Glycocalyx-Shedding and Inflammatory Reactions Occur Yet Do Not Predict Complications Resulting from an Esophagectomy in an Accelerated Recovery After Surgery Program
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Study Registration
2.2. Patient Selection
2.3. ARAS Protocol
2.4. Hemodynamic Protocol
2.5. Measurement of Glycocalyx Shedding
2.6. Measurement of Inflammation Markers
2.7. Measurement of the Veno-Arterial CO2-Difference in Blood
2.8. Grading of Perioperative Complications
2.9. Statistical Analysis
2.10. Use of Generative Artificial Intelligence
3. Results
3.1. Patients’ Characteristics
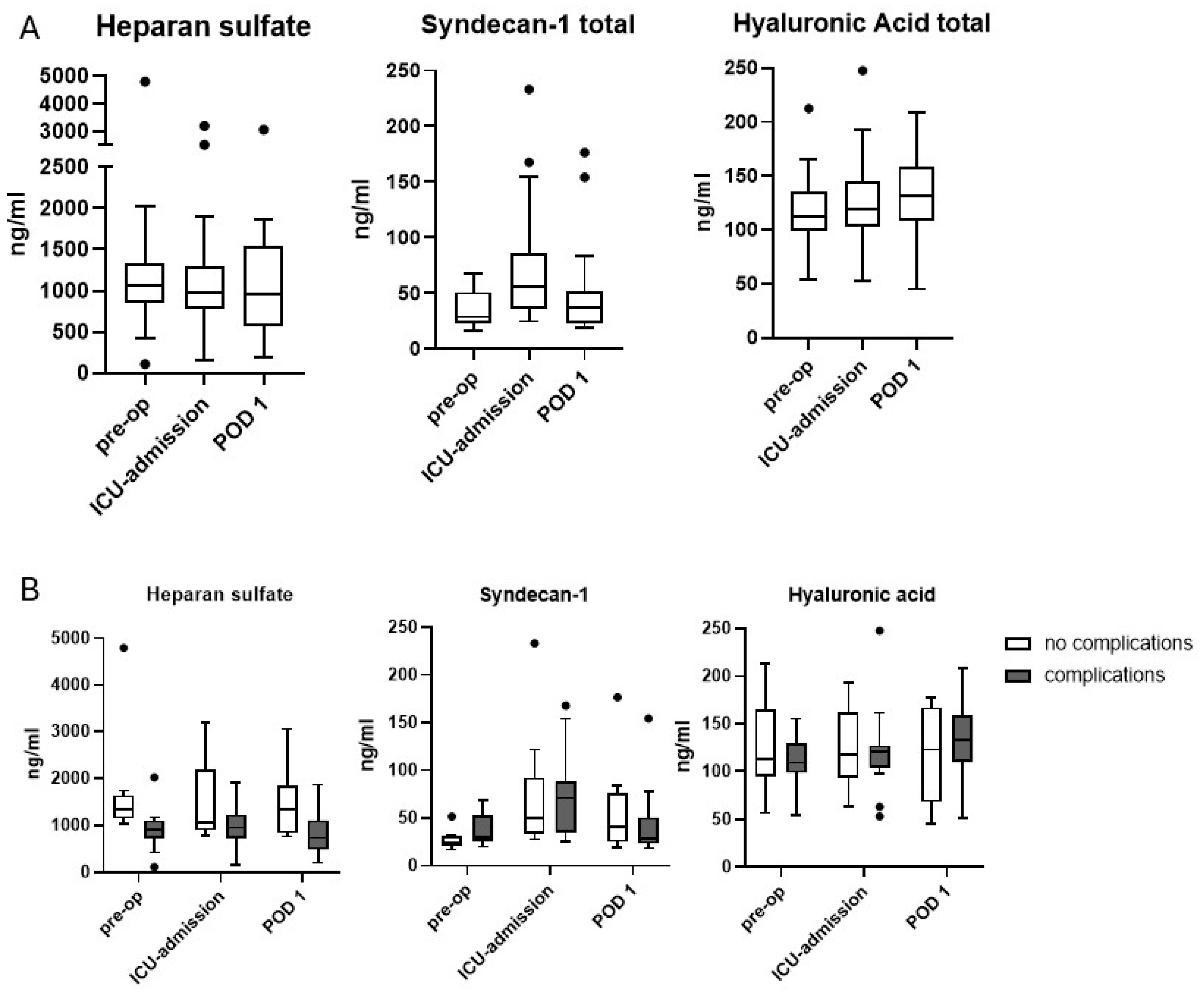
3.2. Glycocalyx Shedding
3.3. Interleukins and Tumor Necrosis Factor
3.4. CO2-Gap at Admission to the Intensive Care Unit
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARAS | Accelerated Recovery after Surgery |
| ASA | American Society of Anesthesiologists |
| CCI® | Comprehensive Complications Index |
| CO2 | Carbon Dioxide |
| ELISA | Enzyme-linked immunosorbent assay |
| ERAS® | Enhanced Recovery after Surgery |
| FEV1 | Forced expiratory volume in one second |
| ICU | Intensive care unit |
| IL | Interleukin |
| IQR | Interquartile range |
| TNF | Tumor necrosis factor |
References
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; de Laval, I.; Geisler, T.; Hinterbuchner, L.; et al. ESCScientific Document Group 2022 ESC Guidelines on cardiovascular assessment management of patients undergoing non-cardiac surgery. Eur. Heart J. 2022, 43, 3826–3924. [Google Scholar] [CrossRef]
- Kutup, A.; Nentwich, M.F.; Bollschweiler, E.; Bogoevski, D.; Izbicki, J.R.; Hölscher, A.H. What should be the gold standard for the surgical component in the treatment of locally advanced esophageal cancer: Transthoracic versus transhiatal esophagectomy. Ann. Surg. 2014, 260, 1016–1022. [Google Scholar] [CrossRef]
- Arlow, R.L.; Moore, D.F.; Chen, C.; Langenfeld, J.; August, D.A. Outcome-volume relationships and transhiatal esophagectomy: Minimizing “failure to rescue”. Ann. Surg. Innov. Res. 2014, 8, 9. [Google Scholar] [CrossRef][Green Version]
- Kim, B.R.; Jang, E.J.; Jo, J.; Lee, H.; Jang, D.Y.; Ryu, H.G. The association between hospital case-volume and postoperative outcomes after esophageal cancer surgery: A population-based retrospective cohort study. Thorac. Cancer 2021, 12, 2487–2493. [Google Scholar] [CrossRef]
- Hallet, J.; Sutradhar, R.; Jerath, A.; d’Empaire, P.P.; Carrier, F.M.; Turgeon, A.F.; McIsaac, D.I.; Idestrup, C.; Lorello, G.; Flexman, A.; et al. Association Between Familiarity of the Surgeon-Anesthesiologist Dyad and Postoperative Patient Outcomes for Complex Gastrointestinal Cancer Surgery. JAMA Surg. 2023, 158, 465–473. [Google Scholar] [CrossRef]
- Liou, D.Z.; Serna-Gallegos, D.; Mirocha, J.; Bairamian, V.; Alban, R.F.; Soukiasian, H.J. Predictors of Failure to Rescue After Esophagectomy. Ann. Thorac. Surg. 2018, 105, 871–878. [Google Scholar] [CrossRef]
- Fuchs, H.F.; Harnsberger, C.R.; Broderick, R.C.; Chang, D.C.; Sandler, B.J.; Jacobsen, G.R.; Bouvet, M.; Horgan, S. Mortality after esophagectomy is heavily impacted by center volume: Retrospective analysis of the Nationwide Inpatient Sample. Surg. Endosc. 2017, 31, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Low, D.E.; Allum, W.; De Manzoni, G.; Ferri, L.; Immanuel, A.; Kuppusamy, M.; Law, S.; Lindblad, M.; Maynard, N.; Neal, J.; et al. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. 2019, 43, 299–330. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.R., 3rd; Abou Chaar, M.K.; Kerfeld, M.H.; Cassivi, S.D.; Hofer, R.E.; Nichols, F.C.; Reisenauer, J.; Saddoughi, S.S.; Shen, K.R.; Stewart, T.M.; et al. Esophagectomy Enhanced Recovery After Surgery Initiative Results in Improved Outcomes. Ann. Thorac. Surg. 2024, 117, 847–857. [Google Scholar] [CrossRef]
- Becker, B.F.; Chappell, D.; Bruegger, D.; Annecke, T.; Jacob, M. Therapeutic strategies targeting the endothelial glycocalyx: Acute deficits, but great potential. Cardiovasc. Res. 2010, 87, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chappell, D.; Annecke, T.; Conzen, P.; Jacob, M.; Welsch, U.; Zwissler, B.; Becker, B.F. Sevoflurane mitigates shedding of hyaluronan from the coronary endothelium, also during ischemia/reperfusion, an ex vivo animal study. Hypoxia 2016, 4, 81–90. [Google Scholar] [CrossRef][Green Version]
- Bogner-Flatz, V.; Braunstein, M.; Ocker, L.E.; Kusmenkov, T.; Tschoep, J.; Ney, L.; Böcker, W.; Annecke, T. On-the-Scene Hyaluronan and Syndecan-1 Serum Concentrations and Outcome after Cardiac Arrest and Resuscitation. Mediat. Inflamm. 2019, 2019, 8071619. [Google Scholar] [CrossRef]
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef]
- Drinhaus, H.; Schroeder, D.C.; Hunzelmann, N.; Herff, H.; Annecke, T.; Böttiger, B.W.; Wetsch, W.A. Shedding of the Endothelial Glycocalyx Independent of Systemic Tryptase Release during Oncologic Oral Surgery: An Observational Study. J. Clin. Med. 2022, 30, 5797. [Google Scholar] [CrossRef]
- Chappell, D.; Bruegger, D.; Potzel, J.; Jacob, M.; Brettner, F.; Vogeser, M.; Conzen, P.; Becker, B.F.; Rehm, M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit. Care 2014, 18, 538. [Google Scholar] [CrossRef]
- Holzmann, M.S.; Winkler, M.S.; Strunden, M.S.; Izbicki, J.R.; Schoen, G.; Greiwe, G.; O Pinnschmidt, H.; Poppe, A.; Saugel, B.; Daum, G.; et al. Syndecan-1 as a biomarker for sepsis survival after major abdominal surgery. Biomark. Med. 2018, 12, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Choi, Y.S.; Park, B.J.; Shin, H.J.; Jeon, S.Y.; Kim, D.J.; Kim, S.Y. Immediate Postoperative High Syndecan-1 is Associated with Short-Term Morbidity and Mortality After Robot-Assisted Esophagectomy: A Prospective Observational Study. Ann. Surg. Oncol. 2023, 30, 5870–5880. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Robin, E.; Jabaudon, M.; Guerin, R.; Petit, A.; Bazin, J.-E.; Constantin, J.-M.; Vallet, B. Central venous O2 saturation and venous-to-arterial CO2 difference as complementary tools for goal-directed therapy during high-risk surgery. Crit. Care 2010, 14, R193. [Google Scholar] [CrossRef] [PubMed]
- Robin, E.; Futier, E.; Pires, O.; Fleyfel, M.; Tavernier, B.; Lebuffe, G.; Vallet, B. Central venous-to-arterial carbon dioxide difference as a prognostic tool in high-risk surgical patients. Crit. Care 2015, 19, 227. [Google Scholar] [CrossRef]
- Huette, P.; Ellouze, O.; Abou-Arab, O.; Guinot, P.G. Venous-to-arterial pCO2 difference in high-risk surgical patients. J. Thorac. Dis. 2019, 11, S1551–S1557. [Google Scholar] [CrossRef]
- Mallmann, C.; Drinhaus, H.; Fuchs, H.; Schiffmann, L.M.; Cleff, C.; Schönau, E.; Bruns, C.J.; Annecke, T.; Schröder, W. Perioperative enhanced recovery after surgery program for Ivor Lewis esophagectomy: First experiences of a high-volume center. Der Chir. 2021, 92, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Lambertz, R.; Drinhaus, H.; Schedler, D.; Bludau, M.; Schröder, W.; Annecke, T. Perioperative management of transthoracic oesophagectomies: Fundamentals of interdisciplinary care and new approaches to accelerated recovery after surgery. Der Anaesthesist 2016, 65, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Slankamenac, K.; Nederlof, N.; Pessaux, P.; de Jonge, J.; Wijnhoven, B.P.; Breitenstein, S.; Oberkofler, C.E.; Graf, R.; Puhan, M.A.; Clavien, P.A. The comprehensive complication index: A novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg. 2014, 260, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, J.S.; Bazancir, L.A.; Johansson, P.I.; Sørensen, H.; Achiam, M.P.; Olsen, A.A. Major open abdominal surgery is associated with increased levels of endothelial damage and interleukin-6. Microvasc. Res. 2023, 148, 104543. [Google Scholar] [CrossRef]
- Wang, J.; Wu, A.; Wu, Y. Endothelial Glycocalyx Layer: A Possible Therapeutic Target for Acute Lung Injury during Lung Resection. BioMed Res. Int. 2017, 2017, 5969657. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, E.; Baek, S.H.; Kim, H.Y.; Kim, J.-Y.; Park, J.; Choi, E.J. Sevoflurane did not show better protective effect on endothelial glycocalyx layer compared to propofol during lung resection surgery with one lung ventilation. J. Thorac. Dis. 2018, 10, 1468–1475. [Google Scholar] [CrossRef]
- Szarvas, T.; Reis, H.; Vom Dorp, F.; Tschirdewahn, S.; Niedworok, C.; Nyirady, P.; Schmid, K.W.; Rübben, H.; Kovalszky, I. Soluble Syndecan-1 (SDC1) serum level as an independent pre-operative predictor of cancer-specific survival in prostate cancer. Prostate 2016, 76, 977–985. [Google Scholar] [CrossRef]
- Bertrand, J.; Bollmann, M. Soluble Syndecans: Biomarkers for diseases and therapeutic options. Br. J. Pharmacol. 2019, 176, 67–81. [Google Scholar] [CrossRef]
- Szumilo, J.; Burdan, F.; Zinkiewicz, K.; Dudka, J.; Klepacz, R.; Dabrowski, A.; Korobowicz, E. Expression of Syndecan-1 and cathepsins D and K in advanced esophageal squamous cell carcinoma. Folia Histochem. Cytobiol. 2009, 47, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Brun, R.; Naroditsky, I.; Waterman, M.; Ben-Izhak, O.; Groisman, G.; Ilan, N.; Vlodavsky, I. Heparanase expression by Barrett’s epithelium and during esophageal carcinoma progression. Mod. Pathol. 2009, 22, 1548–1554. [Google Scholar] [CrossRef]
- Fuchs, A.; Dederichs, J.; Arjune, S.; Todorova, P.; Wöstmann, F.; Antczak, P.; Illerhaus, A.; Gathof, B.; Grundmann, F.; Müller, R.-U.; et al. Microvascular perfusion, perfused boundary region and glycocalyx shedding in patients with autosomal dominant polycystic kidney disease: Results from the GlycoScore III study. Clin. Kidney J. 2022, 16, 384–393. [Google Scholar] [CrossRef] [PubMed]
- D’Journo, X.B.; Michelet, P.; Marin, V.; Diesnis, I.; Blayac, D.; Doddoli, C.; Bongrand, P.; Thomas, P.A. An early inflammatory response to oesophagectomy predicts the occurrence of pulmonary complications. Eur. J. Cardiothorac. Surg. 2010, 37, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, L.; Lang, Y.; Wu, J.; Yao, L.; Ning, J.; Zhang, J.; Xu, S. Fast-track surgery improves postoperative clinical recovery and cellular and humoral immunity after esophagectomy for esophageal cancer. BMC Cancer 2016, 16, 449. [Google Scholar] [CrossRef] [PubMed]
- Fares, K.M.; Mohamed, S.A.; Hamza, H.M.; Sayed, D.M.; Hetta, D.F. Effect of thoracic epidural analgesia on pro-inflammatory cytokines in patients subjected to protective lung ventilation during Ivor Lewis esophagectomy. Pain Physician 2014, 17, 305–315. [Google Scholar]
- De Keijzer, I.N.; Kaufmann, T.; de Waal, E.E.C.; Frank, M.; Boer, D.d.K.-D.; Montenij, L.M.; Buhre, W.; Scheeren, T.W. Can perioperative pCO2 gaps predict complications in patients undergoing major elective abdominal surgery randomized to goal-directed therapy or standard care? A secondary analysis. J. Clin. Monit. Comput. 2024, 38, 469–477. [Google Scholar] [CrossRef]
- Flick, M.; Jannsen, G.P.; Krause, L.; Montomoli, J.; Pollok, F.; Moll-Khosrawi, P.; Kouz, K.; Bergholz, A.; Thomsen, K.K.; Hilty, M.P.; et al. The effect of major abdominal surgery on the sublingual microcirculation: An observational study. Can. J. Anaesth. 2025, 72, 768–779. [Google Scholar] [CrossRef]

| nutrition |
|
| anesthesia/surgery management |
|
| Physiotherapy/Mobilization |
|
| Endoscopy |
|
| Chest drain/nasogastric tube |
|
| Discharge from the hospital |
|
| Overall (n = 26) | No Complications (n = 10) | Complications (n = 16) | p-Value | |
|---|---|---|---|---|
| Age [years] | 55 [51; 60] | 57 [53; 64] | 54 [50; 56] | 0.11 |
| Sex (female/male) | 3/23 | 2/8 | 1/15 | 0.54 |
| ASA class (I/II) | 13/13 | 5/5 | 8/8 | >0.999 |
| CCI | 26.2 [0; 26.2] | 0.0 [0.0; 0.0] | 26.2 [26.2; 31.8] | <0.0001 |
| Body mass index [kg/m2] | 25 [23; 28] | 24 [23; 27] | 25.5 [24; 28] | 0.54 |
| Adenocarcinoma/squamous cell carcinoma | 22/4 | 9/1 | 13/3 | >0.999 |
| Duration of surgery [min] | 340 [314; 354] | 345 [336; 351] | 336.5 [310; 363] | 0.71 |
| Maximal Norepinephrine requirement [µg/kg/min] | 0.06 [0.05; 0.1] | 0.06 [0.05; 0.11] | 0.05 [0.05; 0.08] | 0.28 |
| Cardiac Index upon admission to the ICU [L/min/m2) | 3.4 [3.3; 3.5] | 3.2 [2.5; 3.6] | 3.5 [3.1; 4.0] | 0.26 |
| Lowest Cardiac Index | 2.9 [2.8; 3.5] | 2.8 [2.5; 3.6] | 2.9 [2.6; 3.4] | 0.34 |
| Lowest intraoperative MAP [mm Hg] | 68 [65; 73] | 66 [64; 70] | 70 [65.25; 73] | 0.30 |
| Intraoperative fluid balance [L] | +1.2 [0.8; 1.6] | +1.1 [0.9; 1.4] | +1.2 [0.7; 1.6] | 0.71 |
| Fluid balance after 24 h [L] | +1.5 [−0.2; +2.0] | +1.8 [0; +2.2] | +1.4 [−0.2; 1.8] | 0.72 |
| Overall (n = 24) | No Complications (n = 9) | Complications (n = 15) | p-Value | |
|---|---|---|---|---|
| Heparan sulfate [ng/mL] | ||||
| - Pre-operative | 1086 [857; 1334] | 1343 [1146; 1634] | 901 [727; 1098] | 0.001 |
| - ICU-admission | 982 [781; 1292] | 1062 [898; 2175] | 948 [726; 1225] | 0.073 |
| - POD 1 | 952 [566; 1540] | 1351 [829; 1832] | 727 [487; 1107] | 0.034 |
| Syndecan-1 [ng/mL] | ||||
| - Pre-operative | 29 [23; 51] | 24 [21; 31] | 30 [26; 53] | 0.084 |
| - ICU-admission | 56 [36; 87] | 50 [33; 62] | 71 [35; 88] | 0.60 |
| - POD 1 | 37 [23; 52] | 40 [25; 76] | 28 [23; 50] | 0.59 |
| Hyaluronic acid [ng/mL] | ||||
| - Pre-operative | 113 [99; 136] | 113 [95; 164] | 109 [99; 129] | 0.32 |
| - ICU-admission | 119 [103; 145] | 117 [93; 162] | 121 [104; 127] | 0.95 |
| - POD 1 | 131 [108; 159] | 123 [68; 167] | 133 [110; 159] | 0.47 |
| Overall (n = 25) | No Complications (n = 9) | Complications (n = 16) | p-Value | |
|---|---|---|---|---|
| Interleukin 1b [pg/mL] | ||||
| - Pre-operative | 1.2 [0.9; 1.7] | 1.3 [0.9; 1.5] | 1.1 [0.8; 2,1] | 0.97 |
| - ICU-admission | 1.4 [1.0; 2.5] | 1.3 [1.1; 2.2] | 1.6 [1.0; 2.9] | 0.56 |
| - POD 1 | 1.2 [0.9; 2.5] | 1.2 [1.0; 2.2] | 1.2 [0.8; 3.2] | 0.98 |
| Interleukin 6 [pg/mL] | ||||
| - Pre-operative | 1.7 [1.3; 3.2] | 2.5 [1.5; 3.7] | 1.7 [1.1; 2.9] | 0.35 |
| - ICU-admission | 177.0 [100.8; 251.0] | 194.5 [111.4; 379.1] | 151.1 [97.1; 233.9] | 0.30 |
| - POD 1 | 87.2 [54.2; 140.3] | 99.3 [65.4; 147.6] | 86.0 [34.8; 136.9] | 0.38 |
| Interleukin 8 [pg/mL] | ||||
| - Pre-operative | 5.2 [3.6; 8.2] | 5.2 [3.5; 7.0] | 5.2 [3.3; 9.6] | 0.88 |
| - ICU-admission | 19.9 [13.0; 32.9] | 21.5 [18.5; 33.8] | 19.1 [11.3; 33.1] | 0.60 |
| - POD 1 | 20.7 [13.6; 26.6] | 23.6 [15.1; 36.5] | 19.2 [11.8; 23.9] | 0.35 |
| Interleukin 10 [pg/mL] | ||||
| - Pre-operative | 0.50 [0.45; 0.61] | 0.50 [0.43; 0.65] | 0.51 [0.45; 0.59] | 0.94 |
| - ICU-admission | 1.33 [0.84; 3.54] | 1.50 [1.04; 2.09] | 0.98 [0.81; 3.93] | 0.79 |
| - POD 1 | 0.73 [0.64; 1.15] | 0.67 [0.63; 0.73] | 0.86 [0.65; 1.26] | 0.08 |
| Interleukin 12 [pg/mL] | ||||
| - Pre-operative | 0.42 [0.30; 0.54] | 0.49 [0.39; 0.55] | 0.33 [0.22; 0.54] | 0.13 |
| - ICU-admission | 0.39 [0.32; 0.61] | 0.37 [0.35; 0.43] | 0.42 [0.27; 0.82] | 0.48 |
| - POD 1 | 0.33 [0.27; 0.44] | 0.32 [0.29; 0.41] | 0.36 [0.26; 0.47] | 0.92 |
| TNF-alpha [ng/mL] | ||||
| - Pre-operative | 0.39 [0.09; 0.6] | 0.46 [0.21; 0.76] | 0.33 [0.09; 0.7] | 0.50 |
| - ICU-admission | 0.50 [0.21; 0.78] | 0.5 [0.08; 0.81] | 0.49 [0.27; 0.74] | 0.90 |
| - POD 1 | 0.35 [0.05; 0.55] | 0.30 [0.11; 0.43] | 0.42 [0.04; 0.60] | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drinhaus, H.; Mallmann, C.; Cleff, C.; Neumann, T.; Daniels, C.; Bruns, C.J.; Steinbicker, A.U.; Schröder, W.; Annecke, T. Glycocalyx-Shedding and Inflammatory Reactions Occur Yet Do Not Predict Complications Resulting from an Esophagectomy in an Accelerated Recovery After Surgery Program. J. Clin. Med. 2025, 14, 6048. https://doi.org/10.3390/jcm14176048
Drinhaus H, Mallmann C, Cleff C, Neumann T, Daniels C, Bruns CJ, Steinbicker AU, Schröder W, Annecke T. Glycocalyx-Shedding and Inflammatory Reactions Occur Yet Do Not Predict Complications Resulting from an Esophagectomy in an Accelerated Recovery After Surgery Program. Journal of Clinical Medicine. 2025; 14(17):6048. https://doi.org/10.3390/jcm14176048
Chicago/Turabian StyleDrinhaus, Hendrik, Christoph Mallmann, Corvin Cleff, Tobias Neumann, Christina Daniels, Christiane J. Bruns, Andrea U. Steinbicker, Wolfgang Schröder, and Thorsten Annecke. 2025. "Glycocalyx-Shedding and Inflammatory Reactions Occur Yet Do Not Predict Complications Resulting from an Esophagectomy in an Accelerated Recovery After Surgery Program" Journal of Clinical Medicine 14, no. 17: 6048. https://doi.org/10.3390/jcm14176048
APA StyleDrinhaus, H., Mallmann, C., Cleff, C., Neumann, T., Daniels, C., Bruns, C. J., Steinbicker, A. U., Schröder, W., & Annecke, T. (2025). Glycocalyx-Shedding and Inflammatory Reactions Occur Yet Do Not Predict Complications Resulting from an Esophagectomy in an Accelerated Recovery After Surgery Program. Journal of Clinical Medicine, 14(17), 6048. https://doi.org/10.3390/jcm14176048







