1. Introduction
Immune checkpoint inhibitors (ICIs) are a form of immunotherapy that boosts the patient’s immune response to detect and eliminate cancer cells. The immune system and cancer have a dynamic relationship. The immune system normally recognises and eliminates cancerous cells, but cancer cells can evade this surveillance through genetic changes and by producing immunosuppressive signals. This interplay is so critical that boosting the immune system through cancer immunotherapy has become a revolutionary cancer treatment. Thus, immunotherapy is a medical treatment that harnesses the power of the immune system to fight diseases like cancer by stimulating the immune system to attack specific cancer cells or by suppressing an overactive immune response in conditions like autoimmune diseases.
Since the Food and Drug Administration (FDA) approved ipilimumab for metastatic melanoma in 2011, the use of ICI has grown exponentially, becoming a fundamental treatment for locally advanced and advanced non-small cell lung cancer (NSCLC) without target mutations, and even for small cell lung cancer (SCLC) [
1,
2,
3]. However, despite the considerable improvement in outcomes following the incorporation of ICIs [
4], there has been a corresponding increase in complications related to their administration, known as immune-related adverse events (IRAEs), which vary in severity and can affect virtually any organ [
5].
Although pneumonitis is a general term used to refer to lung damage of various aetiologies, ICI pneumonitis specifically refers to a non-infectious inflammatory process in the lungs resulting from the administration of an ICI. ICIs can cause pulmonary toxicity because the activation of T cells and proinflammatory cytokines may trigger an exaggerated immune response against lung tissue. The lungs are particularly vulnerable compared to other organs due to their continuous exposure to inhaled antigens, high vascularisation, and delicate alveolar structures, which amplify immune-mediated injury. While immune-related toxicities can occur in many organs, pulmonary toxicity is distinct in that even mild inflammation can critically impair gas exchange and rapidly become life-threatening, making it a unique and clinically significant manifestation.
The incidence of ICI pneumonitis ranges from 5% to 19% [
6], being higher with programmed cell death protein 1 (PD-1) inhibitors than with programmed cell death ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) inhibitors [
7]. Despite its clinical relevance, ICI pneumonitis continues to be a diagnostic and therapeutic challenge in daily practise, especially due to its non-specific clinical presentation and because it is a diagnosis of exclusion. The available evidence on this complication is heterogeneous and, in many cases, limited to retrospective studies, clinical cases, and non-systematic reviews. In this context, it is essential to have tools that provide an up-to-date summary of the knowledge on its clinical management.
This paper presents a narrative review with an integrated approach, focusing specifically on ICI-induced pulmonary toxicity. We believe it adds value to previous publications by compiling and structuring the most relevant clinical, radiological, pathophysiological, and therapeutic aspects, including diagnostic algorithms and management proposals, based on guidelines and recent evidence. It also highlights the need for a multidisciplinary approach and points to future lines of research, such as the identification of predictive biomarkers and the personalisation of treatment. In short, this article aims to be a useful tool for both clinicians and researchers in the context of the growing use of immunotherapy in cancer treatment.
1.1. Types of Immunotherapy
Immunotherapy is a generic term that encompasses various types of treatments, including ICIs [
8]. The most common types of immunotherapy are the following:
Chimeric antigen receptor T cell therapy (CAR-T): Obtaining T cells from the patient for modification and aggregation of artificial receptors that recognise specific tumour antigens, with the consequent activation of these T cells and selective tumour destruction.
Monoclonal antibodies (mAbs): There are different types of mAbs with the ability to act on various antigens with the ultimate goal of destroying tumour cells.
Immune checkpoint inhibitors (ICIs): Responsible for inhibiting immune checkpoints to promote the immune system’s attack on the tumour. They are the most common type of immunotherapy. The most commonly used in lung cancer are the following:
- ○
T cell-associated protein 4 inhibitors (CTLA-4);
- ○
Programmed cell death protein 1 (PD-1) inhibitors;
- ○
Programmed cell death ligand 1 (PD-L1) inhibitors.
As an example of how ICIs work, checkpoint proteins such as PD-L1 (in tumour cells) and PD-1 (in T cells) play an important role in controlling immune responses. The binding of PD-L1 to PD-1 prevents T cells from becoming “activated” and attacking tumour cells. Therefore, with the administration of ICIs (anti-PD-L1 or anti-PD-1), this PD-L1-PD-1 binding is prevented, keeping T cells active so that they attack tumour cells [
8,
9].
Cytokines (CKs): Small proteins that activate the immune system when secreted. The most commonly used are interleukins and interferons.
Cancer vaccines: Vaccines responsible for activating the immune system so that it can destroy cancer cells.
1.2. Signalling Pathways of Lung Damage Caused by Immune Checkpoint Inhibitors
To date, the pathogenesis and specific signalling pathways of lung damage caused by ICIs have been little explored. This complexity is compounded by the fact that in most cases, ICIs are not administered in isolation but in combination with other cancer therapies with potential synergistic and/or toxic effects, making it difficult to identify specific causal mechanisms [
10]. As mentioned above, ICIs activate T cells to destroy cancer cells, but this is not a harmless mechanism, as they also alter immune homeostasis, which can lead to adverse effects such as autoimmunity and non-specific inflammation [
11].
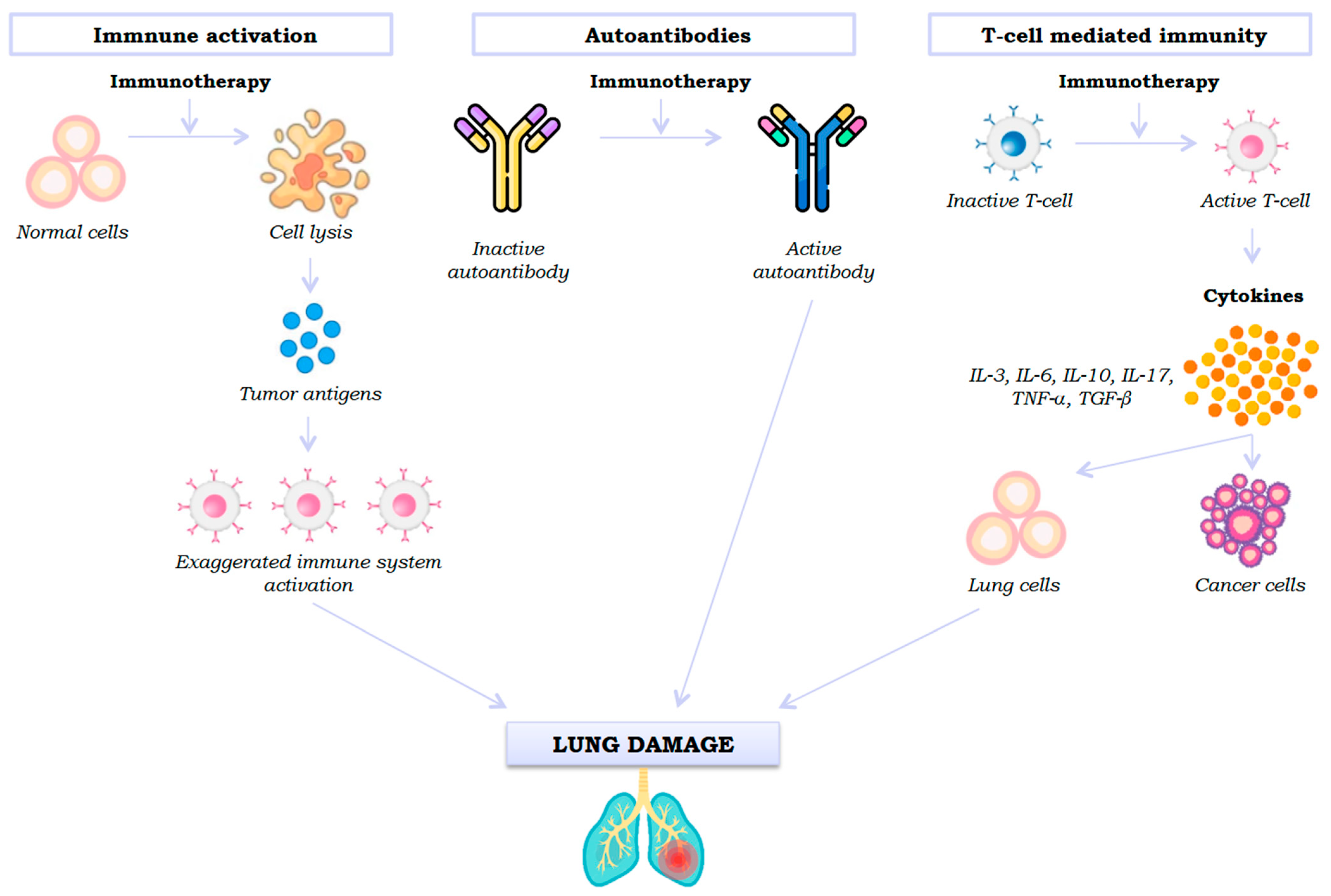
Although evidence is scarce, three mechanisms of lung damage signalling by an ICI have been proposed: generalised immune activation by checkpoint neutralisation, pre-existing autoantibodies, and unwanted effects of T cell-mediated immunity (
Figure 1).
First, ICIs promote a change in the phenotype of inactive T cells to their active effector state, stimulating the immune system, which leads to the release of cytokines (IL-3, IL-6, IL-10, IL-17, TNF-α, and TGF-β) [
12]. However, active T cells not only recognise tumour cells but also shared antigens in healthy lung parenchyma, producing cytotoxic activity. Thus, stimulation of the immune system (Th1 and Th17 lymphocytes) and the production of proinflammatory cytokines will cause inflammation and lung damage [
13].
Secondly, pre-existing autoantibodies also appear to play a role in immunotherapy-induced lung damage, although the mechanism is unknown. Recent studies indicate that patients with autoantibodies who do not have an autoimmune disease prior to administration of an ICI develop immune-mediated adverse effects after taking it [
14].
Thirdly, another mechanism involved in immunotherapy-induced lung toxicity is the release of tumour antigens produced after cell lysis, which leads to an exaggerated activation of the immune system and consequent pneumonitis [
15].
Understanding these pathways is essential for advancing the development of targeted therapeutic strategies, such as the rational use of immunosuppressants, and the identification of biomarkers that would allow for more homogeneous stratification of the risk of pulmonary toxicity.
2. Lung Toxicity from Immune Checkpoint Inhibitors
2.1. Frequency of Toxicities
Retrospective epidemiological studies report an incidence of lung toxicity from ICIs ranging from 3.5% to 19% depending on the series [
6,
16,
17]. Furthermore, the incidence of pneumonitis varies significantly depending on the type of tumour (higher in NSCLC compared to renal cancer or melanoma), patient risk factors (higher in male smokers with previous lung or autoimmune disease), and the specific type of drug administered (higher for anti-PD-1) (
Table 1).
Thus, the classic series describe a higher incidence of pneumonitis after an ICI if the treatment administered is anti-PD1 compared to anti-PD-L1 (3.6% vs. 1.3%, respectively). A recent meta-analysis including 18,715 patients treated with anti-PD-1 or anti-PD-L1 observed an incidence of pneumonitis of 2.8% (95% CI, 2.4–3.2%) [
18]. In contrast, the lowest incidence of pulmonary toxicity after an ICI is observed after administration of anti-CTLA-4. Khoja et al. describe an increased risk of pneumonitis if the drug administered is anti-PD-1 compared to anti-CTLA-4 (OR 6.4; 95% CI: 3.2–12.7) [
19]. In addition, a higher probability of pneumonitis has been reported in patients receiving combination immunotherapy compared to monotherapy (incidence of 10% vs. 3%,
p < 0.001) [
6]. Despite the data described, the incidence of pulmonary toxicity from ICIs in routine clinical practise remains unknown, and it has been postulated that it may be significantly higher than that reported in clinical trials [
20].
2.2. Latency Time
The latency period for the onset of pulmonary toxicity after administration of an ICI varies, with onset most frequently occurring within the first 3 months after initiation. However, it should be noted that there are cases of ICI-induced pneumonitis described up to years after the start of treatment [
21]. In their multicentre study of patients with stage IV NSCLC and melanoma, Delaunay et al. found that pneumonitis after administration of ICIs occurred at a mean of 2.3 months, with earlier onset in cases of NSCLC (2.1 months) compared to cases of melanoma (5.2 months) (
p = 0.02) [
22].
2.3. Grades of Pulmonary Toxicity
Cases of lung toxicity caused by ICIs can vary in severity, and an international grading system should be used to classify them, which will facilitate future comparisons of cases between studies.
Table 2 shows the criteria for the five grades of ICI pneumonitis according to the National Cancer Institute Common Terminology Criteria for Adverse Events [
23].
3. Types of Lung Damage Caused by Immune Checkpoint Inhibitors
In cancer patients, alterations in immune responses induced by ICIs (such as T cell activation, shifts in cytokine profiles, and loss of peripheral tolerance) are closely correlated with both the severity and type of pulmonary toxicity. Enhanced immune reactivity can lead to different histopathological patterns (ICI-related bronchiolitis, sarcoidosis-like granulomatous reaction, and venous thromboembolic disease, among others), each associated with distinct clinical presentations and radiological findings. The intensity of these immune changes often parallels the grade of toxicity, with more pronounced immune dysregulation resulting in higher-grade, potentially life-threatening pulmonary complications.
3.1. Pneumonitis Caused by Immune Checkpoint Inhibitors
ICI pneumonitis is a rare condition (estimated incidence of 4% for anti-PD-1, 2% for anti-PD-L1, and <1% for anti-CTLA-4, reaching up to 10% with combination immunotherapy), but is potentially fatal, resulting from inflammation of the lung parenchyma secondary to the administration of these drugs [
24]. There are patient risk factors that predispose to ICI pulmonary toxicity, such as smoking, older age, pre-existing lung diseases (such as airway diseases but especially fibrotic interstitial lung disease), concomitant treatments (radiotherapy and/or chemotherapy), and specific histological type of NSCLC (epidermoid) [
22]. In addition, these factors have been associated with a poor prognosis for toxic pneumonitis, adding to the severity of the pulmonary condition (greater hypoxemia and/or greater extent of infiltrates), acute onset, comorbidity, previous autoimmune disease, poorer functional status, and greater diagnostic delay [
25]. For this reason, it has been postulated that in the specific subgroup of patients with higher risk factors, a high index of suspicion and active case findings should be maintained when initiating ICI treatment.
The diagnosis of ICI pneumonitis is based on a combination of non-specific respiratory symptoms (dyspnoea, cough, low-grade fever…), a temporal relationship compatible with the onset of treatment, the appearance of new infiltrates on imaging tests (chest CT scan), and the exclusion of other causes (mainly through bronchoscopy with BAL to rule out infection). A diagnostic classification (definite, probable, possible, and indeterminate diagnosis) has been established based on the degree of diagnostic certainty obtained after performing the various complementary tests (
Figure 2). Thus, lung biopsy is reserved for cases where other aetiologies cannot be ruled out. Nevertheless, it should be noted that a definitive diagnosis of ICI pneumonitis is rare even when invasive tests are performed (with lung biopsy by needle aspiration or video-assisted thoracoscopy being the most diagnostically useful) [
25].
From a radiological point of view, chest CT scans can reveal different types of patterns compatible with ICI pneumonitis, such as organised pneumonia (the most common), ground-glass opacities, hypersensitivity pneumonitis, non-specific interstitial pneumonia, eosinophilic pneumonia, or acute alveolar damage. However, there are also other less common imaging patterns (usual interstitial pneumonia, vasculitis, proteinosis, veno-occlusive disease, or constrictive bronchiolitis) [
26]. Similarly, at the histopathological level, several findings compatible with ICI pneumonitis have been described, such as cellular interstitial pneumonitis, chronic interstitial inflammation, granulomatous inflammation, organising pneumonia, and diffuse alveolar damage; there is also a significant frequency of mixed patterns [
19].
3.2. “Sarcoidosis-like” Granulomatous Reaction
The sarcoidosis-like granulomatous reaction is a rare and underdiagnosed type of pulmonary toxicity caused by immunotherapy, as it is often confused with cancer treatment failure or tumour progression due to misinterpretation of imaging findings [
27]. Sarcoidosis-like not only affects the lungs but other commonly affected organs as well, including the skin and lymph nodes. Thus, immunotherapy-induced sarcoid granulomas have been described after administration of ipilimumab, nivolumab, and pembrolizumab, with a variable incidence that is probably underestimated for the reasons explained above [
28].
A diagnosis of sarcoidosis-like is suspected when a chest CT scan is compatible (micronodular pulmonary disease, ground-glass infiltrates with subpleural or perihilar distribution, and/or enlarged hilar–mediastinal lymph nodes), and a suggestive bronchoalveolar lavage (lymphocytosis with increased CD4/CD8 ratio), and is confirmed by compatible histology (non-necrotising granulomas) after ruling out other causes. Thus, at the tissue level, sarcoidosis-like disease is characterised by an immune reaction that induces granulomas with epithelioid cells surrounded by a ring of CD4+ and CD8+ T lymphocytes [
29].
The indications for treatment of sarcoidosis-like disease are not clearly defined, but treatment with corticosteroids is recommended in cases of radiological stage ≥II sarcoidosis, incapacitating symptoms, involvement of vital organs, and radiological and/or functional progression. Overall, the prognosis is good, with regression of symptoms after discontinuation of the ICI, with or without the use of corticosteroids. In addition, sarcoidosis-like disease has been associated with longer response rates to cancer treatment [
22].
3.3. Venous Thromboembolic Disease
Knowledge of cardiovascular complications associated with ICIs is limited, and there are few studies that specifically analyse venous thromboembolic disease (VTD) caused by ICIs, given that this risk may be increased not only by immunotherapy but also by predisposing factors such as cancer itself, immune activation, or combination with other cancer treatments [
30,
31]. Therefore, the actual incidence of VTD following administration of an ICI is unknown. A study of 672 patients treated with an ICI observed 47 episodes of VTE with a cumulative incidence of 12.9%, and another single-centre study of 228 patients with melanoma treated with an ICI identified episodes of VTD in 16.2% of the study subjects [
32,
33]. The management of VTD caused by ICIs does not differ from VTD caused by other factors. In this case, anticoagulation is recommended while the cancer is active, and indefinite anticoagulation should be considered subsequently, depending on the specific characteristics of each patient. In cases of grade 1 to 3 VTD, continuing with ICIs may be considered, while in cases of grade 4 VTD, definitive discontinuation of treatment is recommended [
34].
3.4. Other Types of Lung Damage Caused by Less Common Immune Checkpoint Inhibitors
3.4.1. Bronchiolitis Caused by ICIs
Respiratory bronchiolitis following ICIs is a less common and less well-defined type of pulmonary toxicity, as it has only been reported in case series [
35]. Classically, bronchiolitis is characterised on chest CT by centrilobular nodules, thickening of the bronchial walls, consolidations, ground-glass opacities, and/or a tree-in-bud pattern. For a correct diagnosis, it must be ruled out that these radiological alterations are due to infectious, aspirational, or other inflammatory causes. Thus, suspicion of this entity should be raised in the absence of infectious symptoms and confirmed by imaging tests documenting the resolution of symptoms after maintenance treatment or trial with corticosteroids [
36].
3.4.2. Diaphragmatic Myositis Due to ICIs
There is little evidence regarding other types of pulmonary toxicity caused by ICIs, such as diaphragmatic myositis. There have been reports of case series of myositis following administration of ICIs, both as a monotherapy and in combination. Haddox et al. describe a case of severe necrotising diaphragmatic myositis that resulted in fatal progressive hypoventilation following treatment with anti-PD-1 [
37]. John et al. published another case of fatal progressive hypoventilation due to diaphragmatic myositis in a patient who had received combination therapy (anti-CTLA-4 and anti-PD-1) [
38].
3.4.3. Tuberculosis Caused by ICIs
There is little evidence on the incidence of tuberculosis after starting treatment with an ICI. Some case series have described the reactivation of tuberculosis in patients treated with anti-PD-1 and suggested an immune reactivation syndrome [
39].
3.4.4. Allergic Bronchopulmonary Aspergillosis Caused by ICIs
Allergic bronchopulmonary aspergillosis following ICI administration is poorly understood. Pradere et al. published a case of diagnosed allergic bronchopulmonary aspergillosis treated with immunotherapy due to elevated serum immunoglobulin E (up to 2514 kU/L) and the presence of positive IgE antibodies against Aspergillus fumigatus. This case was treated with antifungals (itraconazole) and corticosteroids, with resolution of the condition [
40].
4. Diagnostic Approach to Lung Toxicity Caused by Immune Checkpoint Inhibitors
For the correct diagnosis of ICI pulmonary toxicity, it is necessary to maintain a high index of suspicion given that the respiratory symptoms are non-specific. Therefore, the correct diagnosis of lung toxicity due to ICIs will follow a series of guiding steps: (1) newly appearing pulmonary infiltrates in imaging tests, normally bilateral and non-segmental in distribution; (2) a compatible temporal relationship between the start of treatment with the ICI and the onset of symptoms; and (3) exclusion of alternative causes.
As mentioned above, given that pneumonitis is more common in the first few months of ICI treatment, extra vigilance is required during this period. If pulmonary toxicity due to the ICI is suspected, imaging tests (initial chest X-ray and, subsequently, chest CT scan) are indicated in order to make a correct differential diagnosis (tumour progression, infection, pulmonary thromboembolism…) and thus guide the rest of the complementary diagnostic tests. Thus, after performing a chest CT scan, bronchoscopy with bronchoalveolar lavage (which, in the case of ICI pneumonitis, would be predominantly lymphocytic) would be indicated if the patient’s clinical condition allows it, primarily to rule out infectious aetiology. Lung biopsies may be indicated in cases where other causes (such as tumour progression) cannot be ruled out despite the aforementioned complementary tests [
22].
The recommendations of the European Society for Medical Oncology (ESMO) establish surgical lung biopsy by video-assisted thoracoscopy as the gold standard in patients who require a histological lung sample to ensure diagnostic certainty [
34]. However, the clinical practise guidelines of the American Society of Clinical Oncology (ASCO) are more conservative and recommend endoscopic transbronchial biopsies in cases requiring an anatomopathological sample [
41]. Therefore, given that there is no clear consensus, the indication for invasive procedures should be discussed in a multidisciplinary meeting (including pulmonologists, oncologists, thoracic surgeons, pathologists, and radiologists).
Figure 2 shows the diagnostic approach algorithm for suspected lung toxicity due to ICIs.
5. Management of Lung Damage Caused by Immune Checkpoint Inhibitors
Respiratory toxicity caused by ICIs can have devastating and potentially irreversible consequences (such as pulmonary fibrosis) if not managed early [
42]. Therefore, early action to discontinue the causative drug will improve the prognosis for patients.
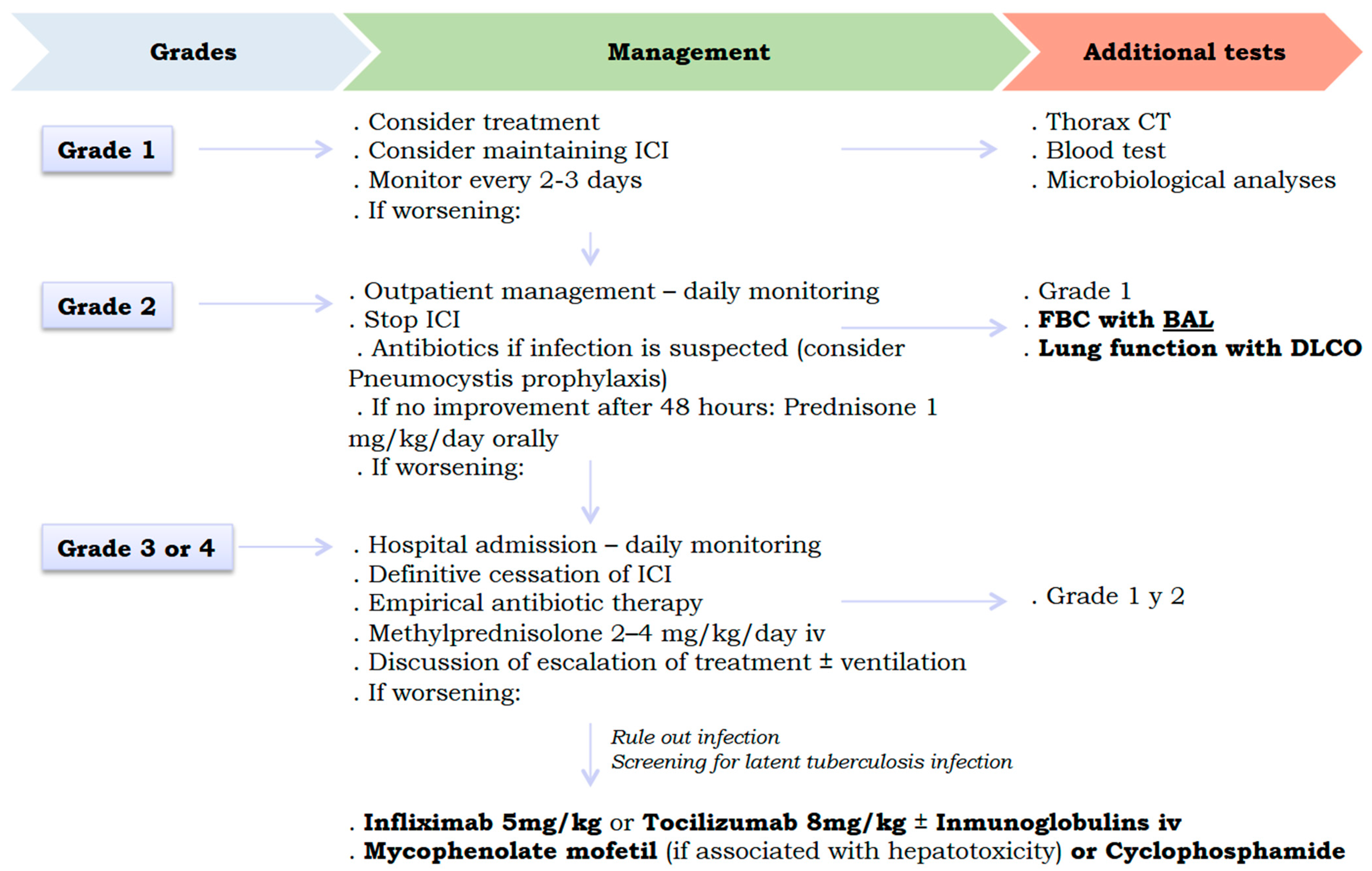
Figure 3 shows the proposed therapeutic algorithm for pulmonary toxicity caused by ICIs. International consensus (National Comprehensive Cancer Network, American Society of Clinical Oncology, Society for Immunotherapy of Cancer, and European Society for Medical Oncology) proposes discontinuing ICIs at grade 2 pulmonary toxicity, with no possibility of reintroducing it (after resolution of the condition) in cases of grade 3 or higher. In addition, it is recommended to start corticosteroid therapy at grade 2 (higher doses of corticosteroids for higher grades), with the possibility of escalating to biological and/or immunosuppressive therapy at grade 3 if there is no response to corticosteroids [
34,
41,
43,
44,
45]. Thus, the drugs available in the latter case would be infliximab (anti-TNF-α), tocilizumab (anti-IL6), intravenous immunoglobulins, mycophenolate mofetil (purine synthesis inhibitor in lymphocytes, especially in cases with associated hepatotoxicity), and cyclophosphamide [
41,
43,
44,
45]. The specific use of these drugs is deduced in order to neutralise the cytokines involved in the ICI lung damage signalling pathways previously explained (IL-6, IL-10, IL-17, TNF-α and TGF-β, …). These treatments should also be combined with the necessary supportive measures for each case, such as supplemental oxygen, non-invasive and invasive mechanical ventilation, and prophylactic antibiotic therapy. It should be noted that these therapeutic recommendations are based on retrospective studies and expert opinions, as no clinical trials have been conducted to demonstrate the efficacy of glucocorticoids and other alternative therapies in cases of lung toxicity due to ICIs [
46].
6. Resolution Time
A favourable prognosis is reported following ICI-induced pulmonary toxicity, especially if early and appropriate management is provided. Overall, the estimated time for resolution of ICI-induced pulmonary damage is between 6 and 10 weeks [
22]. It should be noted that in cases where reintroduction of an ICI is indicated after a case of pneumonitis (grades 1 and 2), there is a possibility of recurrence, estimated at between 17% and 29%, depending on the series, and therefore, it is not indicated to change the specific type of ICI for a different one [
34,
45].
7. Effectiveness Markers
Retrospective studies indicate higher rates of cancer control in patients who experienced immune-related adverse effects [
47]. Pawel et al. observed that patients who received anti-PD-L1 treatment and experienced immune-related adverse effects had higher overall survival compared to cases without toxicity (CRI 0.79; 95% CI: 0.60–1.05) [
48]. Sato et al. observed that patients with advanced NSCLC treated with anti-PD-1 who had immune-mediated adverse effects had a higher objective response rate (63.6% vs. 7.4%,
p < 0.01) and longer progression-free survival (HR 0.10; 95% CI 0.04–1.46;
p = 0.13) compared to those who did not have immune-related adverse effects [
49]. The exact mechanisms explaining how immune-related toxicity can be a subsequent marker of efficacy are unknown. It has been postulated that patients who respond to immunotherapy are those who actually have longer survival and, therefore, a longer treatment duration and consequently more time to develop immune-related adverse events [
50].
On the other hand, it is worth mentioning the importance of researching future biomarkers as potential indicators of efficacy. In this regard, there have been promising advances in the use of serum biomarkers such as KL-6 (Krebs von den Lungen-6), which is a glycoprotein expressed by type II pneumocytes in response to cell damage. There is evidence linking increased circulating levels of KL-6 with greater interstitial lung toxicity [
51]. Another potential biomarker in lung cancer patients treated with ICPI is IL-6. Naqash et al. report that C-reactive protein and IL-6 levels are elevated in ICI pneumonitis following treatment with atezolizumab [
52]. Similarly, recent studies have observed that the presence of certain pre-existing antibodies is associated with the development of immune-mediated adverse effects in NSCLC patients treated with nivolumab or pembrolizumab, although the mechanism of action remains unclear [
53]. Therefore, further studies are needed to define the role of these biomarkers in the diagnosis and monitoring of ICI-induced pulmonary toxicity.
8. Conclusions
Pulmonary toxicity associated with treatment with immune checkpoint inhibitors is a significant immune-mediated complication with a non-negligible incidence, especially during the first months of treatment. Although respiratory symptoms are usually non-specific, radiological findings can guide diagnosis, particularly in patients with known risk factors. This paper provides an overview of the main types of ICI-related pulmonary toxicity, addressing pathophysiological, diagnostic, and therapeutic aspects based on current scientific evidence. In this context, it is essential to maintain a high index of suspicion with at-risk patients (older male smokers with squamous cell lung cancer, respiratory or autoimmune comorbidity, and exposure to anti-PD1, combinations of immunotherapy, or previous thoracic radiotherapy) to facilitate early diagnosis and treatment that could improve prognosis. Fibrobronchoscopy with bronchoalveolar lavage plays a key role in ruling out infections or other alternative causes, and lung biopsy, when indicated, can provide relevant pathological information, such as the presence of lymphocytic alveolitis. Despite advances, gaps in knowledge remain, especially regarding the chronology of the onset of adverse effects, their pathophysiology, and the predictive value of biomarkers, so further studies are needed. In short, this article aims to contribute to structuring the clinical approach to these complications, support decision-making, and stimulate future lines of research in this growing field of treatment.
Author Contributions
A.C., author and drafting, and conception and design. V.L.-F., co-author. L.V.-A., co-author. M.C.-G., co-author. M.M.-A., co-author. M.B.-R., co-author. C.R.-R., co-author. M.T.-D., co-author. A.F.-V., co-author. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
We declare no conflicts of interest associated with this publication. This project did not receive financial support that could have influenced its outcome.
References
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.H.; Schneider, B.J.; Temin, S.; Baker, S., Jr.; Brahmer, J.; Ellis, P.M.; Gaspar, L.E.; Haddad, R.Y.; Hesketh, P.J.; Jain, D.; et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2020, 38, 1608–1632. [Google Scholar] [CrossRef]
- Calles, A.; Aguado, G.; Sandoval, C.; Álvarez, R. The role of immunotherapy in small cell lung cancer. Clin. Transl. Oncol. 2019, 21, 961–976. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in patients treated with anti- programmed death-1/programmed death ligand 1 therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef]
- Khunger, M.; Rakshit, S.; Pasupuleti, V.; Hernandez, A.V.; Mazzone, P.; Stevenson, J.; Pennell, N.A.; Velcheti, V. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: A systematic review and meta-analysis of trials. Chest 2017, 152, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.G.; Yuan, H.; Deng, W.; Li, J.; Huang, Y.; Kim, B.Y.S.; Story, M.D.; Jiang, W. The Reciprocity Between Radiotherapy and Cancer Immunotherapy. Clin. Cancer Res. 2019, 25, 1709–1717. [Google Scholar] [CrossRef]
- Nishino, M.; Sholl, L.M.; Hodi, F.S.; Hatabu, H.; Ramaiya, N.H. Anti-PD-1-Related Pneumonitis During Cancer Immunotherapy. N. Engl. J. Med. 2015, 373, 288–290. [Google Scholar] [CrossRef]
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B.; et al. T-Cell Invigoration to Tumour Burden Ratio Associated with Anti-PD-1 Response. Nature 2017, 545, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Manson, G.; Norwood, J.; Marabelle, A.; Kohrt, H.; Houot, R. Biomarkers Associated with Checkpoint Inhibitors. Ann. Oncol. 2016, 27, 1199–1206. [Google Scholar] [CrossRef]
- Cooling, L.L.; Sherbeck, J.; Mowers, J.C.; Hugan, S.L. Development of Red Blood Cell Autoantibodies Following Treatment with Checkpoint Inhibitors: A New Class of Anti-Neoplastic, Immunotherapeutic Agents Associated with Immune Dysregulation. Immunohematology 2017, 33, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Ramaiya, N.H.; Awad, M.M.; Sholl, L.M.; Maattala, J.A.; Taibi, M.; Hatabu, H.; Ott, P.A.; Armand, P.F.; Hodi, F.S. PD-1 inhibitor-related pneumonitis in advanced cancer patients: Radiographic patterns and clinical course. Clin. Cancer Res. 2016, 22, 6051–6060. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, J.; Lee, J.S.; Kim, Y.J.; Kim, S.H.; Lee, Y.J.; Cho, Y.-J.; Yoon, H.I.; Lee, J.H.; Lee, C.-T.; et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 2018, 125, 150–156. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Chen, T.W.-W.; Siu, L.L.; Hansen, A.R. Tumour and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Suresh, K.; Voong, K.R.; Shankar, B.; Forde, P.M.; Ettinger, D.S.; Marrone, K.A.; Kelly, R.J.; Hann, C.L.; Levy, B.; Feliciano, J.L.; et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: Incidence and risk factors. J. Thorac. Oncol. 2018, 13, 1930–1939. [Google Scholar] [CrossRef]
- Kato, T.; Masuda, N.; Nakanishi, Y.; Takahashi, M.; Hida, T.; Sakai, H.; Atagi, S.; Fujita, S.; Tanaka, H.; Takeda, K.; et al. Nivolumab-induced interstitial lung disease analysis of two phase II studies patients with recurrent or advanced non-small-cell lung cancer. Lung Cancer 2017, 104, 111–118. [Google Scholar] [CrossRef]
- Delaunay, M.; Prévot, G.; Collot, S.; Guilleminault, L.; Didier, A.; Mazières, J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur. Respir. Rev. 2019, 28, 190012. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). National Institutes of Health, National Cancer Institute. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 1 May 2019).
- Nishino, M.; Giobbie-Hurder, A.; Hatabu, H.; Ramaiya, N.H.; Hodi, F.S. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1607–1616. [Google Scholar] [CrossRef]
- Atchley, W.T.; Álvarez, C.; Saxena-Beem, S.; Schwartz, T.A.; Ishizawar, R.C.; Patel, K.P.; Rivera, M.P. Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer Real-World Incidence, Risk Factors, and Management Practices Across Six Health Care Centers in North Carolina. Chest 2021, 160, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Cousin, S.; Toulmonde, M.; Kind, M.; Cazeau, A.-L.; Bechade, D.; Coindre, J.-M.; Italiano, A. Pulmonary sarcoidosis induced by the anti-PD1 monoclonal antibody pembrolizumab. Ann. Oncol. 2016, 27, 1178–1179. [Google Scholar] [CrossRef]
- Faviez, G.; Bousquet, E.; Rabeau, A.; Rouquette, I.; Collot, S.; Goumarre, C.; Meyer, N.; Prevot, G.; Mazieres, J. Sarcoid-like granulomatosis in cancer patients treated with immune checkpoints inhibitors. Rev. Mal. Respir. 2018, 35, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Sholl, L.M.; Awad, M.M.; Hatabu, H.; Armand, P.F.; Hodi, F.S. Sarcoid-like granulomatosis of the lung related to immune-checkpoint inhibitors: Distinct clinical and imaging features of a unique immune-related adverse event. Cancer Immunol. Res. 2018, 6, 630–635. [Google Scholar] [CrossRef]
- Awadalla, M.; Mahmood, S.S.; Groarke, J.D.; Hassan, M.Z.O.; Nohria, A.; Rokicki, A.; Murphy, S.P.; Mercaldo, N.D.; Zhang, L.; Zlotoff, D.A.; et al. Global Longitudinal Strain and Cardiac Events in Patients with Immune Checkpoint Inhibitor-Related Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 467–478. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T.; Büller, H.R. Bidirectional relation between inflammation and coagulation. Circulation 2004, 109, 2698–2704. [Google Scholar] [CrossRef]
- Sussman, T.A.; Li, H.; Hobbs, B.; Funchain, P.; McCrae, K.R.; Khorana, A.A. Incidence of thromboembolism in patients with melanoma on immune checkpoint inhibitor therapy and its adverse association with survival. J. Immunother. Cancer. 2021, 9, e001719. [Google Scholar] [CrossRef]
- Moik, F.; Chan, W.-E.; Wiedemann, S.; Hoeller, C.; Tuchmann, F.; Aretin, M.-B.; Fuereder, T.; Zöchbauer-Müller, S.; Preusser, M.; Pabinger, I.; et al. Incidence, risk factors and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood 2021, 137, 1669–1678. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Balagani, A.; Arain, M.H.; Sheshadri, A. Bronchiolitis obliterans after combination immunotherapy with pembrolizumab and ipilimumab. J. Immunother. Precis. Oncol. 2018, 1, 49–52. [Google Scholar] [CrossRef]
- Blanchard, A.; Bouchard, N.; Larivée, P. Pembrolizumab Induced Bronchiolitis in a Patient with Stage IV Non-Small Cell Lung Cancer. Case Rep. Thorac. Oncol. 2018, 197, A4008. [Google Scholar] [CrossRef]
- Haddox, C.L.; Shenoy, N.; Shah, K.K.; Kao, J.C.; Jain, S.; Halfdanarson, T.R.; Wijdicks, E.F.; Goetz, M.P. Pembrolizumab-induced bulbar myopathy and respiratory failure with
necrotizing myositis of the diaphragm. Ann. Oncol. 2017, 28, 673–675. [Google Scholar] [CrossRef]
- John, S.; Antonia, S.J.; Rose, T.A.; Seifert, R.P.; Centeno, B.A.; Wagner, A.S.; Creelan, B.C. Progressive hypoventilation due to mixed CD8 (+) and CD4 (+) lymphocytic
polymyositis following tremelimumab–durvalumab treatment. J. Immunother. Cancer 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Picchi, H.; Mateus, C.; Chouaid, C.; Besse, B.; Marabelle, A.; Michot, J.M.; Champiat, S.; Voisin, A.L.; Lambotte, O. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: Reactivation of tuberculosis after anti PD-1 treatment. Clin. Microbiol. Infect. 2018, 24, 216–218. [Google Scholar] [CrossRef]
- Pradere, P.; Michot, J.M.; Champiat, S.; Danlos, F.X.; Marabelle, A.; Lambotte, O.; Albiges, L.; Le Pavec, J. Allergic broncho-pulmonary aspergillosis following treatment with an anti-programmed cell death protein 1 monoclonal antibody therapy. Eur. J. Cancer 2017, 75, 308–309. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Kubo, K.; Azuma, A.; Kanazawa, M.; Kameda, H.; Kusumoto, M.; Genma, A.; Saijo, Y.; Sakai, F.; Sugiyama, Y.; Tatsumi, K.; et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir. Investig. 2013, 51, 260–277. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham III, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Trinh, S.; Le, A.; Gowani, S.; La-Beck, N.M. Management of immune- related adverse events associated with immune checkpoint inhibitor therapy: A minireview of current clinical guidelines. Asia Pac. J. Oncol. Nurs. 2019, 6, 154–160. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Hou, X.; Zhang, Y.; Zhou, T.; Liu, J.; Lin, Z.; Fang, W.; Yang, Y.; Zhou, C.; et al. Immune-related pneumonitis associated with immune checkpoint inhibitors in lung cancer: A network meta-analysis. J. Immunother. Cancer 2020, 8, e001170. [Google Scholar] [CrossRef]
- Sears, C.R.; Peikert, T.; Possick, J.D.; Naidoo, J.; Nishino, M.; Patel, S.P.; Camus, P.; Gaga, M.; Garon, E.B.; Gould, M.K.; et al. Knowledge gaps and research priorities in immune checkpoint inhibitor-related pneumonitis. An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e31–e43. [Google Scholar] [CrossRef]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
- von Pawel, J.; Syrigos, K.; Mazieres, J.; Cortinovis, D.; Dziadziuszko, R.; Gandara, D.R.; Conkling, P.; Goldschmidt, J.; Thomas, C.A.; Bordoni, R.; et al. Association between immune-related adverse events (irAEs) and atezolizumab efficacy in advanced NSCLC: Analyses from the phase III study OAK. Ann. Oncol. 2017, 28 (Suppl. 5), mdx380.017. [Google Scholar] [CrossRef]
- Sato, K.; Akamatsu, H.; Murakami, E.; Sasaki, S.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; Koh, Y.; Ueda, H.; et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 2018, 115, 71–74. [Google Scholar] [CrossRef]
- June, C.H.; Warshauer, J.T.; Bluestone, J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017, 23, 540–547. [Google Scholar] [CrossRef]
- Ohnishi, H.; Yokoyama, A.; Yasuhara, Y.; Watanabe, A.; Naka, T.; Hamada, H.; Abe, M.; Nishimura, K.; Higaki, J.; Ikezoe, J.; et al. Circulating KL-6 levels in patients with drug induced pneumonitis. Thorax 2003, 58, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Naqash, A.R.; Yang, L.V.; Sanderlin, E.J.; Atwell, D.C.; Walker, P.R. Interleukin-6 as One of the Potential Mediators of Immune-Related Adverse Events in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Blockade: Evidence From a Case Report. Acta Oncol. 2018, 57, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Toi, Y.; Sugawara, S.; Sugisaka, J.; Ono, H.; Kawashima, Y.; Aiba, T.; Kawana, S.; Saito, R.; Aso, M.; Tsurumi, K.; et al. Profiling Preexisting Antibodies in Patients Treated with Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 376–383. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).