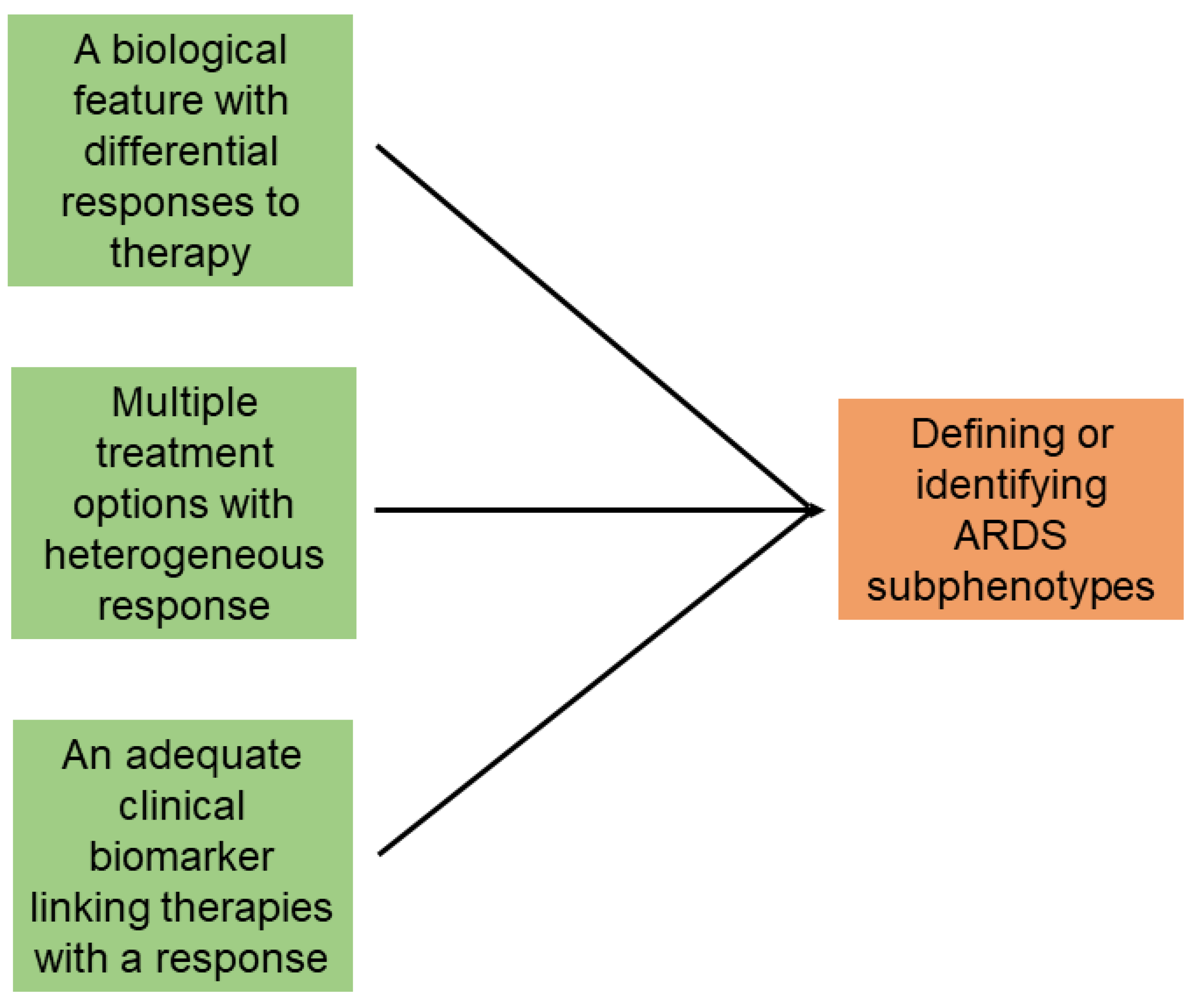
ARDS Subphenotypes as a Guide to Therapy and Enrollment into Therapeutic Trials: Not So Fast
Abstract
1. Background
2. General Terms
3. Heterogeneity of Disease Processes
4. Treatment Effects of Feature Targets in Mechanically Ventilated ARDS Patients
5. Subphenotyping by Clinical Parameters and Its Problems
6. Biomarkers and Inflammatory Subphenotypes
- Blood samples for biomarker analysis were collected within 36–72 h of meeting ARDS diagnostic criteria.
- Patients with mild ARDS were included, but outcomes stratified by ARDS severity were not reported.
- External validation in independent cohorts was lacking.
- Identifying these subphenotypes at the bedside in real time is challenging.
- As ARDS shares molecular pathways with sepsis and severe pneumonia, and more than 70% of the patients analyzed had sepsis or pneumonia, the hyper/hypo-inflammatory classification may simply reflect the host response to infection rather than to specific ARDS.
- Although no specific ventilator management scheme was promulgated, in the simvastatin trial [41], adherence to lung-protective ventilation ranged from only 20 to 39% across study time-points [42] despite protocol recommendations, raising concerns that ventilator-induced lung injury [43] may have contributed to the hyper-inflammatory subphenotype [38].
- It remains unclear why only two subphenotypes were selected, rather than three or more, as is common in other syndromes [32]. The limited number of patients in some groups and imbalances in clinical features may have influenced this two-class model, creating “pseudo-cohorts”. Due to the potential selection bias for enrollment into the trials, it seems that the number of final subphenotypes was the result of an insufficient number of patients in some of the subpopulations, as well as being related to an imbalance of clinical features in the two “pseudo-cohorts”. In clinical practice, many disease processes are more naturally classified into “hyper–normo–hypo”, “severe–moderate–mild”, or “high–normal–low” categories [31,33].
- The timing of data collection was not standardized, which may significantly impact patient classification [24].
- It is not specified whether the analysis used data collected at ARDS onset, at randomization, or after randomization. Furthermore, it is unclear how many patients failed to meet ARDS criteria after randomization.
- Blood for biomarker measurements were collected within 36–72 h of meeting ARDS criteria.
- Patients with mild ARDS were included, but outcomes relating to lung severity were not reported.
- Those subphenotypes were never validated in external cohorts.
- There is a real challenge in identifying these subphenotypes at the bedside in real time.
- ARDS shares the same molecular pathways as sepsis or severe pneumonia. More than 70% of patients in the trials used for developing the inflammatory subphenotypes had sepsis or pneumonia.
- During the screening period of the trials, there were more ARDS patients excluded than included. The hyper-inflammatory class could be a reflection of host response to infections causing ARDS.
- In some trials, compliance with lung-protective ventilation was reported in less than 40% of patients across all time-points. It is plausible that inflammatory responses to ventilator-induced lung injury were part of the hyper-inflammatory subphenotype.
- Due to potential selection bias for enrollment into the trials, it seems than the number of final subphenotypes was the result of an insufficient number of patients in some of the subpopulations.
- The specific time for modeling was not standardized
- It is unclear if the data for analysis are at ARDS onset, at randomization, or after randomization. Also, it is unknown how many patients did not meet ARDS criteria after randomization.
- It is unclear whether those subphenotypes are treatable traits.
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slutsky, A.S.; Villar, J.; Pesenti, A. Happy 50th birthday ARDS! Intensive Care Med. 2016, 42, 637–639. [Google Scholar] [CrossRef][Green Version]
- Villar, J.; Szakmany, T.; Graselli, G.; Camporota, L. Redefining ARDS: A paradigm shift. Crit. Care 2023, 27, 416. [Google Scholar] [CrossRef]
- Villar, J.; Blanco, J.; del Campo, R.; Andaluz-Ojeda, D.; Díaz-Domínguez, F.J.; Muriel, A.; Córcoles, V.; Suárez-Sipmann, F.; Tarancon, C.; González-Higueras, E.; et al. Assessment of PaO2/FiO2 for stratification of patients with moderate and severe acute respiratory distress syndrome. BMJ Open 2015, 5, e006812. [Google Scholar] [CrossRef]
- Ashbaugh, D.G.; Bigelow, D.B.; Petty, T.L.; Levine, B.E. Acute respiratory distress syndrome in adults. Lancet 1967, 290, 319–323. [Google Scholar] [CrossRef]
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM guidelines on acute respiratory distress syndrome: Definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023, 49, 727–759. [Google Scholar] [CrossRef] [PubMed]
- Maslove, D.M.; Tang, B.; Shankar-Hari, M.; Lawler, P.R.; Angus, D.C.; Baillie, J.K.; Baron, R.M.; Bauer, M.; Buchmann, T.G.; Calfee, C.S.; et al. Redefinining critical illness. Nat. Med. 2022, 28, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.R.; Reddy, K.; Munshi, L.; Bos, L.D.J. Towards precision medicine in respiratory failure. Crit. Care Med. 2025, 53, e656–e664. [Google Scholar] [CrossRef]
- Villar, J.; Mora-Ordoñez, J.M.; Soler, J.A.; Mosteiro, F.; Vidal, A.; Ambrós, A.; Fernández, L.; Murcia, I.; Civantos, B.; Romera, M.A.; et al. The PANDORA study: Prevalence and outcome of acute hypoxemic respiratory failure in the pre-COVID-19 era. Crit. Care Explor. 2022, 4, e0684. [Google Scholar] [CrossRef] [PubMed]
- Niven, A.S.; Herasevich, S.; Pickering, B.W.; Gajic, O. The future of critical care lies in quality improvement and education. Ann. Am. Thorac. Soc. 2019, 16, 649–656. [Google Scholar] [CrossRef]
- Churpek, M.M.; Gupta, S.; Spicer, A.B.; Parker, W.F.; Fahrenbach, J.; Brenner, S.K.; Leaf, D.E. Hospital-level variation in death for critically ill patients with COVID-19. Am. J. Respir. Crit. Care Med. 2021, 204, 403–411. [Google Scholar] [CrossRef]
- Stefanicka-Wojtas, D.; Kwurpas, D. Personalised medicine—Implementation to the healthcare system in Europe (Focus Group Discussions). J. Pers. Med. 2023, 13, 380. [Google Scholar] [CrossRef]
- Wildi, K.; Livingstone, S.; Palmieri, C.; LiBassi, G.; Suen, J.; Fraser, J. The discovery of biological subphenotypes in ARDS: A novel approach to targeted medicine? J. Intensive Care 2021, 9, 14. [Google Scholar] [CrossRef]
- Villar, J. Genetics and the pathogenesis of adult respiratory distress syndrome. Curr. Opin. Crit. Care 2002, 8, 1–5. [Google Scholar] [CrossRef]
- Thusheim, M.R.; Berndt, E.R.; Douglas, F.L. Stratified medicine: Strategic and economic implications of combining drugs and clinical biomarkers. Nat. Rev. 2007, 6, 287–293. [Google Scholar]
- Vranas, K.C.; Jopling, J.K.; Sweeney, T.E.; Ramsey, M.C.; Milstein, A.S.; Slatore, C.G.; Escobar, G.J.; Liu, V.X. Identifying distinct subgroups of ICU patients: A machine learning approach. Crit. Care Med. 2017, 45, 1607–1615. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin definition. JAMA J. Am. Med. Assoc. 2012, 307, 2526–2533. [Google Scholar]
- Philips, C.R. The Berlin definition: Real change or the emperor’s new clothes? Crit. Care 2013, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Pérez-Méndez, L.; Aguirre-Jaime, A.; Kacmarek, R.M. Why are physicians so skeptical about positive randomized controlled trials in critical care medicine? Intensive Care Med. 2005, 31, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Hall, J.B.; Slutsky, A.S. Ten big mistakes in intensive care medicine. Intensive Care Med. 2015, 41, 505–507. [Google Scholar] [CrossRef]
- Buell, K.G.; Spicer, A.B.; Casey, J.D.; Seitz, K.P.; Qian, E.T.; Linck, E.J.G.; Self, W.H.; Rice, T.W.; Sinha, P.; Young, P.J.; et al. Individualized treatment effects of oxygen targets in mechanically ventilated critically ill adults. JAMA 2024, 33, 1195–1204. [Google Scholar] [CrossRef]
- Villar, J.; Ferrando, C.; Tusman, G.; Berra, L.; Rodríguez-Suárez, P.; Suárez-Sipmann, F. Unsuccessful and successful clinical trials in acute respiratory distress syndrome: Addressing physiology-based gaps. Front. Physiol. 2021, 12, 774025. [Google Scholar] [CrossRef] [PubMed]
- Sacks, C.A. The evidence is in. NEJM Evid. 2024, 3, EVIDe2400018. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.A.; Fan, E.; Ferguson, N.D. Precision medicine and heterogeneity of treatment effect in therapies for ARDS. Chest 2021, 160, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Fernández, C.; González-Martin, J.M.; Ferrando, C.; Añón, J.M.; del Saz-Ortiz, A.M.; Díaz-Lamas, A.; Bueno-González, A.; Fernández, L.; Domínguez-Berrot, A.M.; et al. Respiratory subsets in patients with moderate to severe acute respiratory distress syndrome for early prediction of death. J. Clin. Med. 2022, 11, 5724. [Google Scholar] [CrossRef]
- Guerin, C.; Reignier, J.; Richard, M.; Beuret, P.; Gacouin, A.; Boulain, T.; Clavel, M.; Chatellier, D.; Jaber, S.; Rosselli, S.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Relly, J.P.; Meyer, N. Pattern recognition in ARDS: A crucial first step toward personalized threatment. Lancet Respir. Med. 2014, 2, 594–595. [Google Scholar] [CrossRef]
- Urner, M.; Barnett, A.G.; Bassi, G.L.; Brodie, D.; Dalton, H.J.; Ferguson, N.D.; Heinsar, S.; Hodgson, C.L.; Peek, G.; Shekar, K.; et al. COVID-19 Critical Care Consortium Investigators. Venovenous extracorporeal membrane oxygenation in patients with acute COVID-19 associated respiratory failure: Comparative effectiveness study. BMJ 2022, 377, e068723. [Google Scholar] [CrossRef]
- Siuba, M.T.; Bulgarelli, L.; Duggal, A.; Cavalcanti, A.B.; Zampieri, F.G.; Rey, D.A.; Lucena, W.; Maia, I.S.; Paisani, D.M.; Laranjeira, L.N.; et al. Differential effect of positive end-expiratory pressure strategies in patients with ARDS. Chest 2024, 166, 754–764. [Google Scholar] [CrossRef]
- Kacmarek, R.M.; Berra, L. Prediction of ARDS outcome: What tool should I use? Lancet Respir. Med. 2018, 6, 253–254. [Google Scholar] [CrossRef]
- Petty, T.L. The adult respiratory distress syndrome. Confessions of a “lumper”. Am. Rev. Respir. Dis. 1975, 111, 713–715. [Google Scholar]
- Villar, J.; Fernández, R.L.; Ambrós, A.; Parra, L.; Blanco, J.; Dominguez-Berrot, A.M.; Gutierrez, J.M.; Blanch, L.; Añón, J.M.; Martín, C.; et al. A clinical classification of the acute respiratory distress síndrome for predicting outcome and guiding therapy. Crit. Care Med. 2015, 43, 346–353. [Google Scholar] [CrossRef]
- Forrester, J.S.; Diamond, G.A.; Swan, H.J.C. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am. J. Cardiol. 1977, 39, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.; Cremer, O.L.; Ong, D.S.Y.; Caser, E.B.; Barbas, C.S.V.; Villar, J.; Kacmarek, R.M.; Schultz, M.J.; MARS Consortium. External validation confirms the legitimacy of a new classification of ARDS for predicting outcome. Intensive Care Med. 2015, 41, 2004–2005. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.P. What is acute lung injury? What is ARDS? Chest 1995, 107, 1721–1726. [Google Scholar] [CrossRef]
- Abbot, M.; Li, Y.; Brochard, L.; Zhang, H. Precision medicine using simultaneous monitoring and assessment with imaging and biomarkers to manage mechanical ventilation in ARDS. Intensive Care Res. 2023, 3, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A.; NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomized controlled trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef]
- Famous, K.R.; Delucchi, K.; Ware, L.B.; Kangelaris, K.N.; Liu, K.D.; Thompson, B.T.; Calfee, C.S. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am. J. Respir. Crit. Care Med. 2017, 195, 331–338, Erratum in Am. J. Respir. Crit. Care Med. 2018, 198, 1590. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; MCDowell, C.; Laffey, J.G.; O’Kane, C.M.; McAuley, D.F.; et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomized controlled trial. Lancet Respir. Med. 2018, 6, 691–698. [Google Scholar] [CrossRef]
- Hashem, M.; Hopkins, T.O.; Colantuoni, E.; Dinglas, V.D.; Sinha, P.; Friedman, L.A.; Morris, P.E.; Jackson, J.C.; Hough, C.L.; Calfee, C.S.; et al. Six month and 12-month patient outcomes based on inflammatory subphenotypes in sepsis-associated ARDS: Secondary analysis of SAILS-ALTOS trial. Thorax 2022, 77, 22–30. [Google Scholar] [CrossRef]
- McKelvey, M.C.; Bradbury, I.; McDowell, C.; Calfee, C.S.; Welden, S.; O’Kane, C.M.; McAuley, D.F.; Taggart, C.C. The relationship between plasma cystatin C, mortality and acute respiratory distress syndrome subphenotype in the HARP-s trial. Crit. Care Resusc. 2023, 24, 251–258. [Google Scholar] [CrossRef]
- McAuley, D.F.; Laffey, J.G.; O’ Kane, C.M.; Perkins, G.D.; Mullon, B.; Trinder, T.J.; Johnston, P.; Hopkins, P.A.; Johnston, A.J.; McDowell, C.; et al. Simvastatin in the acute respiratory distress syndrome. N. Engl. J. Med. 2014, 371, 1695–1703. [Google Scholar] [CrossRef]
- Poole, J.; McDowell, C.; Lall, R.; Perkins, G.; McAuley, D.; Gao, F.; Young, D. Individual patient data analysis of tidal volumes used in three large randomized control trials involving patients with acute respiratory distress syndrome. Br. J. Anesth. 2017, 118, 570–575. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Nasa, P.; Bos, L.D.; Estenssoro, E.; van Haren, F.M.P.; Neto, A.S.; Rocco, P.R.M.; Slutsky, A.S.; Schultz, M.J.; DIOS Investigators. Defining and subphenotyping ARDS: Insights from an international Delphi expert panel. Lancet Respir. Med. 2025, 13, 638–650. [Google Scholar] [CrossRef]
- Smit, J.M.; Jonkman, A.H.; Krijthe, J. Sub-phenotyping in critical care: A valuable strategy or methodologically fragile path? Intensive Care Med. Exp. 2025, 13, 59. [Google Scholar] [CrossRef]
- Santacruz, C.A.; Pereira, A.J.; Celis, E.; Vincent, J.L. Which multicenter randomized controlled trials in critical care medicine have shown reduced mortality? A systematic review. Crit. Care Med. 2019, 47, 1680–1691. [Google Scholar] [CrossRef]
- Bos, L.D.; Schuten, L.R.; van Vught, L.A.; Wiewel, M.A.; Ong, D.S.Y.; Cremer, O.; Artigas, A.; Martin-Loeches, I.; Hoogendijk, A.J.; van del Poll, T.; et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax 2017, 72, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Scherger, S.J.; Kalil, A.C. Sepsis phenotypes, subphenotypes, and endotypes: Are they ready for bedside care? Curr. Opin. Crit. Care 2024, 30, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.J.; Reddy, K.; Conlon, J.; Auzinger, G.; Bannard-Smith, J.; Barrett, N.A.; Camporota, L.; Gillies, M.A.; Jackson, C.; McDowell, C.; et al. Evaluation of plasma biomarkers to understand the biology and heterogeneity of treatment effect in lower tidal volume ventilation facilitated by extracorporeal CO2 removal in acute hypoxemic respiratory failure: A secondary analysis of the REST Trial. Crit. Care Explorat 2025, 7, e1246. [Google Scholar] [CrossRef]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomized controlled trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]
- Doran, G.T. There’s a SMART way to write management’s goals and objectives. Manag. Rev. 1981, 70, 35–36. [Google Scholar]
- SMART Criteria. Available online: https://en.wikipedia.org/wiki/SMART_criteria (accessed on 4 June 2024).
- Taylor, S.P.; Kowalkowski, M. Failure to rescue as a quality measure in sepsis. JAMA 2024, 332, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, Y.E.; Chalmers, S.J.; Barreto, E.F.; Jentzer, J.C.; Gajic, O.; Yadav, H. Early, biomarker-guided steioid dosing in COVID-19 pneumonia: A pilot randomized controlled trial. Crit. Care 2022, 26, 9. [Google Scholar] [CrossRef]
- Wieland, E.; Shipkova, M. Pharmacokinetic and pharmacodynamics drug monitoring of direct-acting oral anticoagulants: Where do we stand? Ther. Drug Monit. 2019, 41, 180–191. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villar, J.; Szakmany, T.; Gajic, O.; Casali, D.; Cazorla-Rivero, S.; Niven, A.S. ARDS Subphenotypes as a Guide to Therapy and Enrollment into Therapeutic Trials: Not So Fast. J. Clin. Med. 2025, 14, 6088. https://doi.org/10.3390/jcm14176088
Villar J, Szakmany T, Gajic O, Casali D, Cazorla-Rivero S, Niven AS. ARDS Subphenotypes as a Guide to Therapy and Enrollment into Therapeutic Trials: Not So Fast. Journal of Clinical Medicine. 2025; 14(17):6088. https://doi.org/10.3390/jcm14176088
Chicago/Turabian StyleVillar, Jesús, Tamas Szakmany, Ognjen Gajic, Diego Casali, Sara Cazorla-Rivero, and Alexander S. Niven. 2025. "ARDS Subphenotypes as a Guide to Therapy and Enrollment into Therapeutic Trials: Not So Fast" Journal of Clinical Medicine 14, no. 17: 6088. https://doi.org/10.3390/jcm14176088
APA StyleVillar, J., Szakmany, T., Gajic, O., Casali, D., Cazorla-Rivero, S., & Niven, A. S. (2025). ARDS Subphenotypes as a Guide to Therapy and Enrollment into Therapeutic Trials: Not So Fast. Journal of Clinical Medicine, 14(17), 6088. https://doi.org/10.3390/jcm14176088






