Clinical Versus Dermoscopic Evaluation of Tumor Margins Prior to Surgical Excision—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
- Study design: Interventional studies (randomized and non-randomized controlled trials) and observational studies (such as cohort studies, cross-sectional studies, and case–control studies).
- Population: Adult patients (≥18 years) undergoing surgical excision of histologically confirmed non-melanoma skin cancers (BBC and/or SCC). Intervention: Preoperative margin evaluation using dermoscopy.
- Comparator: Preoperative margin evaluation using clinical visual inspection alone (naked-eye examination).
- Outcomes: Primary outcome was the rate of complete excision (histologically clear margins).
- Language: Only studies published in English were included.
- Publication type: Peer-reviewed articles. Abstracts, case reports, case-series < 5 patients editorials, and reviews were excluded.
2.3. Information Sources and Search Strategy
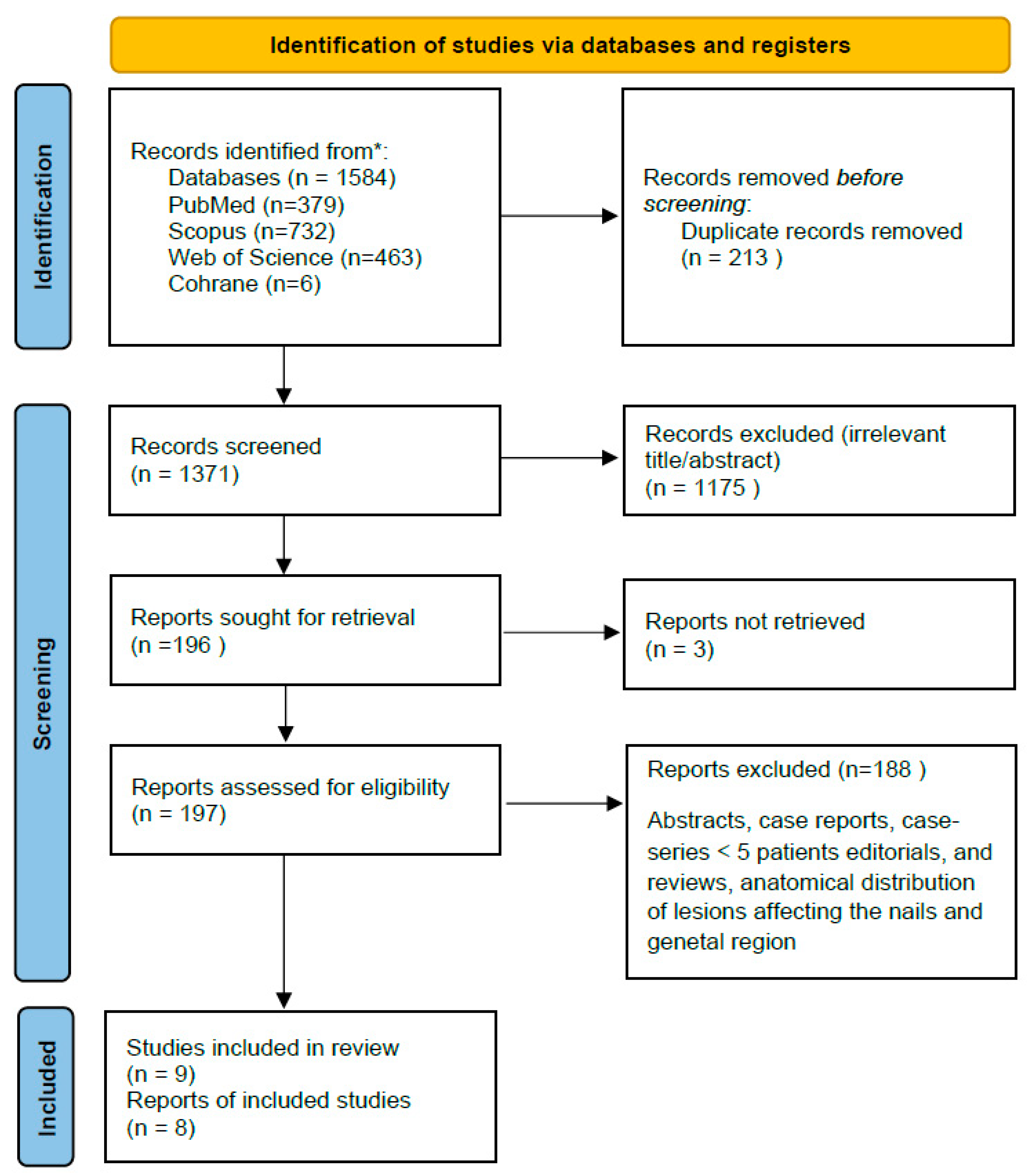
2.4. Study Selection
2.5. Data Extraction
- Study characteristics: author, year, country, study design;
- Patient demographics and tumor characteristics;
- Method of margin evaluation (dermoscopy vs. clinical);
- Surgical technique (e.g., standard excision);
- Primary outcomes as defined above;
- Where data were missing or unclear, study authors were contacted for clarification.
2.6. Risk of Bias Assessment
2.7. Data Synthesis and Statistical Analysis
3. Results
3.1. Study Characteristics
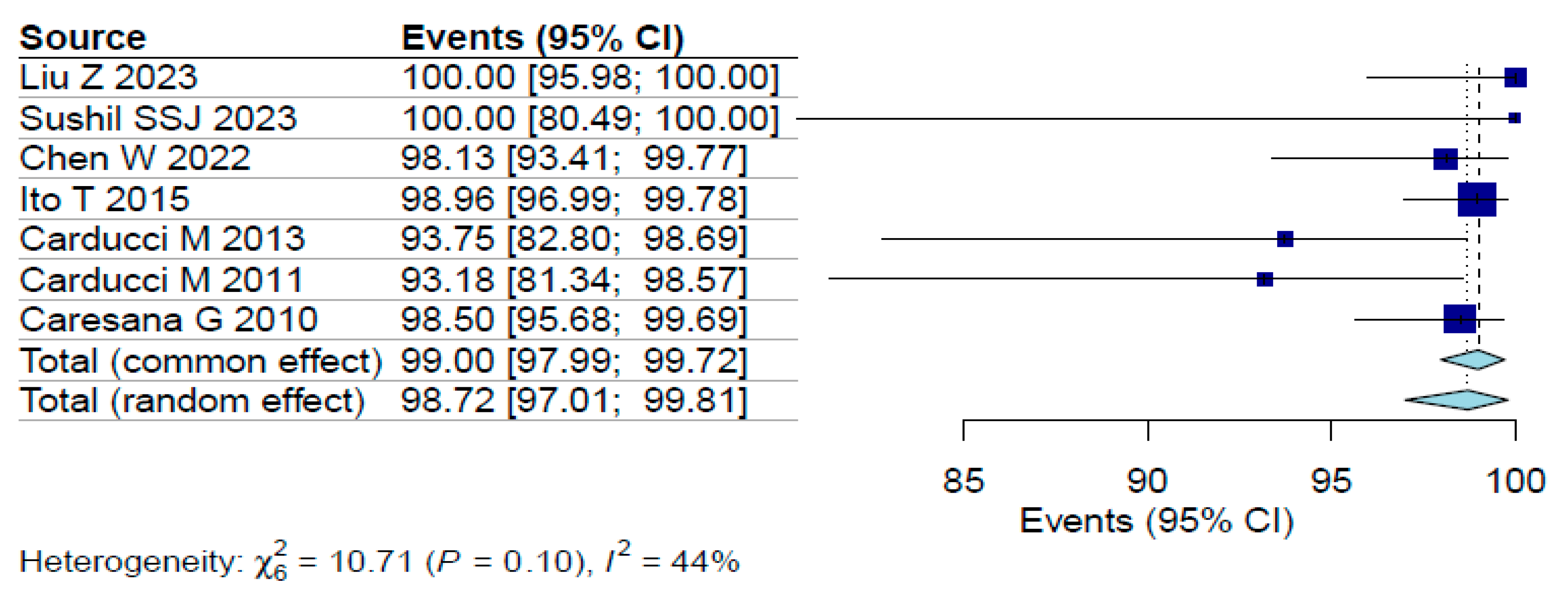
3.2. Margin Clearance Rates
3.3. Risk of Bias
4. Discussion
Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gray, D.T.; Suman, V.J.; Su, W.P.D.; Clay, R.P.; Harmsen, W.S.; Roenigk, R.K. Trends in the population-based incidence of squamous cell carcinoma of the skin first diagnosed between 1984 and 1992. Arch. Dermatol. 1997, 133, 735–740. [Google Scholar] [CrossRef]
- Zalaudek, I.; Kreusch, J.; Giacomel, J.; Ferrara, G.; Catricalà, C.; Argenziano, G. How to diagnose nonpigmented skin tumors: A review of vascular structures seen with dermoscopy: Part I. Melanocytic skin tumors. J. Am. Acad. Dermatol. 2010, 63, 361. [Google Scholar] [CrossRef]
- Hurley, A.R.; Totty, J.P.; Pinder, R.M. Dermoscopy as an adjunct to surgical excision of nonmelanoma Skin lesions: A systematic review and Meta-analysis. J. Clin. Aesthet. Dermatol. 2022, 15, 45. [Google Scholar]
- Chen, W.; Liu, Z.; Zhou, Y.; Liu, M.; Wang, X.; Wang, D. The effect of dermoscopy in assisting on defining surgical margins of basal cell carcinoma. Dermatol. Ther. 2022, 35, e15711. [Google Scholar] [CrossRef]
- Litaiem, N.; Karray, M.; Jones, M.; Rammeh, S.; Zeglaoui, F. Effectiveness of dermoscopy in the demarcation of surgical margins in slow Mohs surgery. Dermatol. Ther. 2020, 33, e14196. [Google Scholar] [CrossRef] [PubMed]
- QS130; Quality Statement 3: Dermoscopy. NICE Quality Standards for Skin Cancer; National Institute for Health and Care Excellence (NICE): London, UK, 2016; Updated 2024.
- Camela, E.; Anca, P.I.; Kyrgidis, A.; Lallas, K.; Scalvenzi, M.; Papageorgiou, C.; Manoli, S.-M.; Gkentsidi, T.; Eftychidou, P.; Delli, F.S.; et al. Dermatoscopic predictors of histopathologically aggressive basal cell carcinoma and their positive impact of subtype prediction by human readers. J. Am. Acad. Dermatol. 2024, 91, 1236–1239. [Google Scholar] [CrossRef]
- Lallas, A.; Argenziano, G.; Kyrgidis, A.; Apalla, Z.; Moscarella, E.; Longo, C.; Ferrara, G.; Piana, S.; Benati, E.; Zendri, E.; et al. Dermoscopy uncovers clinically undetectable pigmentation in basal cell carcinoma. Br. J. Dermatol. 2014, 170, 192–195. [Google Scholar] [CrossRef]
- Lallas, A.; Tzellos, T.; Kyrgidis, A.; Apalla, Z.; Zalaudek, I.; Karatolias, A.; Ferrara, G.; Piana, S.; Longo, C.; Moscarella, E.; et al. Accuracy of dermoscopic criteria for discriminating superficial from other subtypes of basal cell carcinoma. J. Am. Acad. Dermatol. 2014, 70, 303–311. [Google Scholar] [CrossRef]
- Longo, C.; Specchio, F.; Ribero, S.; Coco, V.; Kyrgidis, A.; Moscarella, E.; Ragazzi, M.; Peris, K.; Argenziano, G. Dermoscopy of small-size basal cell carcinoma: A case-control study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e273. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Giuffrida, R.; Zalaudek, I.; Guarneri, F.; Cannavò, S.P.; Pizzichetta, M.A.; Bonin, S.; Corneli, P.; Bussani, R.; Bazzacco, G.; et al. Dermoscopic Findings in the Presurgical Evaluation of Basal Cell Carcinoma. A Prospective Study. Dermatol. Surg. 2021, 47, e37. [Google Scholar] [CrossRef] [PubMed]
- Menzies, S.W.; Kreusch, J.; Byth, K.; Pizzichetta, M.A.; Marghoob, A.; Braun, R.; Malvehy, J.; Puig, S.; Argenziano, G.; Zalaudek, I.; et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch. Dermatol. 2008, 144, 1120–1127. [Google Scholar] [CrossRef]
- Nolan, G.; Kiely, A.; Totty, J.; Wormald, J.; Wade, R.; Arbyn, M.; Jain, A. Incomplete surgical excision of keratinocyte skin cancers: A systematic review and meta-analysis. Br. J. Dermatol. 2021, 184, 1033–1044. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, S.; Li, F.; Wang, X.; Liu, M.; Wong, H.S.; Jiang, J.; Zhou, Y.; Wang, D. The efficacy of dermoscopy in defining the surgical margins of cutaneous squamous cell carcinoma: A retrospective study. Front. Oncol. 2023, 13, 1141820. [Google Scholar] [CrossRef]
- Wojtowicz, I.; Żychowska, M. Dermoscopy of Basal Cell Carcinoma Part 3: Differential Diagnosis, Treatment Monitoring and Novel Technologies. Cancers 2025, 17, 1025. [Google Scholar] [CrossRef]
- Leibovitch, I.; Huilgol, S.C.; Selva, D.; Richards, S.; Paver, R. Basal cell carcinoma treated with Mohs surgery in Australia II. Outcome at 5-year follow-up. J. Am. Acad. Dermatol. 2005, 53, 452. [Google Scholar] [CrossRef]
- Longo, C.; Pampena, R.; Bombonato, C.; Gardini, S.; Piana, S.; Mirra, M.; Raucci, M.; Kyrgidis, A.; Pellacani, G.; Ragazzi, M. Diagnostic accuracy of ex vivo fluorescence confocal microscopy in Mohs surgery of basal cell carcinomas: A prospective study on 753 margins. Br. J. Dermatol. 2019, 180, 1473–1480. [Google Scholar] [CrossRef]
- Aoki, K.C.M.; Lazzara, D.D.; Bartos, G.D.; Weiss, E.M.; Saleeby, E.R.M. Surgical Margins of Nonmelanoma Skin Cancers in Mohs Surgery: Dermoscopy Versus Naked Eye. Dermatol. Surg. 2025, 51, 236–239. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Carducci, M.; Bozzetti, M.; de Marco, G.; Foscolo, A.M.; Betti, R. Preoperative margin detection by digital dermoscopy in the traditional surgical excision of cutaneous squamous cell carcinomas. J. Dermatol. Treat. 2013, 24, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Carducci, M.; Bozzetti, M.; Foscolo, A.M.; Betti, R. Margin detection using digital dermatoscopy improves the performance of traditional surgical excision of basal cell carcinomas of the head and neck. Dermatol. Surg. 2011, 37, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Caresana, G.; Giardini, R. Dermoscopy-guided surgery in basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1395. [Google Scholar] [CrossRef]
- Ito, T.; Inatomi, Y.; Nagae, K.; Nakano-Nakamura, M.; Nakahara, T.; Furue, M.; Uchi, H. Narrow-margin excision is a safe, reliable treatment for well-defined, primary pigmented basal cell carcinoma: An analysis of 288 lesions in Japan. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1828–1831. [Google Scholar] [CrossRef]
- Lupu, M.; Voiculescu, V.M.; Caruntu, A.; Tebeica, T.; Caruntu, C. Preoperative Evaluation through Dermoscopy and Reflectance Confocal Microscopy of the Lateral Excision Margins for Primary Basal Cell Carcinoma. Diagnostics 2021, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Savant, S.S., Jr. Use of preoperative and perioperative ex vivo dermoscopy for precise mapping of margins for standard surgical excision of primary basal cell carcinoma. Indian J. Dermatol. Venereol. Leprol. 2023, 89, 793. [Google Scholar] [CrossRef] [PubMed]
- Bakos, R.M.; Blumetti, T.P.; Roldán-Marín, R.; Salerni, G. Noninvasive Imaging Tools in the Diagnosis and Treatment of Skin Cancers. Am. J. Clin. Dermatol. 2018, 19, 3. [Google Scholar] [CrossRef]
- Lallas, A.; Martínez, G.; Arceu, M.; Kyrgidis, A.; Liopyris, K.; Brancaccio, G.; Longo, C.; Errichetti, E.; Sgouros, D.; Papageorgiou, C.; et al. Clinical and dermatoscopic predictors of squamous cell carcinoma of the lips: A case-control, multicentric study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 222. [Google Scholar] [CrossRef]
- Lallas, A.; Pyne, J.; Kyrgidis, A.; Andreani, S.; Argenziano, G.; Cavaller, A.; Giacomel, J.; Longo, C.; Malvestiti, A.; Moscarella, E.; et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br. J. Dermatol. 2015, 172, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Lallas, A.; Tzellos, T.; Sidiropoulos, T.; Lefaki, I.; Trakatelli, M.; Sotiriou, E.; Lazaridou, E.; Evangelou, G.; Patsatsi, A.; et al. Applicability of dermoscopy for evaluation of patients’ response to nonablative therapies for the treatment of superficial basal cell carcinoma. Br. J. Dermatol. 2014, 170, 809–815. [Google Scholar] [CrossRef]
- Reiter, O.; Mimouni, I.; Gdalevich, M.; Marghoob, A.A.; Levi, A.; Hodak, E.; Leshem, Y.A. The diagnostic accuracy of dermoscopy for basal cell carcinoma: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 1380–1388. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Chuchu, N.; Matin, R.N.; Wong, K.Y.; Aldridge, R.B.; Durack, A.; Gulati, A.; Chan, S.A.; Johnston, L.; et al. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst. Rev. 2018, 12, CD011901. [Google Scholar] [CrossRef]
- Pampena, R.; Benati, E.; Borsari, S.; Bombonato, C.; Lombardi, M.; Raucci, M.; Mirra, M.; Lallas, A.; Apalla, Z.; Papadimitriou, I.; et al. Tracking actinic keratosis of face and scalp treated with 0.015% ingenol mebutate to identify clinical and dermoscopic predictors of treatment response. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Kittler, H.; Marghoob, A.A.; Argenziano, G.; Carrera, C.; Curiel-Lewandrowski, C.; Hofmann-Wellenhof, R.; Malvehy, J.; Menzies, S.; Puig, S.; Rabinovitz, H.; et al. Standardization of terminology in dermoscopy/dermatoscopy: Results of the third consensus conference of the International Society of Dermoscopy. J. Am. Acad. Dermatol. 2016, 74, 1093–1106. [Google Scholar] [CrossRef]
- Tran, T.; Ternov, N.K.; Weber, J.; Barata, C.; Berry, E.G.; Doan, H.Q.; Marghoob, A.A.; Seiverling, E.V.; Sinclair, S.; Stein, J.A.; et al. Instructional Strategies to Enhance Dermoscopic Image Interpretation Education: A Review of the Literature. Dermatol. Pract. Concept. 2022, 12, e2022189. [Google Scholar] [CrossRef]
- Halip, I.-A.; Vâţă, D.; Statescu, L.; Salahoru, P.; Patraşcu, A.I.; Olinici, D.T.; Tarcau, B.; Popescu, I.-A.; Mocanu, M.; Constantin, A.-M.; et al. Assessment of Basal Cell Carcinoma Using Dermoscopy and High Frequency Ultrasound Examination. Diagnostics 2022, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Janowska, A.; Oranges, T.; Granieri, G.; Romanelli, M.; Fidanzi, C.; Iannone, M.; Dini, V. Non-invasive imaging techniques in presurgical margin assessment of basal cell carcinoma: Current evidence. Ski. Res. Technol. 2023, 29, e13271. [Google Scholar] [CrossRef] [PubMed]
| First Author | Year of Publication | Country | Study Design | Study Period | Sample Size | Lesions | Mean Age (Years) | Males (%) | Cancer Type (BCC, SCC) | Comparison Group (Standard Visual, etc.) | Margin Clearence | Margin Clearance Rate (%) | Recurrences | Risk of Bias Assessment | Comments/Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liu Z [4] | 2023 | China | Cohort | 2016–2022 | 90 | 90 | 74 | 56.7 | SCC | NA | 90 | 100% | 0% | Moderate | Wider dermoscopic borders compared to those identified visually (38.9%) |
| Sushil SSJ [7] | 2023 | India | Cohort | 2015–2020 | 17 | 17 | 60.8 | 64.7 | BCC | NA | 17 | 100% | 0% | Moderate | Preoperative dermoscopy was useful in correlating the clinical subtypes with the final histopathological diagnosis in all the cases |
| Chen W [8] | 2022 | China | Cohort | 2016–2020 | 107 | 107 | 64.2 | 61.7 | BCC | NA | 105 | 98.1 | 0 | Moderate | Eighten of 107 (16.8%) patients showed that the visual margin was inadequate compared to the dermoscopy-detected margin |
| Lupu M [9] | 2021 | Romania | Cohort | 2018–2020 | 18 | 20 | 71.5 | 33.3 | BCC | NA | 21 | 72.41 | NA | Moderate | 32 margins in 20 BCC were explored, 3 margins destroyed during tissue processing, leaving 29 margins in the final analysis |
| Conforti C [3] | 2020 | Italy | Cohort | 2018–2019 | 88 | 88 | 72.8 | 57.9 | BCC | NA | NA | NA | NA | Moderate | Differences between Clinical and Dermoscopic Margins |
| Ito T [10] | 2015 | Japan | Cohort | 2006–2013 | 263 | 288 | 71.2 | 46 | BCC | NA | 285 | 99.3 | 0% | Moderate | Dermoscopically determined borders almost exactly corresponded to the histopathological findings |
| Carducci M [11] | 2013 | Italy | Cohort | 2008–2011 | 48 | 48 | 81 | 54.1 | SCC | 46 (clinical) | 45 | 94% | 0% | Moderate | Margin possitivity rate in clinical detection group was significantly higher (17%) than in dermoscopic group (6%) |
| Carducci M [12] | 2011 | Italy | Cohort | 2008–2009 | 44 | 44 | 71.8 | 52.2 | BCC | 40 (clinical) | 41 | 93% | NA | Moderate | Margin possitivity rate in clinical detection group was significantly higher (20%) than in dermoscopic group (7%) |
| Caresana G [13] | 2010 | Italy | Cohort | 2007–2009 | 200 | 200 | NA | NA | BCC | NA | 197 | 98.50% | 0.00% | Moderate | In 69 cases (34.5%) dermoscopic evaluation showed a larger peripheral extension, compared to clinical measurements |
| Liu Z [4] | 2023 | China | Cohort | 2016–2022 | 90 | 90 | 74 | 56.7 | SCC | NA | 90 | 100% | 0% | Moderate | Wider dermoscopic borders compared to those identified visually (38.9%) |
| Sushil SSJ [7] | 2023 | India | Cohort | 2015–2020 | 17 | 17 | 60.8 | 64.7 | BCC | NA | 17 | 100% | 0% | Moderate | Preoperative dermoscopy was useful in correlating the clinical subtypes with the final histopathological diagnosis in all the cases |
| Chen W [8] | 2022 | China | Cohort | 2016–2020 | 107 | 107 | 64.2 | 61.7 | BCC | NA | 105 | 98.1 | 0 | Moderate | Eighten of 107 (16.8%) patients showed that the visual margin was inadequate compared to the dermoscopy-detected margin |
| Lupu M [9] | 2021 | Romania | Cohort | 2018–2020 | 18 | 20 | 71.5 | 33.3 | BCC | NA | 21 | 72.41 | NA | Moderate | 32 margins in 20 BCC were explored, 3 margins destroyed during tissue processing, leaving 29 margins in the final analysis |
| Conforti C [3] | 2020 | Italy | Cohort | 2018–2019 | 88 | 88 | 72.8 | 57.9 | BCC | NA | NA | NA | NA | Moderate | Differences between Clinical and Dermoscopic Margins |
| Ito T [10] | 2015 | Japan | Cohort | 2006–2013 | 263 | 288 | 71.2 | 46 | BCC | NA | 285 | 99.3 | 0% | Moderate | Dermoscopically determined borders almost exactly corresponded to the histopathological findings |
| Carducci M [11] | 2013 | Italy | Cohort | 2008–2011 | 48 | 48 | 81 | 54.1 | SCC | 46 (clinical) | 45 | 94% | 0% | Moderate | Margin possitivity rate in clinical detection group was significantly higher (17%) than in dermoscopic group (6%) |
| Carducci M [12] | 2011 | Italy | Cohort | 2008–2009 | 44 | 44 | 71.8 | 52.2 | BCC | 40 (clinical) | 41 | 93% | NA | Moderate | Margin possitivity rate in clinical detection group was significantly higher (20%) than in dermoscopic group (7%) |
| Caresana G [13] | 2010 | Italy | Cohort | 2007–2009 | 200 | 200 | NA | NA | BCC | NA | 197 | 98.50% | 0.00% | Moderate | In 69 cases (34.5%) dermoscopic evaluation showed a larger peripheral extension, compared to clinical measurements |
| Parameter | Clinical (Visual/Naked-Eye) Inspection | Dermoscopy-Guided Assessment |
| Tool used | Unassisted visual examination | Handheld dermoscope (polarized or non-polarized light) |
| Magnification | None or minimal (unaided eye) | Typically 10× magnification |
| Light source | Ambient/room light | Polarized or cross-polarized light |
| Border definition | Based on visible color, texture, elevation | Enhanced visualization of subclinical tumor margins |
| Common features evaluated | Lesion size, color, induration, ulceration, surface irregularity | Arborizing vessels, blue-gray ovoid nests, leaf-like areas, pink-white areas, short telangiectasias, pigment network, peripheral structures |
| Margin determination | Estimation based on lesion appearance and palpation | Identification of subtle extensions beyond visible border |
| Operator dependency | Subjective, based on clinical experience | Subjective but aided by pattern recognition and dermoscopic criteria |
| Documentation | Often non-standardized | Can be photo-documented and reproducible |
| Limitations | May miss subclinical extensions; prone to underestimation in cosmetically sensitive sites | Requires training; interobserver variability in interpretation |
| Clinical utility | Standard method; quick and widely used | Adjunctive method; enhances margin precision especially in BCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysostomidis, A.; Kostares, E.; Saramantos, A.; Lallas, K.; Lallas, A.; Kantzanou, M.; Tilaveridis, I.; Kyrgidis, A. Clinical Versus Dermoscopic Evaluation of Tumor Margins Prior to Surgical Excision—A Systematic Review. J. Clin. Med. 2025, 14, 6014. https://doi.org/10.3390/jcm14176014
Chrysostomidis A, Kostares E, Saramantos A, Lallas K, Lallas A, Kantzanou M, Tilaveridis I, Kyrgidis A. Clinical Versus Dermoscopic Evaluation of Tumor Margins Prior to Surgical Excision—A Systematic Review. Journal of Clinical Medicine. 2025; 14(17):6014. https://doi.org/10.3390/jcm14176014
Chicago/Turabian StyleChrysostomidis, Anestis, Evangelos Kostares, Antonios Saramantos, Konstantinos Lallas, Aimilios Lallas, Maria Kantzanou, Ioannis Tilaveridis, and Athanassios Kyrgidis. 2025. "Clinical Versus Dermoscopic Evaluation of Tumor Margins Prior to Surgical Excision—A Systematic Review" Journal of Clinical Medicine 14, no. 17: 6014. https://doi.org/10.3390/jcm14176014
APA StyleChrysostomidis, A., Kostares, E., Saramantos, A., Lallas, K., Lallas, A., Kantzanou, M., Tilaveridis, I., & Kyrgidis, A. (2025). Clinical Versus Dermoscopic Evaluation of Tumor Margins Prior to Surgical Excision—A Systematic Review. Journal of Clinical Medicine, 14(17), 6014. https://doi.org/10.3390/jcm14176014