Pilot Evaluation of Intestinal Current Measurement in Cystic Fibrosis and CRMS/CFSPID Patients in Poland
Abstract
1. Introduction
Aim
2. Materials and Methods
2.1. Ethics
2.2. Statistical Analysis
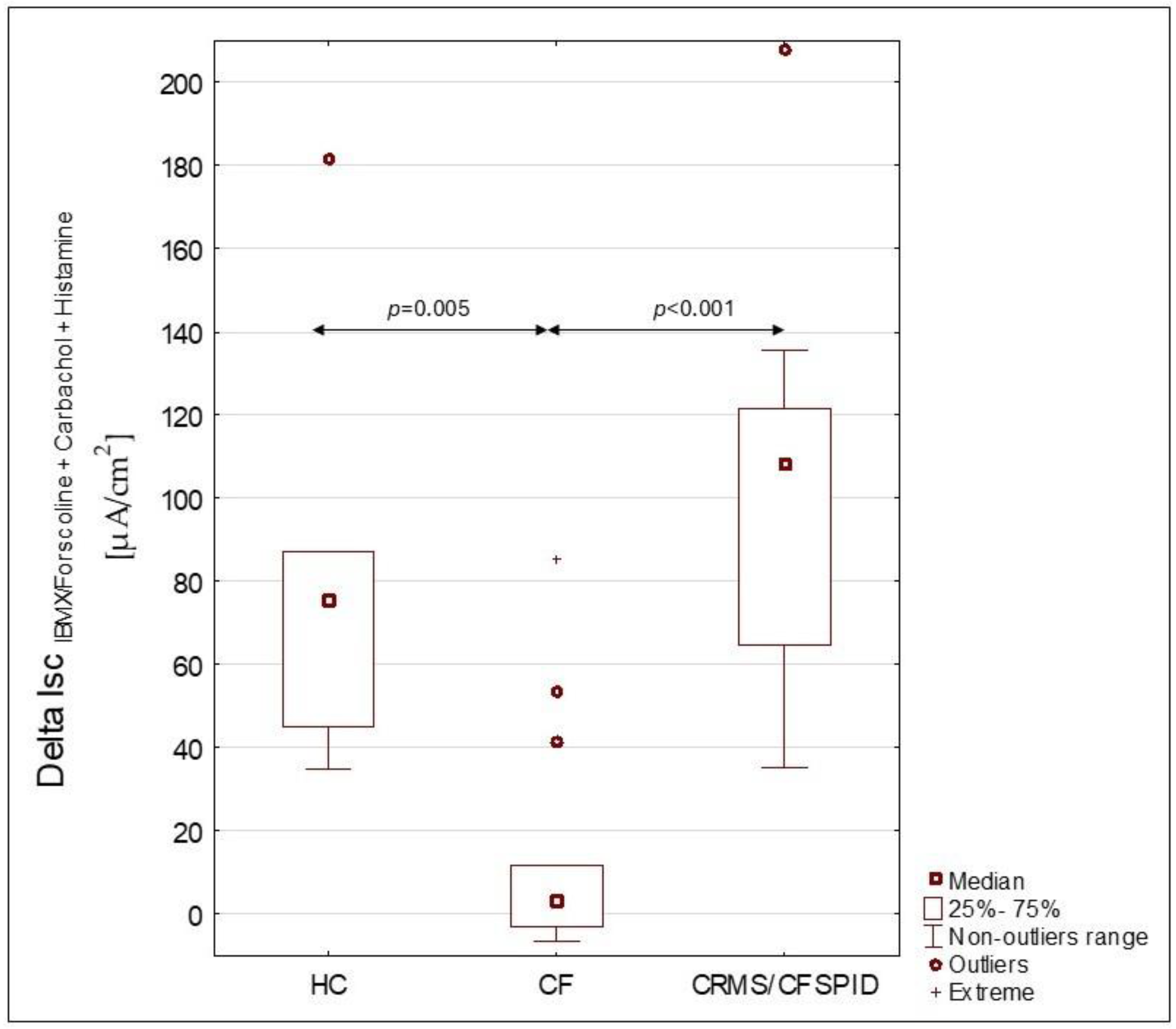
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Cystic Fibrosis |
| CF NBS | Cystic Fibrosis Newborn Bloodspot Screening |
| CF TDN | Cystic Fibrosis Therapeutics Development Network |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CRMS/CFSPID | Cystic fibrosis transmembrane conductance regulator-related metabolic syndrome/cystic fibrosis screen positive, inconclusive diagnosis |
| ECFS DNWG | European Cystic Fibrosis Society Diagnostic Network Working Group |
| HC | Healthy control |
| ICM | Intestinal Current Measurement |
| IRT | Immunoreactive Trypsin |
| IRT/DNA/EGA | Immunoreactive Trypsin Analysis/Analysis of genetic material, detection of variants in the CFTR gene/Genotypic analysis, identification of variants in the CFTR gene |
| ISC | Short circuit-current |
| NPD | Nasal Potential Difference |
| PBS | Phosphate-Buffered Saline |
| PI-CF | Cystic fibrosis with pancreatic insufficiency |
| PS-CF | Cystic fibrosis with pancreatic sufficiency |
| SOP | Standard operating procedure |
| VUC | Variant of unknown clinical significance |
| VVCC | Variant with varying clinical consequence |
References
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Sands, D.; Zybert, K.; Mierzejewska, E.; Ołtarzewski, M. Diagnosing cystic fibrosis in newborn screening in Poland—15 years of experience. Dev. Period. Med. 2015, 19, 16–24. [Google Scholar]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castanos, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef]
- Esther, C.R., Jr.; Muhlebach, M.S.; Ehre, C.; Hill, D.B.; Wolfgang, M.C.; Kesimer, M.; Ramsey, K.A.; Markovetz, M.R.; Garbarine, I.C.; Forest, M.G.; et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci. Transl. Med. 2019, 11, eaav3488. [Google Scholar] [CrossRef]
- Ranganathan, S.C.; Hall, G.L.; Sly, P.D.; Stick, S.M.; Douglas, T.A. Early Lung Disease in Infants and Preschool Children with Cystic Fibrosis. What Have We Learned and What Should We Do about It? Am. J. Respir. Crit. Care Med. 2017, 195, 1567–1575. [Google Scholar] [CrossRef]
- Nguyen, T.T.-D.; Thia, L.P.; Hoo, A.-F.; Bush, A.; Aurora, P.; Wade, A.; Chudleigh, J.; Lum, S.; Stocks, J. Evolution of lung function during the first year of life in newborn screened cystic fibrosis infants. Thorax 2014, 69, 910–917. [Google Scholar] [CrossRef]
- Bush, A.; Sly, P.D. Evolution of cystic fibrosis lung function in the early years. Curr. Opin. Pulm. Med. 2015, 21, 602–608. [Google Scholar] [CrossRef]
- Mall, M.A.; Mayer-Hamblett, N.; Rowe, S.M. Cystic Fibrosis: Emergence of Highly Effective Targeted Therapeutics and Potential Clinical Implications. Am. J. Respir. Crit. Care Med. 2020, 201, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- Minso, R.; Schulz, A.; Dopfer, C.; Alfeis, N.; Barneveld, A.V.; Makartian-Gyulumyan, L.; Hansen, G.; Junge, S.; Müller, C.; Ringshausen, F.C.C.; et al. Intestinal current measurement and nasal potential difference to make a diagnosis of cases with inconclusive CFTR genetics and sweat test. BMJ Open Respir. Res. 2020, 7, e000736. [Google Scholar] [CrossRef] [PubMed]
- Drumm, M.L.; Ziady, A.G.; Davis, P.B. Genetic variation and clinical heterogeneity in cystic fibrosis. Annu. Rev. Pathol. 2012, 7, 267–282. [Google Scholar] [CrossRef]
- Sobczyńska-Tomaszewska, A.; Ołtarzewski, M.; Czerska, K.; Wertheim-Tysarowska, K.; Sands, D.; Walkowiak, J.; Bal, J.; Mazurczak, T. Newborn screening for cystic fibrosis. Polish 4 years experience with CFTR sequencing strategy. Eur. J. Hum. Genet. 2013, 21, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181, S4–S15.e11. [Google Scholar] [CrossRef] [PubMed]
- Barben, J.; Castellani, C.; Dankert-Roelse, J.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Mayell, S.; Munck, A.; Sands, D.; Sommerburg, O.; et al. The expansion and performance of national newborn screening programmes for cystic fibrosis in Europe. J. Cyst. Fibros. 2017, 16, 207–213. [Google Scholar] [CrossRef]
- Levy, H.; Nugent, M.; Schneck, K.; Stachiw-Hietpas, D.; Laxova, A.; Lakser, O.; Rock, M.; Dahmer, M.K.; Biller, J.; Nasr, S.Z.; et al. Refining the continuum of CFTR-associated disorders in the era of newborn screening. Clin. Genet. 2016, 89, 539–549. [Google Scholar] [CrossRef]
- Loeber, J.G.; Burgard, P.; Cornel, M.C.; Rigter, T.; Weinreich, S.S.; Rupp, K.; Hoffmann, G.F.; Vittozzi, L. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 1—From blood spot to screening result. J. Inherit. Metab. Dis. 2012, 35, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Zybert, K.; Wozniacki, L.; Tomaszewska-Sobczyńska, A.; Wertheim-Tysarowska, K.; Czerska, K.; Ołtarzewski, M.; Sands, D. Clinical characteristics of rare CFTR mutations causing cystic fibrosis in Polish population. Pediatr. Pulmonol. 2020, 55, 2097–2107. [Google Scholar] [CrossRef]
- Ussing, H.H.; Zerahn, K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Reprinted from Acta. Physiol. Scand. 23: 110–127, 1951. J. Am. Soc. Nephrol. 1999, 10, 2056–2065. [Google Scholar]
- Frizzell, R.A.; Rechkemmer, G.; Shoemaker, R.L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science 1986, 233, 558–560. [Google Scholar] [CrossRef]
- Bronsveld, I.; Mekus, F.; Bijman, J.; Ballmann, M.; Greipel, J.; Hundrieser, J.; Halley, D.J.J.; Laabs, U.; Busche, R.; De Jonge, H.R.; et al. Residual chloride secretion in intestinal tissue of ΔF508 homozygous twins and siblings with cystic fibrosis. Gastroenterology 2000, 119, 32–40. [Google Scholar] [CrossRef]
- De Jonge, H.R.; Ballmann, M.; Veeze, H.; Bronsveld, I.; Stanke, F.; Tümmler, B.; Sinaasappel, M. Ex vivo CF diagnosis by intestinal current measurements (ICM) in small aperture, circulating Ussing chambers. J. Cyst. Fibros. 2004, 3, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Berschneider, H.M.; Knowles, M.R.; Azizkhan, R.G.; Boucher, R.C.; Tobey, N.A.; Orlando, R.C.; Powell, D.W. Altered intestinal chloride transport in cystic fibrosis. Faseb J. 1988, 2, 2625–2629. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.; Bleich, M.; Kuehr, J.; Brandis, M.; Greger, R.; Kunzelmann, K. CFTR-mediated inhibition of epithelial Na+ conductance in human colon is defective in cystic fibrosis. Am. J. Physiol. 1999, 277, G709–G716. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sheppard, D.N.; Hug, M.J. Transepithelial electrical measurements with the Ussing chamber. J. Cyst. Fibros. 2004, 3, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Veeze, H.J.; Halley, D.J.; Bijman, J.; de Jongste, J.C.; de Jonge, H.R.; Sinaasappel, M. Determinants of mild clinical symptoms in cystic fibrosis patients. Residual chloride secretion measured in rectal biopsies in relation to the genotype. J. Clin. Investig. 1994, 93, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Veeze, H.J.; Sinaasappel, M.; Bijman, J.; Bouquet, J.; de Jonge, H.R. Ion transport abnormalities in rectal suction biopsies from children with cystic fibrosis. Gastroenterology 1991, 101, 398–403. [Google Scholar] [CrossRef]
- Clarke, L.L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1151–G1166. [Google Scholar] [CrossRef]
- Brown, D.R.; O’Grady, S.M. The Ussing Chamber and Measurement of Drug Actions on Mucosal Ion Transport. Curr. Protoc. Pharmacol. 2008, 41, 7.12.11–17.12.17. [Google Scholar] [CrossRef]
- Graeber, S.Y.; Vitzthum, C.; Mall, M.A. Potential of Intestinal Current Measurement for Personalized Treatment of Patients with Cystic Fibrosis. J. Pers. Med. 2021, 11, 384. [Google Scholar] [CrossRef]
- Southern, K.W.; Barben, J.; Gartner, S.; Munck, A.; Castellani, C.; Mayell, S.J.; Davies, J.C.; Winters, V.; Murphy, J.; Salinas, D.; et al. Inconclusive diagnosis after a positive newborn bloodspot screening result for cystic fibrosis; clarification of the harmonised international definition. J. Cyst. Fibros. 2019, 18, 778–780. [Google Scholar] [CrossRef]
- Munck, A. Inconclusive Diagnosis after Newborn Screening for Cystic Fibrosis. Int. J. Neonatal Screen. 2020, 6, 19. [Google Scholar] [CrossRef]
- Oliver, K.N.; Free, M.L.; Bok, C.; McCoy, K.S.; Lemanek, K.L.; Emery, C.F. Stigma and optimism in adolescents and young adults with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 737–744. [Google Scholar] [CrossRef]
- Johnson, F.; Southern, K.W.; Ulph, F. Psychological Impact on Parents of an Inconclusive Diagnosis Following Newborn Bloodspot Screening for Cystic Fibrosis: A Qualitative Study. Int. J. Neonatal Screen. 2019, 5, 23. [Google Scholar] [CrossRef]
- Ramalho, A.S.; Boon, M.; Proesmans, M.; Vermeulen, F.; Carlon, M.S.; Boeck, K. Assays of CFTR Function In Vitro, Ex Vivo and In Vivo. Int. J. Mol. Sci. 2022, 23, 1437. [Google Scholar] [CrossRef]
- De Boeck, K.; Derichs, N.; Fajac, I.; de Jonge, H.R.; Bronsveld, I.; Sermet, I.; Vermeulen, F.; Sheppard, D.N.; Cuppens, H.; Hug, M.; et al. New clinical diagnostic procedures for cystic fibrosis in Europe. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2011, 10, S53–S66. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Mall, M. Electrolyte Transport in the Mammalian Colon: Mechanisms and Implications for Disease. Physiol. Rev. 2002, 82, 245–289. [Google Scholar] [CrossRef]
- Mall, M.; Bleich, M.; Schurlein, M.; Kuhr, J.; Seydewitz, H.H.; Brandis, M.; Greger, R.; Kunzelmann, K. Cholinergic ion secretion in human colon requires coactivation by cAMP. Am. J. Physiol. 1998, 275, G1274–G1281. [Google Scholar] [CrossRef]
- Hatton, A.; Bergougnoux, A.; Zybert, K.; Chevalier, B.; Mesbahi, M.; Altéri, J.P.; Walicka-Serzysko, K.; Postek, M.; Taulan-Cadars, M.; Edelman, A.; et al. Reclassifying inconclusive diagnosis after newborn screening for cystic fibrosis. Moving forward. J. Cyst. Fibros. 2022, 21, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.P.; Szczesniak, R.D.; Ashlock, M.A.; Ernst, S.E.; Fan, L.; Hornick, D.B.; Karp, P.H.; Khan, U.; Lymp, J.; Ostmann, A.J.; et al. Multicenter Intestinal Current Measurements in Rectal Biopsies from CF and Non-CF Subjects to Monitor CFTR Function. PLoS ONE 2013, 8, e73905. [Google Scholar] [CrossRef]
- Cohen-Cymberknoh, M.; Yaakov, Y.; Shoseyov, D.; Shteyer, E.; Schachar, E.; Rivlin, J.; Bentur, L.; Picard, E.; Aviram, M.; Israeli, E.; et al. Evaluation of the intestinal current measurement method as a diagnostic test for cystic fibrosis. Pediatr. Pulmonol. 2013, 48, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Graeber, S.Y.; Sommerburg, O.; Yu, Y.; Berges, J.; Hirtz, S.; Scheuermann, H.; Berger, J.; Duerr, J.; Mall, M.A. Intestinal current measurement detects age-dependent differences in CFTR function in rectal epithelium. Front. Pharmacol. 2025, 16, 1537095. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, T.E.; Broerman, M.J.; Kliment, C.R.; Lo, C. CFTR expression decreases with age in several airway cell types. Sci. Rep. 2024, 14, 28832. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Roehmel, J.; Eichinger, M.; Doellinger, F.; Naehrlich, L.; Kopp, M.V.; Dittrich, A.M.; Lee, C.; Sommerburg, O.; Tian, S.; et al. Effects of Lumacaftor/Ivacaftor on Cystic Fibrosis Disease Progression in Children 2 through 5 Years of Age Homozygous for F508del-CFTR: A Phase 2 Placebo-controlled Clinical Trial. Ann. Am. Thorac. Soc. 2023, 20, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Roehmel, J.; Eichinger, M.; Doellinger, F.; Naehrlich, L.; Kopp, M.V.; Dittrich, A.M.; Sommerburg, O.; Ray, P.; Maniktala, A.; et al. Long-Term Impact of Lumacaftor/Ivacaftor Treatment on Cystic Fibrosis Disease Progression in Children 2–5 Years of Age Homozygous for F508del-CFTR: A Phase 2, Open-Label Clinical Trial. Ann. Am. Thorac. Soc. 2024, 21, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
| CRMS/CFSPID | HC | CF | PS-CF | PI-CF | |
|---|---|---|---|---|---|
| N (number of patients) | 16 | 7 | 17 | 8 | 9 |
| Male/Female [%] | 7/9 [44/56] | 2/5 [29/71] | 9/8 [53/47] | 4/4 [50/50] | 5/4 [56/44] |
| Age [years] | |||||
| Mean ± SD | 6.66 ± 4.83 | 23.7 ± 9.50 | 9.10 ± 4.18 | 7.85 ± 4.38 | 9.04 ± 3.91 |
| Min–max | [0.6–18] | [7.8–34.6] | [0.7–17.2] | [2.5–17.2] | [0.7–15.8] |
| ΔIsc response [µA/cm2] * | CRMS/CFSPID | HC | CF | PS-CF | PI-CF |
|---|---|---|---|---|---|
| Amiloride | |||||
| Mean ± SD | −9.71 ± 13.32 | −1.15 ± 0.78 | −11.03 ± 10.90 | −7.18 ± 7.14 | −14.46 ± 12.85 |
| [min–max] | [−51.80–2.65] | [−2.70–0.60] | [−37.40–0.30] | [−5.30–20.70] | [−37.40–0.30] |
| IBMX **/forskolin | |||||
| Mean ± SD | 28.56 ± 13.95 | 7.55 ± 5.22 | 1.65 ± 7.66 | 5.24 ± 8.49 | −1.53 ± 5.48 |
| [min–max] | [8.60–59.30] | [0.00–15.00] | [−13.90–23.90] | [−3.80–23.90] | [−13.90–3.80] |
| Carbachol | |||||
| Mean ± SD | 33.04 ± 17.53 | 36.47 ± 24.04 | 1.78 ± 6.10 | 3.28 ± 8.05 | 0.44 ± 3.68 |
| [min–max] | [−11.50–65.50] | [19.70–88.10] | [−7.30–21.80] | [−3.20–21.80] | [−7.30–5.50] |
| Histamine | |||||
| Mean ± SD | 24.64 ± 14.78 | 36.10 ± 22.72 | −0.19 ± 2.50 | 0.69 ± 2.91 | −0.97 ± 1.92 |
| [min–max] | [−10.60–66.40] | [16.80–83.60] | [−4.00–7.40] | [−2.10–7.40] | [−4.00–3.10] |
| ΔIsc IBMX/Forskolin + Carbachol + Histamine | |||||
| Mean ± SD | 86.84 ± 37.84 | 80.16 ± 48.54 | 15.31 ± 15.47 | 9.18 ± 18.95 | −1.67 ± 10.11 |
| [min–max] | [35.40–166.80] | [43.70–181.40] | [−24.60–53.10] | [−6.50–53.10] | [−24.60–11.90] |
| P values between groups in ΔIsc IBMX/Forskolin + Carbachol + Histamine | |||||
| CRMS/CFSPID | n/a | >0.05 | <0.001 | <0.001 | <0.001 |
| HC | >0.05 | n/a | 0.005 | >0.05 | 0.02 |
| CF | <0.001 | 0.005 | n/a | n/a | n/a |
| PS-CF | <0.001 | >0.05 | n/a | n/a | n/a |
| PI-CF | <0.001 | 0.02 | n/a | n/a | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postek, M.; Zybert, K.; Wozniacki, L.; Woynarowski, M.; Sands, D. Pilot Evaluation of Intestinal Current Measurement in Cystic Fibrosis and CRMS/CFSPID Patients in Poland. J. Clin. Med. 2025, 14, 6020. https://doi.org/10.3390/jcm14176020
Postek M, Zybert K, Wozniacki L, Woynarowski M, Sands D. Pilot Evaluation of Intestinal Current Measurement in Cystic Fibrosis and CRMS/CFSPID Patients in Poland. Journal of Clinical Medicine. 2025; 14(17):6020. https://doi.org/10.3390/jcm14176020
Chicago/Turabian StylePostek, Magdalena, Katarzyna Zybert, Lukasz Wozniacki, Marek Woynarowski, and Dorota Sands. 2025. "Pilot Evaluation of Intestinal Current Measurement in Cystic Fibrosis and CRMS/CFSPID Patients in Poland" Journal of Clinical Medicine 14, no. 17: 6020. https://doi.org/10.3390/jcm14176020
APA StylePostek, M., Zybert, K., Wozniacki, L., Woynarowski, M., & Sands, D. (2025). Pilot Evaluation of Intestinal Current Measurement in Cystic Fibrosis and CRMS/CFSPID Patients in Poland. Journal of Clinical Medicine, 14(17), 6020. https://doi.org/10.3390/jcm14176020





