05, Cohen’s h = –0.45) and less ketorolac on day 1 (27%, 95% CI: 19.2–35.7 in SUG vs. 46%, 95% CI: 36.4–55.2 in NEO; p < 0.05, Cohen’s h = –0.40). Interestingly, the overall use of analgesics was 119 doses in the SUG group and 178 doses in the NEO group (p < 0.001).
PONV incidences were significantly lower in the SUG group during PACU (21%, 95% CI: 13.7–28.8 vs. 49%, 95% CI: 39.1–58.1, p < 0.05, Cohen’s h = –0.60) and remained lower until 6 h post-surgery (e.g., 30 min: SUG 19%, 95% CI: 11.4–25.8 vs. NEO 57%, 95% CI: 47.6–66.4, p < 0.05, Cohen’s h = –0.81).
Correlation analyses between postoperative pain, PONV, recovery time, and analgesic consumption did not reveal any statistically significant associations. Pearson and Spearman correlation coefficients ranged from –0.11 to 0.09, with all p-values > 0.69, suggesting no meaningful linear or monotonic relationships among these variables in our cohort.
The length of hospital stay did not differ significantly between the two groups.
No cases of hypersensitivity or anaphylaxis related to sugammadex were observed in our study. No intraoperative complications, including bleeding, visceral injury, or need for conversion to laparotomy, occurred in either group. Additionally, no adverse events related to deep NMB were reported.
4. Discussion
Effective pain management is essential to enhance patient recovery following laparoscopic procedures. Evidence suggests that deep NMB may provide significant advantages over moderate NMB. Our study contributes to this growing body of knowledge by demonstrating that deep NMB, reversed with sugammadex, is associated with reduced postoperative pain, faster recovery, and a lower incidence of PONV compared to moderate NMB reversed with neostigmine.
Our findings align only in part with the current literature. While some randomized trials and meta-analyses [
5,
13,
14] report improved surgical conditions and faster recovery with deep NMB, others show minimal clinical benefit, particularly when standard-pressure pneumoperitoneum is used. These discrepancies may reflect methodological heterogeneity, including variation in NMB depth monitoring, reversal agents, surgical type, and pressure settings. On the other hand, our findings are consistent with recent data by Kathopoulis et al. [
15], who similarly demonstrated improved pain control and recovery parameters in patients receiving deep NMB during gynecologic laparoscopic procedures. This alignment reinforces the clinical relevance of our results.
Postoperative pain following laparoscopic surgery originates from multiple sources, including intra-abdominal pain, superficial incisional pain, and referred shoulder pain mainly due to diaphragmatic irritation from CO
2 insufflation [
4,
14]. While postoperative pain after open surgery is mostly of somatic origin, postoperative pain after laparoscopic surgery consists of both somatic and visceral elements. The insertion of trocars and sutures is responsible of somatic pain, which is referred to as abdominal pain. In contrast, visceral referred pain after laparoscopy is described as moderate-to-severe dull pain in the shoulder, scapula, and abdomen. Peritoneal stretching, inflammation, postoperative gas retention, and muscle spasm mainly cause this type of pain. Some of these mechanisms can be attenuated, for example, by correct surgical manipulation, optimizing the temperature and humidity of the gas, and decreasing the insufflation pressure. As regards the pathophysiology of pain related to muscle stretching, it may be further explained by a recent study in a rat model [
12], which demonstrated that muscle contraction significantly activated nociceptive dorsal horn neurons (DHN). When NMB was induced with pancuronium, muscle contractions and DHN activation were inhibited, confirming that contraction-induced activation was in part responsible for pain. These findings suggest a potential analgesic effect of deep NMB, as it reduces muscle stretching, which triggers pain stimuli.
Our study showed that patients receiving deep NMB (SUG) reported lower pain scores within the first 6 h compared to those in the moderate NMB group (NEO), after Bonferroni correction for multiple comparisons. The mean pain scores were consistently lower in the SUG group across different conditions (rest, palpation, movement, and cough), suggesting a tangible benefit of deep NMB in minimizing postoperative discomfort. Although pain scores showed statistically significant differences between groups, the magnitude of reduction (2–3 points on the NRS) during the early postoperative period is also considered clinically meaningful in the surgical setting. More specifically, the observed reductions in pain scores (2–3 NRS points) exceed the Minimal Clinically Important Difference (MCID) for postoperative pain, which has been estimated at approximately 1.3–1.5 points on the NRS in surgical populations [
16,
17]. Therefore, these findings not only reached statistical significance but also fulfilled the accepted threshold for clinical relevance, reinforcing their impact for patient recovery and analgesic management.
Importantly, the analgesic benefit of deep neuromuscular blockade with sugammadex was confirmed in the early postoperative period, where differences remained significant after Bonferroni correction for multiple comparisons. Beyond 6 h, the observed reductions in pain scores did not retain statistical significance once corrected for multiplicity, although they showed a consistent trend in favor of sugammadex. These findings suggest that the most robust advantage of this anesthetic strategy is concentrated in the immediate postoperative phase, while later differences should be interpreted with caution as exploratory results.
The observed benefits in recovery and analgesic sparing in the SUG group may also be explained by more rapid and complete reversal of NMB, reducing residual paralysis and facilitating earlier extubation. Additionally, sugammadex avoids the cholinergic effects of neostigmine, which may contribute to reduced incidence of PONV and ileus. Enhanced postoperative bowel function may also relate to lower opioid requirements in the immediate postoperative period.
These findings align with prior research, such as Madsen et al.’s work, which demonstrated that deep NMB and low-pressure pneumoperitoneum together reduce postoperative pain after laparoscopic hysterectomy [
5]. Unlike Madsen’s study, our trial maintained a standardized pneumoperitoneum pressure of 12 mmHg in both groups, confirming that the observed pain reduction was attributable to the NMB depth rather than differences in intra-abdominal pressure. Reduced intra-abdominal pressure alone is, however, correlated with less postoperative pain, as demonstrated in numerous studies [
15,
18,
19]: the physio-pathological causes, as previously stated, are multiple, but an important role is played by the different degree of muscular inflammation correlated to a different tension of the abdominal muscles.
Kathopoulis et al. demonstrated that deep NMB was associated with lower pain scores and reduced analgesic consumption in gynecologic laparoscopic procedures [
15]. In his study, Kathopoulis included patients receiving laparoscopy for various gynecologic procedures, while we included only patients undergoing laparoscopic hysterectomy to have a more homogeneous population of study. Moreover, in our study, analgesic consumption was significantly lower in the SUG group, particularly regarding opioid use. Morphine administration was required in only 2% of patients in the SUG group compared to 13% in the NEO group on day 0, and ketorolac use on day 1 was also significantly lower. These findings are consistent with those reported by Castro et al., who observed that patients reversed with sugammadex after bariatric surgery experienced less postoperative pain and lower PONV rates compared to those reversed with neostigmine [
10].
While improved surgical conditions were anticipated with deep NMB, our findings indicate that surgeon satisfaction, measured by the Surgical Rating Scale (SRS), did not differ significantly between the two groups, as recently demonstrated by Kathopoulis et al. [
15]. This result contrasts with previous studies that reported better surgical working conditions using deep NMB; for example, Dubois et al. [
4] demonstrated superior surgical field in patients under deep NMB. Similarly, Raval et al. observed enhanced surgical visualization and marginally shorter operative times with deep NMB during abdominal procedures [
13]. Additionally, Esa et al. found improved surgical workspace conditions with deep NMB in the context of low-pressure pneumoperitoneum, emphasizing its potential benefit in such settings [
19].
A possible explanation for this discrepancy lies in the different pneumoperitoneum pressures adopted across studies. In most of the above-mentioned trials, surgeries were performed under low intra-abdominal pressures (typically 6–10 mmHg), where the impact of deep muscle relaxation on abdominal wall compliance and workspace expansion is more pronounced. In contrast, our study was conducted under a constant and standard pneumoperitoneum pressure of 12 mmHg, which is already sufficient to provide an adequate surgical field. At this pressure level, the incremental benefit of deep NMB on surgical visibility might be minimal or clinically irrelevant, thus explaining the lack of difference in surgeon satisfaction in our findings. Therefore, we believe that the effect of deep NMB on surgical field conditions is likely pressure-dependent and becomes more evident only in low-pressure settings.
A key finding of our study was the significantly shorter neuromuscular recovery time in the deep NMB group. The time to achieve a TOF ratio of ≥ 0.9 was markedly reduced in the SUG group (147.58 ± 82.26 sec) compared to the NEO group (488.02 ± 223.07 sec,
p < 0.05). This rapid recovery facilitated faster emergence from anesthesia, as evidenced by a shorter extubation time (∆T1), awakening time (∆T2), and transfer to the recovery room (∆T3) in the SUG group. Similar results were observed by Putz et al., who reported that patients receiving deep NMB reversed with sugammadex experienced significantly faster operating room discharge compared to those reversed with neostigmine (22 min vs. 72 min,
p < 0.05) [
20].
The incidence of PONV was significantly lower in the SUG group, both in the PACU and throughout the 48 h postoperative period. This finding aligns with previous research by Ledowski et al., who found that sugammadex use was associated with lower PONV rates [
21]. Similar findings were reported by Yagan et al., who observed a lower incidence of PONV in patients reversed with sugammadex compared to neostigmine [
22].
Effect sizes for categorical outcomes were calculated using Cohen’s h and ranged from small to large. Notably, moderate-to-large effects were observed for analgesic use and PONV reduction, supporting the clinical relevance of the findings beyond statistical significance.
Although sugammadex has been associated with rare but potentially severe adverse events—particularly in older and pediatric populations—it remains a widely used and generally well-tolerated agent. Clinicians should be aware of these potential reactions to ensure prompt and appropriate management when necessary [
23]. On the other hand, large-scale studies such as that by Reutzler et al. have reported similar safety profiles between sugammadex and neostigmine, with only a small proportion of patients experiencing postoperative side effects [
24]. In our cohort, no sugammadex-related adverse events or complications associated with deep NMB were observed, supporting the safety of this strategy in routine gynecologic laparoscopic procedures. Nonetheless, the possibility of rare hypersensitivity reactions should always be considered when assessing the overall risk–benefit profile of sugammadex.
While the duration of surgery itself remained unchanged between groups in our study, the reduced recovery time from anesthesia in the SUG group led to a decreased overall occupancy time in the operating room. In fact, the significantly faster neuromuscular recovery observed in the SUG group (mean difference > 5 min) is particularly relevant in busy surgical settings, where even modest time savings can contribute to improved perioperative workflow and reduced occupancy of operating or recovery rooms. Moreover, faster recovery may reduce the risk of residual paralysis and facilitate earlier patient mobilization and discharge from the PACU. We did not perform a cost-effectiveness analysis; further studies in the future may better analyze this important and interesting aspect.
Although our study provides robust evidence supporting the benefits of deep neuromuscular blockade (NMB), several limitations should be acknowledged. First, while our findings indicate clear advantages in ASA I–II patients undergoing laparoscopic hysterectomy, caution is warranted when extrapolating these results to other surgical populations, higher-risk patients (e.g., ASA III–IV), or procedures performed under low-pressure pneumoperitoneum. Further studies in these subgroups are needed to validate generalizability.
Additionally, recovery times were reported as means and standard deviations; however, future research could incorporate survival analysis techniques to more accurately evaluate time-dependent outcomes, such as reversal time or readiness for discharge.
Investigating deep NMB in procedures conducted exclusively under low pneumoperitoneum pressure could also offer insights, as reduced muscular stretching may further influence postoperative pain.
Lastly, a dedicated cost-effectiveness analysis comparing sugammadex and neostigmine would be valuable to better understand the economic implications of each strategy in clinical practice.
5. Conclusions
Our findings reinforce the clinical benefits of deep NMB in gynecologic laparoscopic surgery, demonstrating reduced postoperative pain, faster recovery, and lower PONV incidence compared to moderate NMB. The use of sugammadex for NMB reversal enhances these advantages by facilitating rapid recovery and minimizing residual curarization. Given these findings, deep NMB with sugammadex should be considered a component of anesthetic management for major laparoscopic gynecologic procedures, with potential implications for fast-track surgery protocols. Further research is warranted to confirm these benefits in larger and more heterogeneous patient populations to assess the long-term impact on healthcare efficiency.
Author Contributions
Conceptualization, C.T., L.S., F.P., F.C., R.A. and C.D.C.N.; data curation, C.T., F.C. and R.A.; formal analysis, C.T., L.S., F.P., F.C., R.A. and C.D.C.N.; investigation, C.T., L.S., F.C., L.F., S.R., F.F., R.M., F.G., A.M., R.A. and C.D.C.N.; methodology, C.T., L.S., F.P., F.C., R.A. and C.D.C.N.; project administration, C.T., R.A. and C.D.C.N.; resources, F.P.; software, C.T. and R.A.; supervision, C.T., L.S., F.P., D.L., R.A. and C.D.C.N.; validation, C.T., R.M., R.A. and C.D.C.N.; visualization, L.S., R.M. and V.D.D.; writing—original draft, L.F., S.R. and F.F.; writing—review and editing, C.T., L.S., F.P., F.C., L.F., F.F., R.M., F.G., D.L., V.D.D., A.M., R.A. and C.D.C.N. All the authors approved the final version of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (of Campus Bio-Medico of Rome (No 39/17 INT ComEtCBM, date 12 July 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author (data are not publicly available due to privacy).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NMB | Neuromuscular blockade |
| PONV | Postoperative Nausea and Vomiting |
| IAP | Intra-abdominal pressure |
| CO2 | Carbon dioxide |
| TOF | Train-of-four |
| PTC | Post tetanic count |
| BMI | Body mass index |
| PACU | Post-anesthesia care unit |
| SRS | Surgical Rating Scale |
| Hb | Hemoglobin |
| WBCs | White blood cells |
| PCR | C-reactive protein |
References
- Rockall, T.; Demartines, N. Laparoscopy in the era of enhanced recovery. Best Pr. Res. Clin. Gastroenterol. 2014, 28, 133–142. [Google Scholar] [CrossRef]
- Brunschot, D.M.D.Ö.-V.; van Laarhoven, K.C.J.H.M.; Scheffer, G.-J.; Pouwels, S.; Wever, K.E.; Warlé, M.C. What is the evidence for the use of low-pressure pneumoperitoneum? A systematic review. Surg. Endosc. 2015, 30, 2049–2065. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.W. Residual intraperitoneal carbon dioxide gas following laparoscopy for adnexal masses: Residual gas volume assessment and postoperative outcome analysis. Medicine 2022, 101, e30142. [Google Scholar] [CrossRef] [PubMed]
- Dubois, P.E.; Putz, L.; Jamart, J.; Marotta, M.-L.; Gourdin, M.; Donnez, O. Deep neuromuscular block improves surgical conditions during laparoscopic hysterectomy. Eur. J. Anaesthesiol. 2014, 31, 430–436. [Google Scholar] [CrossRef]
- Madsen, M.V.; Staehr-Rye, A.K.; Gätke, M.R.; Claudius, C. Neuromuscular blockade for optimising surgical conditions during abdominal and gynaecological surgery: A systematic review. Acta Anaesthesiol Scand. 2015, 59, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Weiss, C.; Hertel, H.; Hillemanns, P.; Klapdor, R.; Soergel, P. Association between intraabdominal pressure during gynaecologic laparoscopy and postoperative pain. Arch. Gynecol. Obstet. 2017, 295, 1191–1199. [Google Scholar] [CrossRef]
- Honing, M.; Reijnders-Boerboom, G.; Dell-Kuster, S.; van Velzen, M.; Martini, C.; Valenza, F.; Proto, P.; Cambronero, O.D.; Broens, S.; Panhuizen, I.; et al. The impact of deep versus standard neuromuscular block on intraoperative safety during laparoscopic surgery: An international multicenter randomized controlled double-blind strategy trial—EURO-RELAX TRIAL. Trials 2021, 22, 1–13. [Google Scholar] [CrossRef]
- Kim, J.E.; Min, S.K.; Ha, E.; Lee, D.; Kim, J.Y.; Kwak, H.J. Effects of deep neuromuscular block with low-pressure pneumoperitoneum on respiratory mechanics and biotrauma in a steep Trendelenburg position. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Huh, H.; Choi, S.I.; Jang, Y.-J.; Park, J.-M.; Kwon, O.K.; Jung, M.R.; Jeong, O.; Min, J.S.; Kim, J.-J.; An, L.; et al. Impact of the Deep Neuromuscular Block on Oncologic Quality of Laparoscopic Surgery in Obese Gastric Cancer Patients: A Randomized Clinical Trial. J. Am. Coll. Surg. 2022, 234, 326–339. [Google Scholar] [CrossRef]
- Castro, D.S.; Leão, P.; Borges, S.; Gomes, L.; Pacheco, M.; Figueiredo, P. Sugammadex Reduces Postoperative Pain After Laparoscopic Bariatric Surgery. Surg. Laparosc. Endosc. Percutaneous Tech. 2014, 24, 420–423. [Google Scholar] [CrossRef]
- Golzari, S.E.J.; Nader, N.D.; Mahmoodpoor, A. Underlying mechanisms of postoperative pain after laparoscopic surgery. JAMA Surg. 2016, 151, 295–296. [Google Scholar] [CrossRef]
- Gu, H.; Sugiyama, D.; Kang, S.; Brennan, T.J. Deep Tissue Incision Enhances Spinal Dorsal Horn Neuron Activity During Static Isometric Muscle Contraction in Rats. J. Pain 2019, 20, 301–314. [Google Scholar] [CrossRef]
- Raval, A.D.; Deshpande, S.; Rabar, S.; Koufopoulou, M.; Neupane, B.; Iheanacho, I.; Bash, L.D.; Horrow, J.; Fuchs-Buder, T.; Farag, E. Does deep neuromuscular blockade during laparoscopy procedures change patient, surgical, and healthcare resource outcomes? A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2020, 15, e0231452. [Google Scholar] [CrossRef]
- Bala, D.; Shields, D.; Shukri, S. Prospective randomized trial of low-pressure pneumoperitoneum for reduction of shoulder-tip pain following laparoscopy. Br. J. Surg. 2001, 88, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Kathopoulis, N.; Protopapas, A.; Stamatakis, E.; Chatzipapas, I.; Zacharakis, D.; Grigoriadis, T.; Athanasiou, S.; Valsmidis, D. Deep versus Moderate Neuromuscular Blockade in Gynecologic Laparoscopic Operations: Randomized Controlled Trial. J. Pers. Med. 2022, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Myles, D.B.; Galagher, W.; Boyd, D.; Chew, C.; MacDonald, N.; Dennis, A. Measuring acute postoperative pain using the visual analog scale: The minimal clinically important difference and patient acceptable symptom state. Br. J. Anaesth. 2017, 118, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Gerbershagen, H.J.; Rothaug, J.; Kalkman, C.J.; Meissner, W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: A cut-off point analysis applying four different methods. Br. J. Anaesth. 2011, 107, 619–626. [Google Scholar] [CrossRef]
- Madsen, M.V.; Istre, O.; Staehr-Rye, A.K.; Springborg, H.H.; Rosenberg, J.; Lund, J.; Gätke, M.R. Postoperative shoulder pain after laparoscopic hysterectomy with deep neuromuscular blockade and low-pressure pneumoperitoneum: A randomised controlled trial. Eur. J. Anaesthesiol. 2016, 33, 341–347. [Google Scholar] [CrossRef]
- Esa, U.; Zaini, R.H.M.; Mazlan, M.Z.; Omar, A.A.; Omar, S.C.; Rosedi, A. Evaluation of surgical condition during laparoscopic gynaecological surgery in patients with moderate vs. deep neuromuscular block in low-pressure pneumoperitoneum. Anaesthesiol. Intensiv. Ther. 2024, 56, 121–128. [Google Scholar] [CrossRef]
- Putz, L.; Dransart, C.; Jamart, J.; Marotta, M.-L.; Delnooz, G.; Dubois, P.E. Operating room discharge after deep neuromuscular block reversed with sugammadex compared with shallow block reversed with neostigmine: A randomized controlled trial. J. Clin. Anesthesia 2016, 35, 107–113. [Google Scholar] [CrossRef]
- Ledowski, T.; Falke, L.; Johnston, F.; Gillies, E.; Greenaway, M.; De Mel, A.; Tiong, W.S.; Phillips, M. Retrospective investigation of postoperative outcome after reversal of residual neuromuscular blockade: Sugammadex, neostigmine or no reversal. Eur. J. Anaesthesiol. 2014, 31, 423–429. [Google Scholar] [CrossRef]
- Yağan, Ö.; Taş, N.; Mutlu, T.; Hancı, V. Comparison of the effects of sugammadex and neostigmine on postoperative nausea and vomiting. Braz. J. Anesthesiol. 2017, 67, 147–152. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Q.; Li, Z.; Yan, S.; Ming, S. Systematic analysis of sugammadex-related adverse drug reaction signals using FAERS database. Int. J. Surg. 2024, 111, 1988–1994. [Google Scholar] [CrossRef]
- Ruetzler, K.; Li, K.; Chhabada, S.; Maheshwari, K.; Chahar, P.; Khanna, S.; Schmidt, M.T.; Yang, D.; Turan, A.; Sessler, D.I. Sugammadex Versus Neostigmine for Reversal of Residual Neuromuscular Blocks After Surgery: A Retrospective Cohort Analysis of Postoperative Side Effects. Anesthesia Analg. 2022, 134, 1043–1053. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).