Primary and Revision Reverse Shoulder Arthroplasty Using Custom-Made 3D-Printed Baseplates for Severe Multiplanar Glenoid Bone Defects: A Retrospective Study of Clinical and Radiographic Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
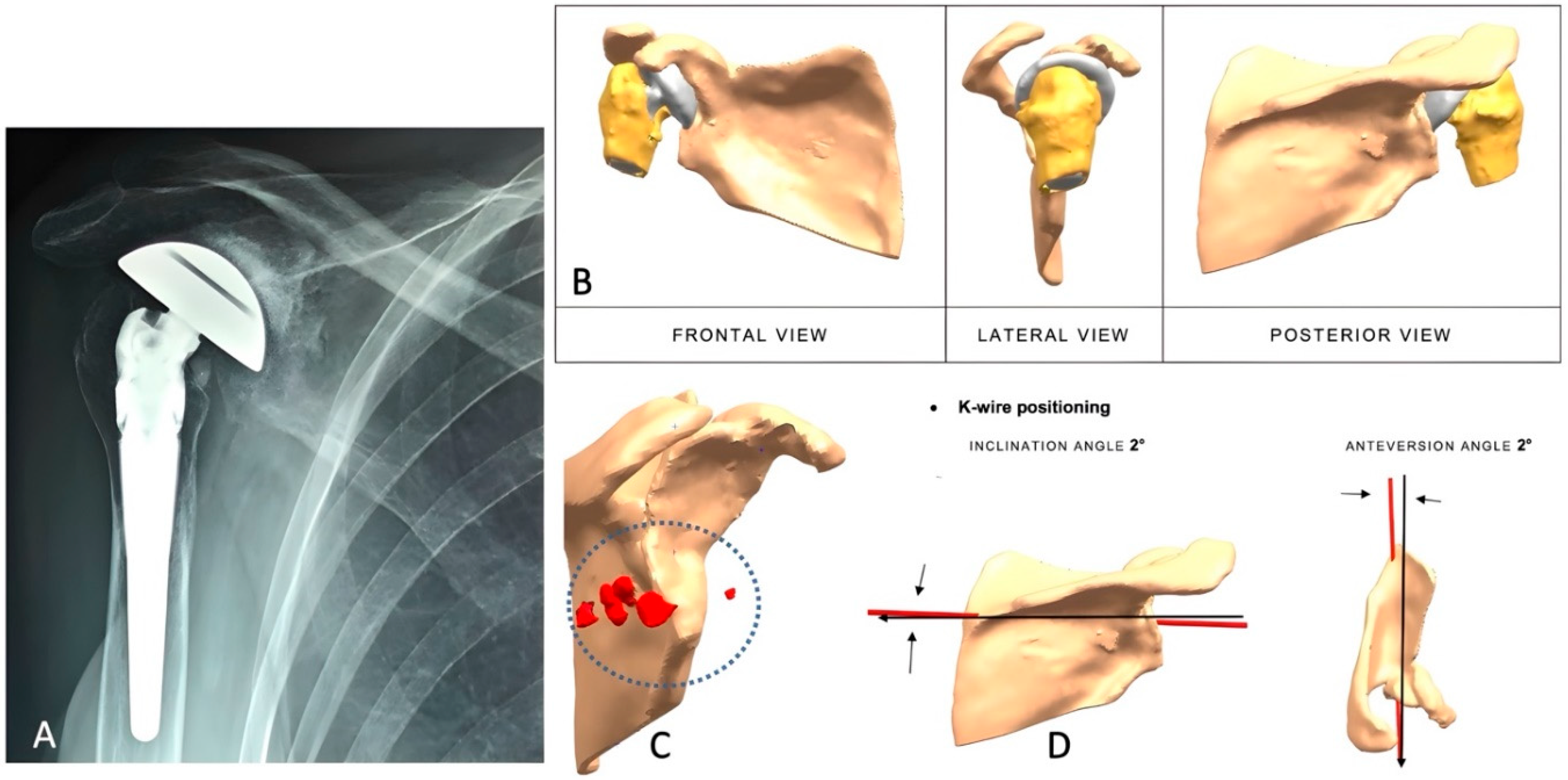
2.2. Preoperative Imaging
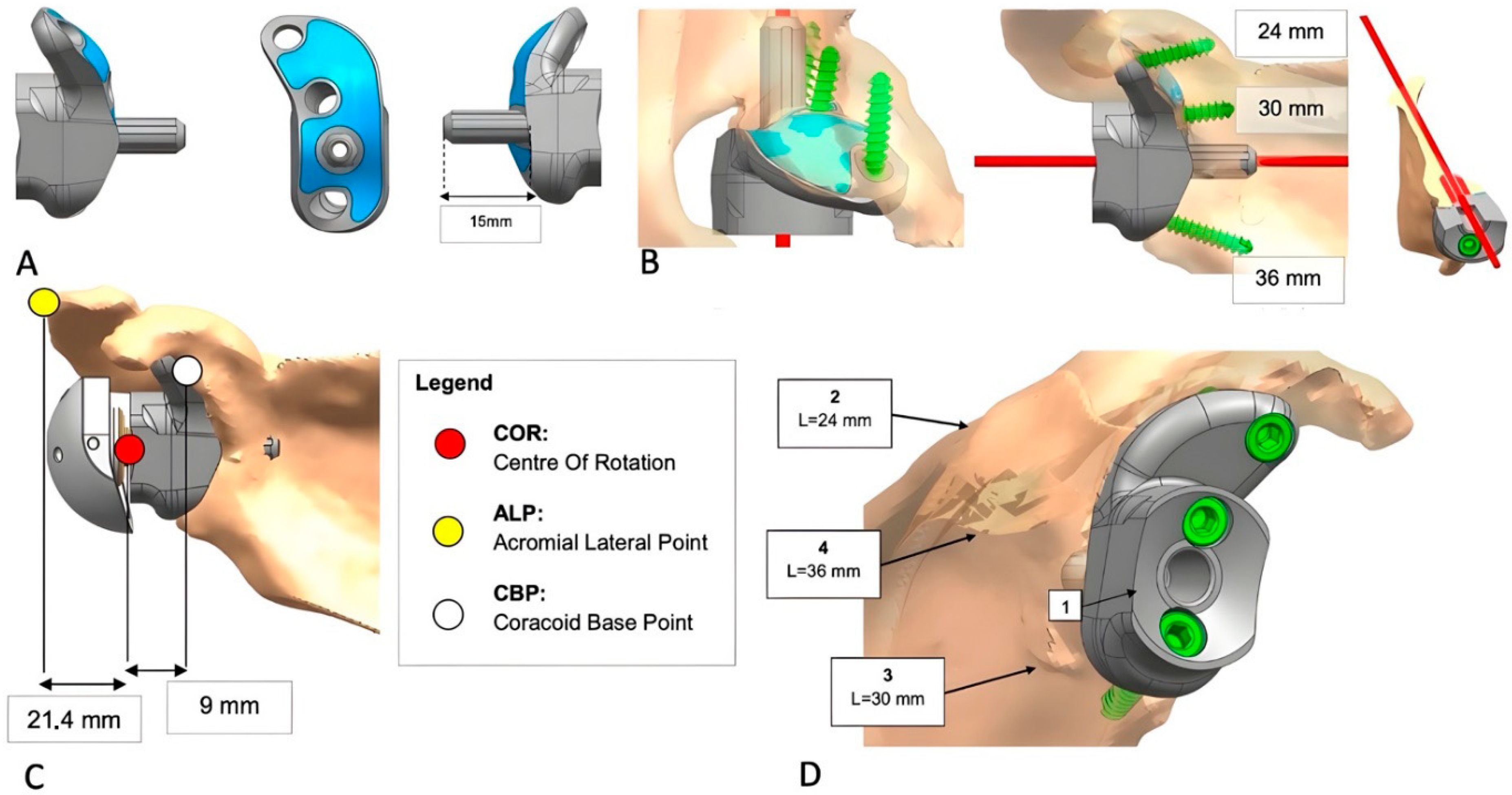
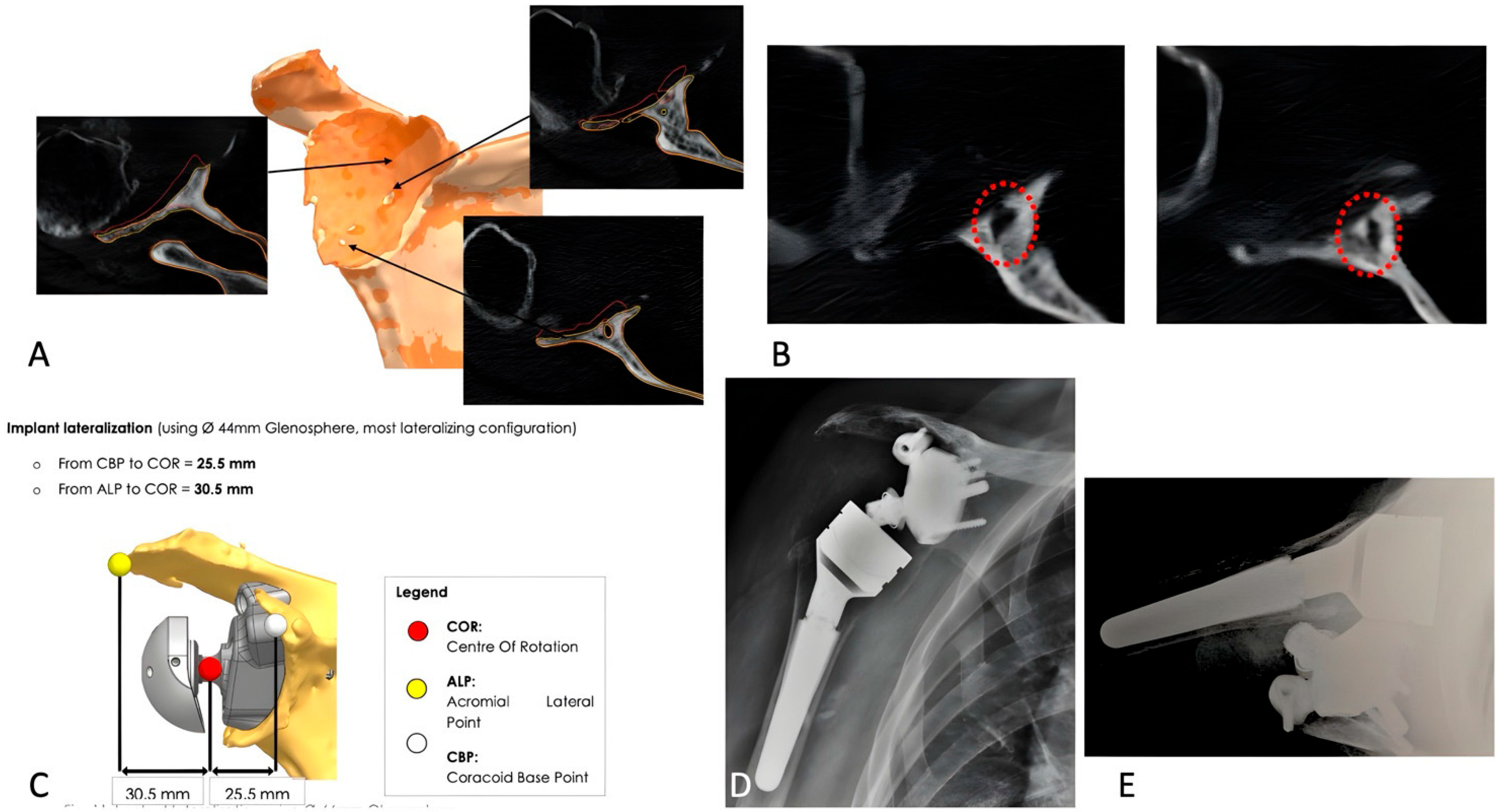
2.3. Implant Design and Custom-Made Development Process
2.4. Clinical Evaluation and Outcome Measures
2.5. Operative Procedure
2.6. Postoperative Imaging
2.7. Statistical Analysis
3. Results
3.1. Clinical Outcomes
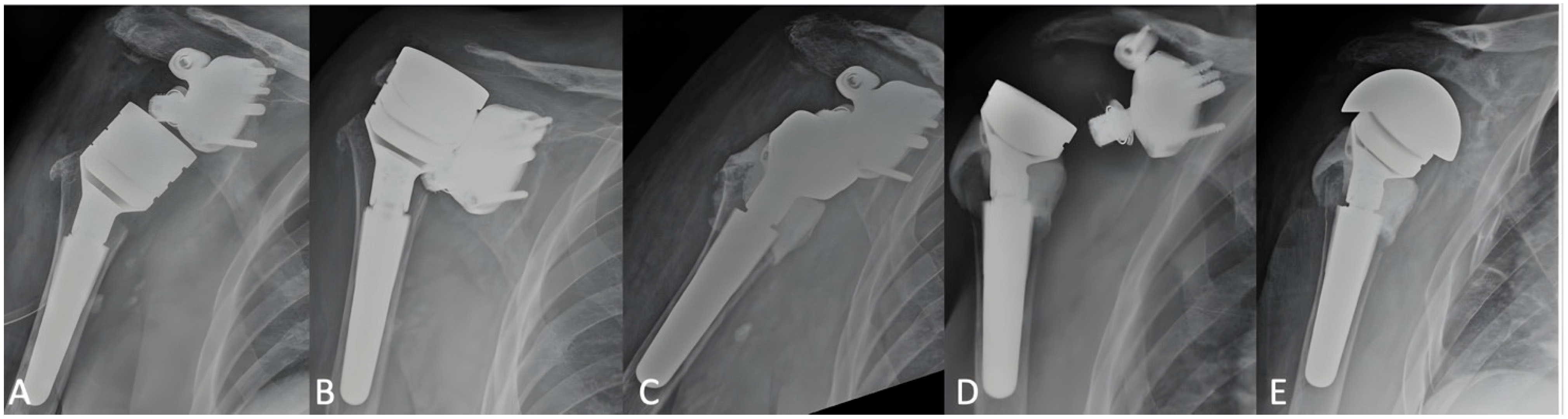
3.2. Radiographic Outcomes
3.3. Complications and Revisions
4. Discussion
Conclusions and Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lübbeke, A.; Rees, J.L.; Barea, C.; Combescure, C.; Carr, A.J.; Silman, A.J. International variation in shoulder arthroplasty. Acta Orthop. 2017, 88, 592–599. [Google Scholar] [CrossRef]
- Seidl, A.J.; Williams, G.R.; Boileau, P. Challenges in Reverse Shoulder Arthroplasty: Addressing Glenoid Bone Loss. Orthopedics 2016, 39, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Rachuene, P.A.; Dey, R.; Sivarasu, S.; Du Plessis, J.-P.; Roche, S.; Vrettos, B. A narrative review of treatment strategies for major glenoid defects during primary reverse shoulder arthroplasty, with a focus on the use of structural bone graft. EFORT Open Rev. 2023, 8, 759–770. [Google Scholar] [CrossRef]
- Sanchez-Sotelo, J. Glenoid Bone Loss: Etiology, Evaluation, and Classification. Instr. Course Lect. 2019, 68, 65–78. [Google Scholar]
- Gupta, A.; Thussbas, C.; Koch, M.; Seebauer, L. Management of glenoid bone defects with reverse shoulder arthroplasty—Surgical technique and clinical outcomes. J. Shoulder Elb. Surg. 2018, 27, 853–862. [Google Scholar] [CrossRef]
- Antuna, S.A.; Sperling, J.W.; Cofield, R.H.; Rowland, C.M. Glenoid revision surgery after total shoulder arthroplasty. J. Shoulder Elb. Surg. 2001, 10, 217–224. [Google Scholar] [CrossRef]
- Bercik, M.J.; Kruse, K.; Yalizis, M.; Gauci, M.-O.; Chaoui, J.; Walch, G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J. Shoulder Elb. Surg. 2016, 25, 1601–1606. [Google Scholar] [CrossRef]
- Boileau, P.; Moineau, G.; Roussanne, Y.; O’Shea, K. Bony Increased-offset Reversed Shoulder Arthroplasty: Minimizing Scapular Impingement While Maximizing Glenoid Fixation. Clin. Orthop. 2011, 469, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Merolla, G.; Giorgini, A.; Bonfatti, R.; Micheloni, G.M.; Negri, A.; Catani, F.; Tarallo, L.; Paladini, P.; Porcellini, G. BIO-RSA vs. metal-augmented baseplate in shoulder osteoarthritis with multiplanar glenoid deformity: A comparative study of radiographic findings and patient outcomes. J. Shoulder Elb. Surg. 2023, 32, 2264–2275. [Google Scholar] [CrossRef]
- Levine, W.N.; Djurasovic, M.; Glasson, J.-M.; Pollock, R.G.; Flatow, E.L.; Bigliani, L.U. Hemiarthroplasty for glenohumeral osteoarthritis: Results correlated to degree of glenoid wear. J. Shoulder Elb. Surg. 1997, 6, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.E.; Ricchetti, E.T.; Huffman, G.R.; Iannotti, J.P.; Glaser, D.L. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J. Shoulder Elb. Surg. 2013, 22, 1298–1308. [Google Scholar] [CrossRef]
- Levine, W.N.; Fischer, C.R.; Nguyen, D.; Flatow, E.L.; Ahmad, C.S.; Bigliani, L.U. Long-Term Follow-up of Shoulder Hemiarthroplasty for Glenohumeral Osteoarthritis. J. Bone Jt. Surg.-Am. 2012, 94, e164. [Google Scholar] [CrossRef]
- Hill, J.M.; Norris, T.R. Long-Term Results of Total Shoulder Arthroplasty Following Bone-Grafting of the Glenoid. J. Bone Jt. Surg.-Am. 2001, 83, 877–883. [Google Scholar] [CrossRef]
- Klika, B.J.; Wooten, C.W.; Sperling, J.W.; Steinmann, S.P.; Schleck, C.D.; Harmsen, W.S.; Cofield, R.H. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J. Shoulder Elb. Surg. 2014, 23, 1066–1072. [Google Scholar] [CrossRef]
- Campana, V.; Cardona, V.; Vismara, V.; Monteleone, A.S.; Piazza, P.; Messinese, P.; Mocini, F.; Sircana, G.; Maccauro, G.; Saccomanno, M.F. 3D printing in shoulder surgery. Orthop. Rev. 2020, 12 (Suppl. 1), 8681. [Google Scholar] [CrossRef]
- Gruber, M.S.; Schwarz, T.; Lindorfer, M.; Rittenschober, F.; Bischofreiter, M.; Hochreiter, J.; Ortmaier, R. Clinical and Radiological Outcomes after Total Shoulder Arthroplasty Using Custom-Made Glenoid Components: A Systematic Review. J. Clin. Med. 2022, 11, 7268. [Google Scholar] [CrossRef]
- Bodendorfer, B.M.; Loughran, G.J.; Looney, A.M.; Velott, A.T.; Stein, J.A.; Lutton, D.M.; Wiesel, B.B.; Murthi, A.M. Short-term outcomes of reverse shoulder arthroplasty using a custom baseplate for severe glenoid deficiency. J. Shoulder Elb. Surg. 2021, 30, 1060–1067. [Google Scholar] [CrossRef]
- Ortmaier, R.; Wierer, G.; Gruber, M.S. Functional and Radiological Outcomes after Treatment with Custom-Made Glenoid Components in Revision Reverse Shoulder Arthroplasty. J. Clin. Med. 2022, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Porcellini, G.; Micheloni, G.M.; Tarallo, L.; Paladini, P.; Merolla, G.; Catani, F. Custom-made reverse shoulder arthroplasty for severe glenoid bone loss: Review of the literature and our preliminary results. J. Orthop. Traumatol. 2021, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.S.; Cunningham, L.; Shields, D.W.; Walton, M.J.; Monga, P.; Bale, R.S.; Trail, I.A. Clinical and radiologic outcomes of Lima ProMade custom 3D-printed glenoid components in primary and revision reverse total shoulder arthroplasty with severe glenoid bone loss: A minimum 2-year follow-up. J. Shoulder Elb. Surg. 2023, 32, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Sirveaux, F.; Favard, L.; Oudet, D.; Huquet, D.; Walch, G.; Mole, D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: Results of a multicentre study of 80 shoulders. J. Bone Jt. Surg. Br. 2004, 86-B, 388–395. [Google Scholar] [CrossRef]
- Goutallier, D.; Postel, J.M.; Bernageau, J.; Lavau, L.; Voisin, M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin. Orthop. 1994, 304, 78–83. [Google Scholar] [CrossRef]
- Melis, B.; DeFranco, M.; Lädermann, A.; Molé, D.; Favard, L.; Nérot, C.; Maynou, C.; Walch, G. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J. Bone Jt. Surg. Br. 2011, 93-B, 1240–1246. [Google Scholar] [CrossRef]
- Levy, J.C.; Anderson, C.; Samson, A. Classification of Postoperative Acromial Fractures Following Reverse Shoulder Arthroplasty. J. Bone Jt. Surg.-Am. 2013, 95, e104. [Google Scholar] [CrossRef]
- Michelin, R.M.; Manuputy, I.; Rangarajan, R.; Lee, B.K.; Schultzel, M.; Itamura, J.M. Primary and revision reverse total shoulder arthroplasty using a patient-matched glenoid implant for severe glenoid bone deficiency. J. Shoulder Elb. Surg. 2024, 33, S93–S103. [Google Scholar] [CrossRef]
- Abboud, J.A.; Anakwenze, O.A.; Hsu, J.E. Soft-tissue Management in Revision Total Shoulder Arthroplasty. J. Am. Acad. Orthop. Surg. 2013, 21, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Baldari, A.; Saccone, L.; Caldaria, A.; De Sanctis, E.G.; De Angelis D’Ossat, G.M.; La Verde, L.; Palumbo, A.; Franceschi, F. Revision shoulder arthroplasty and proximal humeral bone loss: A comprehensive review and proposal of a new algorithm of management. J. Orthop. Traumatol. 2024, 25, 40. [Google Scholar] [CrossRef]
- Nolte, P.-C.; Miles, J.W.; Tanghe, K.K.; Brady, A.W.; Midtgaard, K.S.; Cooper, J.D.; Lacheta, L.; Provencher, M.T.; Millett, P.J. The effect of glenosphere lateralization and inferiorization on deltoid force in reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2021, 30, 1817–1826. [Google Scholar] [CrossRef]
- Ott, N.; Alikah, A.; Hackl, M.; Seybold, D.; Müller, L.P.; Wegmann, K. The effect of glenoid lateralization and glenosphere size in reverse shoulder arthroplasty on deltoid load: A biomechanical cadaveric study. J. Orthop. 2021, 25, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Clinker, C.; Ishikawa, H.; Presson, A.P.; Zhang, C.; Joyce, C.; Chalmers, P.N.; Tashjian, R.Z. The effect of lateralization and distalization after Grammont-style reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2024, 33, 2664–2670. [Google Scholar] [CrossRef]
- Longo, U.G.; Franceschetti, E.; Carnevale, A.; Schena, E.; Cozza, G.; Perricone, G.; Cardinale, M.E.; Papalia, R. Influence of Lateralization and Distalization on Joint Function after Primary Reverse Total Shoulder Arthroplasty. Bioengineering 2023, 10, 1409. [Google Scholar] [CrossRef]
- Saccone, L.; Giovannetti De Sanctis, E.; Caldaria, A.; Biagi, N.; Baldari, A.; De Angelis D’Ossat, G.M.; La Verde, L.; Palumbo, A.; Franceschi, F. Modified distalization shoulder angle and lateralization shoulder angle show weakly correlation with clinical outcomes following reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2025, 34, 1650–1657. [Google Scholar] [CrossRef]
- Debeer, P.; Berghs, B.; Pouliart, N.; Van Den Bogaert, G.; Verhaegen, F.; Nijs, S. Treatment of severe glenoid deficiencies in reverse shoulder arthroplasty: The Glenius Glenoid Reconstruction System experience. J. Shoulder Elb. Surg. 2019, 28, 1601–1608. [Google Scholar] [CrossRef]
- Sholtis, C.; Kha, S.T.; Ramakrishnan, A.; Abrams, G.D.; Freehill, M.T.; Cheung, E.V. Glenoid structural bone grafting in reverse total shoulder arthroplasty: Clinical and radiographic outcomes. J. Shoulder Elb. Surg. 2025, 34, e103–e111. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.G.J.; Chao, J.Y.Y.; Leong, C.; Tan, C.H. 3D printed patient specific customised surgical jig for reverse shoulder arthroplasty, a cost effective and accurate solution. J. Clin. Orthop. Trauma. 2021, 21, 101503. [Google Scholar] [CrossRef] [PubMed]
- Ferlauto, H.R.; Wickman, J.R.; Lazarides, A.L.; Hendren, S.; Visgauss, J.D.; Brigman, B.E.; Anakwenze, O.A.; Klifto, C.S.; Eward, W.C. Reverse total shoulder arthroplasty for oncologic reconstruction of the proximal humerus: A systematic review. J. Shoulder Elb. Surg. 2021, 30, e647–e658. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, W.; Zeng, Q.; Wang, S.; Zhang, Z.; Liu, J.; Zhang, Y.; Shao, Z.; Wang, B. The Personalized Shoulder Reconstruction Assisted by 3D Printing Technology After Resection of the Proximal Humerus Tumours. Cancer Manag. Res. 2019, 11, 10665–10673. [Google Scholar] [CrossRef]
| Variable | Primary RSA | Revision RSA | p Value |
|---|---|---|---|
| Shoulders (no.) | 11 | 12 | 0.429 |
| Follow-up duration, mo, median (range) | 24.09 (7.7) | 32.25 (15.29) | 0.174 |
| Mean age (years) (range) | 66.45 (40–80) | 67.58 (48–86) | 0.82 |
| Gender (M/F) (%) | 4/7 | 7/5 | 0.52 |
| Glenoid wear according to Sirveaux et al. [21] (no.) (%) | NA | ||
| E0 | 0 | 0 | |
| E1 | 1 (9.1) | 0 | |
| E2 | 0 | 0 | |
| E3 | 5 (45.5) | 1 (8.3) | |
| Glenoid bone loss according to Bercik-Walch et al. [7] (no.) (%) | NA | ||
| A1 | 0 | 0 | |
| A2 | 0 | 0 | |
| B1 | 0 | 0 | |
| B2 | 0 | 0 | |
| B3 | 0 | 0 | |
| C | 0 | 1 (8.3) | |
| Glenoid bone loss according to Gupta-Seebauer [5] (no.) (%) | 0.565 | ||
| C1 | 0 | 0 | |
| C2 | 0 | 0 | |
| C3 | 1 (9.1) | 0 | |
| C4 | 2 (18.2) | 4 (33.3) | |
| E1 | 0 | 0 | |
| E2 | 0 | 0 | |
| E3 | 1 (9.1) | 1 (8.3) | |
| E4 | 3 (27.3) | 4 (33.3) | |
| Cemented stem | 1 (9.1) | 1 (8.3) |
| Primary Arthroplasty Group | Revision Arthroplasty Group | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Median (IQR) | Mean ± SD | Confidence Interval | Median (IQR) | Mean ± SD | Confidence Interval | p Value (Mann–Witney Test) |
| AAE1 | 70 (60; 90) | 71.8 ± 20.6 | 70 (40; 70) | 59.6 ± 17.4 | 0.19 | ||
| AAE2 | 125 (105; 130) | 118.6 ± 14 | 105 (65; 126) | 99.3 ± 41.1 | 0.18 | ||
| AAE Delta score | −55 (−65; −35) | −46.8 ± 30.1 | −67.0; −26.6 | −31 (−77.5; −10) | −39.7 ± 35.3 | −62.2; −17.3 | 0.57 |
| ALE1 | 50 (45; 70) | 52.7 ± 16.6 | 45 (10; 60) | 39.2 ± 26.7 | 0.24 | ||
| ALE2 | 100 (100; 110) | 101.4 ± 11.4 | 82.5 (65; 100) | 80.4 ± 4.2 | 0.07 | ||
| ALE Delta score | −50 (−60; −40) | −48.6 ± 16.3 | −59.6; −37.7 | −32.5 (−57.5; −5) | −41.2 ± 44.9 | −69.8; −12.8 | 0.17 |
| ER1 | 2 (2; 2) | 1.6 ± 0.8 | 2 (2; 2) | 1.7 ± 0.78 | 0.92 | ||
| ER2 | 2 (2; 6) | 3.3 ± 1.8 | 2 (2; 2) | 2 ± 0.85 | 0.06 | ||
| ER delta score | 0 (+4; 0) | −1.6 ± 2 | −3.0; −0.32 | 0 (0; 0) | −0.33 ± 1.4 | −1.2; −0.58 | 0.08 |
| IR1 | 2 (2; 4) | 2.5 ± 1.3 | 1 (0; 2) | 1.3 ± 1.6 | 0.06 | ||
| IR2 | 4 (4; 4) | 4.5 ± 1.3 | 4 (1; 6) | 3.7 ± 2.9 | 0.40 | ||
| IR delta score | −2 (−4; 0) | −2 ± 1.5 | −3.0; −0.96 | −3 (−4; 0) | −2.3 ± 3.4 | −4.5; −0.18 | 0.61 |
| Primary Arthroplasty Group | Revision Arthroplasty Group | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Median (IQR) | Mean ± SD | Confidence Interval | Median (IQR) | Mean ± SD | Confidence Interval | p Value (Mann–Whitney) |
| CS1 | 22 (20; 30) | 23.3 ± 6.6 | 20 (12; 24) | 19.5 ± 8.7 | 0.17 | ||
| CS2 | 58 (55; 64) | 58.8 ± 4.2 | 46 (29; 61) | 45.2 ± 20.2 | 0.09 | ||
| CS2pain | 12 (10; 5) | 12.4 ± 2.2 | 10 (10; 15) | 10.8 ± 3.6 | 0.23 | ||
| CS2DLA | 16 (14; 20) | 15.9 ± 4.2 | 13 (7; 16) | 11.7 ± 5.1 | 0.04 | ||
| CS2mobility | 22 (20; 26) | 24.2 ± 7.1 | 14.5 (11; 22) | 16.2 ± 8.7 | 0.03 | ||
| CS2strength | 4 (2; 8) | 4.7 ± 2.4 | 4 (1.5; 5) | 4.5 ± 4.2 | 0.39 | ||
| CS delta score | −34 (−37; −28) | −35.5 ± 8.7 | −41.39;−29.7 | −24 (−37.5; −10.5) | −25.7 ± 16 | −35.9; −15.6 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merolla, G.; De Filippo, F.; Magrini Pasquinelli, F.; Micheloni, G.M.; Porcellini, G.; Paladini, P.; Castricini, R. Primary and Revision Reverse Shoulder Arthroplasty Using Custom-Made 3D-Printed Baseplates for Severe Multiplanar Glenoid Bone Defects: A Retrospective Study of Clinical and Radiographic Outcomes. J. Clin. Med. 2025, 14, 6153. https://doi.org/10.3390/jcm14176153
Merolla G, De Filippo F, Magrini Pasquinelli F, Micheloni GM, Porcellini G, Paladini P, Castricini R. Primary and Revision Reverse Shoulder Arthroplasty Using Custom-Made 3D-Printed Baseplates for Severe Multiplanar Glenoid Bone Defects: A Retrospective Study of Clinical and Radiographic Outcomes. Journal of Clinical Medicine. 2025; 14(17):6153. https://doi.org/10.3390/jcm14176153
Chicago/Turabian StyleMerolla, Giovanni, Francesco De Filippo, Fabiana Magrini Pasquinelli, Gian Mario Micheloni, Giuseppe Porcellini, Paolo Paladini, and Roberto Castricini. 2025. "Primary and Revision Reverse Shoulder Arthroplasty Using Custom-Made 3D-Printed Baseplates for Severe Multiplanar Glenoid Bone Defects: A Retrospective Study of Clinical and Radiographic Outcomes" Journal of Clinical Medicine 14, no. 17: 6153. https://doi.org/10.3390/jcm14176153
APA StyleMerolla, G., De Filippo, F., Magrini Pasquinelli, F., Micheloni, G. M., Porcellini, G., Paladini, P., & Castricini, R. (2025). Primary and Revision Reverse Shoulder Arthroplasty Using Custom-Made 3D-Printed Baseplates for Severe Multiplanar Glenoid Bone Defects: A Retrospective Study of Clinical and Radiographic Outcomes. Journal of Clinical Medicine, 14(17), 6153. https://doi.org/10.3390/jcm14176153





