The Role of Biomarkers in Surveillance of Ulcerative Colitis-Associated Colorectal Cancer: A Scoping Review
Abstract
1. Introduction
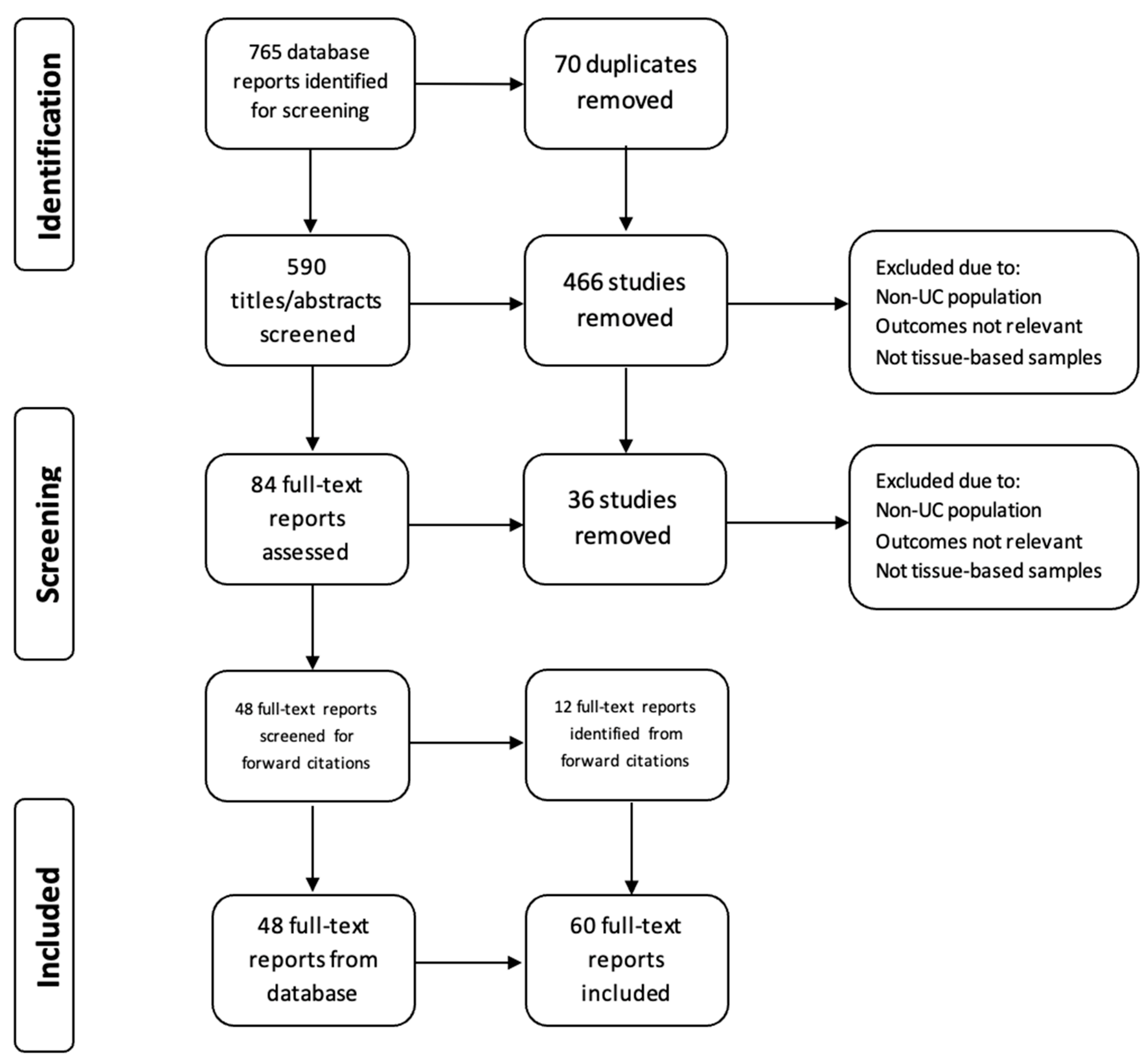
2. Materials and Methods
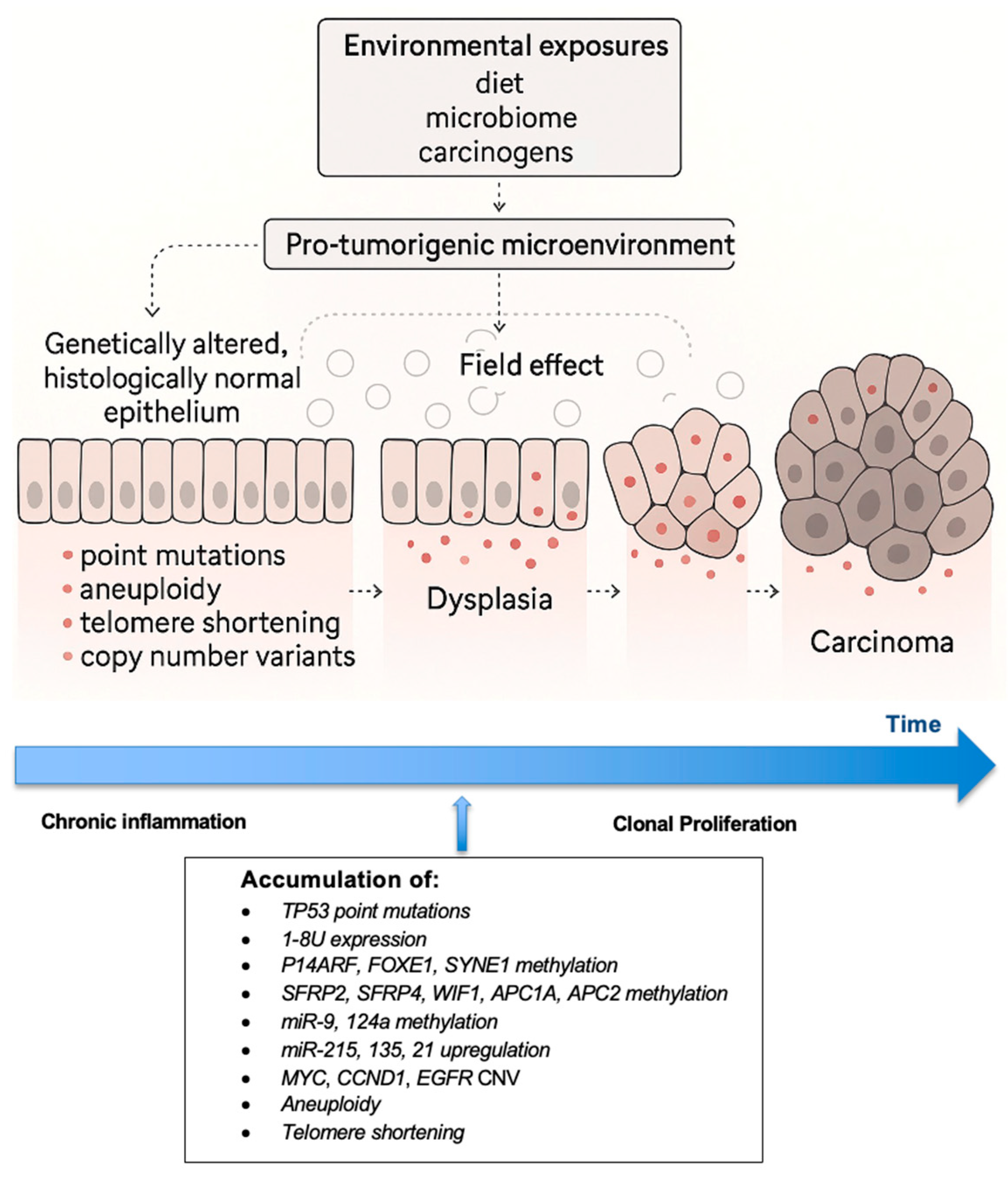
3. Pathogenesis
4. Point Mutations
5. Methylation Patterns
6. microRNA
7. Other Biomarkers
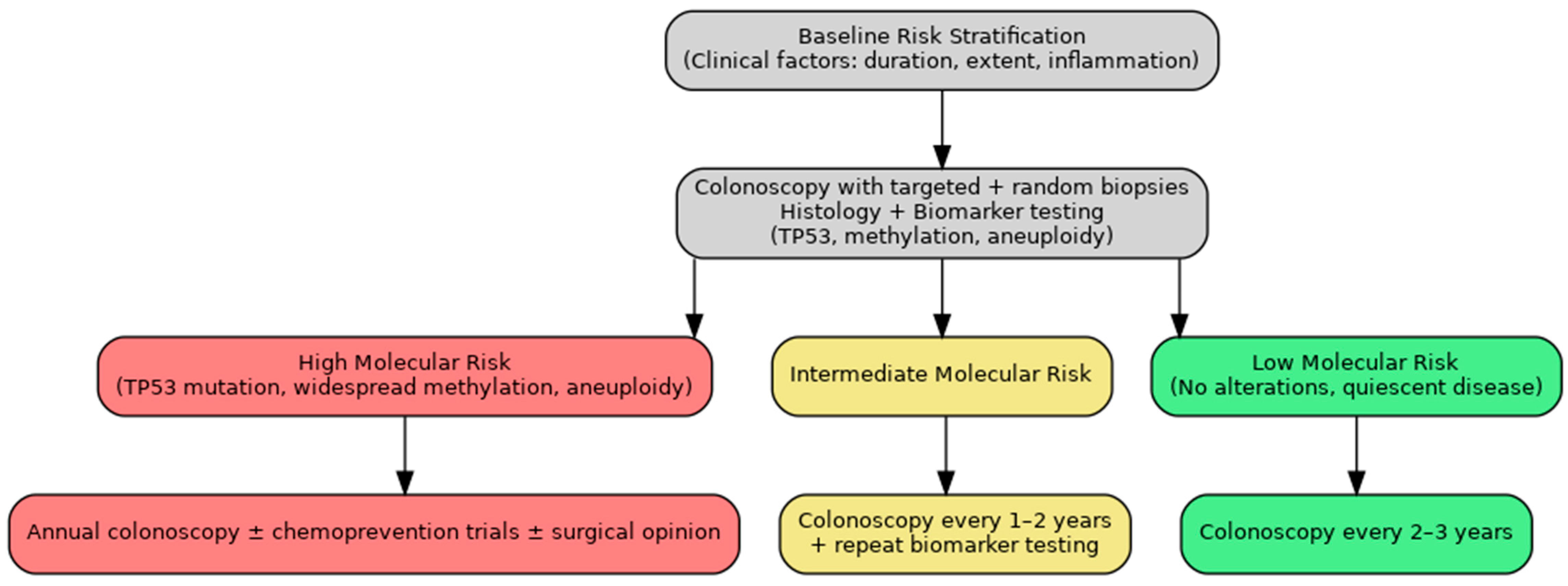
8. Discussion
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UC | Ulcerative colitis |
| UC-CRC | Ulcerative colitis-associated colorectal cancer |
| IBD | Inflammatory bowel disease |
| CRC | Colorectal cancer |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| NGS | Next-generation sequencing |
| IHC | Immunohistochemistry |
| CNV | Copy number variation |
| lpWGS | Low-pass whole genome sequencing |
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Su, T.; Xiao, T.; Xu, H.; Zhao, S. Colorectal cancer risk in ulcerative colitis: An updated population-based systematic review and meta-analysis. eClinicalMedicine 2025, 84, 103269. [Google Scholar] [CrossRef]
- Lutgens, M.W.; van Oijen, M.G.; van der Heijden, G.J.; Vleggaar, F.P.; Siersema, P.D.; Oldenburg, B. Declining risk of colorectal cancer in inflammatory bowel disease: An updated meta-analysis of population-based cohort studies. Inflamm. Bowel Dis. 2013, 19, 789–799. [Google Scholar] [CrossRef]
- Huang, L.C.; Merchea, A. Dysplasia and Cancer in Inflammatory Bowel Disease. Surg. Clin. N. Am. 2017, 97, 627–639. [Google Scholar] [CrossRef]
- Baker, K.T.; Salk, J.J.; Brentnall, T.A.; Risques, R.A. Precancer in ulcerative colitis: The role of the field effect and its clinical implications. Carcinogenesis 2018, 39, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lai, L.A.; Brentnall, T.A.; Pan, S. Biomarkers for colitis-associated colorectal cancer. World J. Gastroenterol. 2016, 22, 7882–7891. [Google Scholar] [CrossRef]
- Scarpa, M.; Castagliuolo, I.; Castoro, C.; Pozza, A.; Scarpa, M.; Kotsafti, A.; Angriman, I. Inflammatory colonic carcinogenesis: A review on pathogenesis and immunosurveillance mechanisms in ulcerative colitis. World J. Gastroenterol. 2014, 20, 6774–6785. [Google Scholar] [CrossRef]
- Riddell, R.H.; Goldman, H.; Ransohoff, D.F.; Appelman, H.D.; Fenoglio, C.M.; Haggitt, R.C.; Hren, C.; Correa, P.; Hamilton, S.R.; Morson, B.C.; et al. Dysplasia in inflammatory bowel disease: Standardized classification with provisional clinical applications. Hum. Pathol. 1983, 14, 931–968. [Google Scholar] [CrossRef]
- Fujii, S.; Fujimori, T.; Chiba, T.; Terano, A. Efficacy of surveillance and molecular markers for detection of ulcerative colitis-associated colorectal neoplasia. J. Gastroenterol. 2003, 38, 1117–1125. [Google Scholar] [CrossRef]
- Bernstein, C.N. Cancer surveillance in inflammatory bowel disease. Curr. Gastroenterol. Rep. 1999, 1, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Risques, R.A.; Rabinovitch, P.S.; Brentnall, T.A. Cancer surveillance in inflammatory bowel disease: New molecular approaches. Curr. Opin. Gastroenterol. 2006, 22, 382–390. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Gudjonsson, T.; Nielsen, O.H.; Vainer, B.; Seidelin, J.B. Pathogenesis and biomarkers of carcinogenesis in ulcerative colitis. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 395–404. [Google Scholar] [CrossRef]
- Romano, M.; DEFrancesco, F.; Zarantonello, L.; Ruffolo, C.; A Ferraro, G.; Zanus, G.; Giordano, A.; Bassi, N.; Cillo, U. From Inflammation to Cancer in Inflammatory Bowel Disease: Molecular Perspectives. Anticancer Res. 2016, 36, 1447–1460. [Google Scholar]
- Hisamatsu, T.; Watanabe, M.; Ogata, H.; Ezaki, T.; Hozawa, S.; Ishii, H.; Kanai, T.; Hibi, T. Interferon-inducible gene family 1-8U expression in colitis-associated colon cancer and severely inflamed mucosa in ulcerative colitis. Cancer Res. 1999, 59, 5927–5931. [Google Scholar]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.R.; Bakir, I.A.; Hart, A.L.; Graham, T.A. Clonal evolution of colorectal cancer in IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.T.; Kővári, B.P.; Lauwers, G.Y. The Significance of Flat/Invisible Dysplasia and Nonconventional Dysplastic Subtypes in Inflammatory Bowel Disease: A Review of Their Morphologic, Clinicopathologic, and Molecular Characteristics. Adv. Anat. Pathol. 2022, 29, 15–24. [Google Scholar] [CrossRef]
- Du, L.; Kim, J.J.; Shen, J.; Chen, B.; Dai, N. KRAS and TP53 mutations in inflammatory bowel disease-associated colorectal cancer: A meta-analysis. Oncotarget 2017, 8, 22175–22186. [Google Scholar] [CrossRef]
- Itzkowitz, S.H. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2006, 35, 553–571. [Google Scholar] [CrossRef] [PubMed]
- George, N.E.; Sarojini Thomas, G. Role of immunohistochemical expression of p53 in intestinal epithelial cells to detect dysplasia in patients with inflammatory bowel disease. J. Clin. Diagn. Res. 2022, 16, EC26–EC29. [Google Scholar] [CrossRef]
- Yin, J.; Harpaz, N.; Tong, Y.; Huang, Y.; Laurin, J.; Greenwald, B.D.; Hontanosas, M.; Newkirk, C.; Meltzer, S.J. p53 point mutations in dysplastic and cancerous ulcerative colitis lesions. Gastroenterology 1993, 104, 1633–1639. [Google Scholar] [CrossRef]
- Harpaz, N.; Peck, A.L.; Yin, J.; Fiel, I.; Hontanosas, M.; Tong, T.R.; Laurin, J.N.; Abraham, J.M.; Greenwald, B.D.; Meltzer, S.J. p53 protein expression in ulcerative colitis-associated colorectal dysplasia and carcinoma. Hum. Pathol. 1994, 25, 1069–1074. [Google Scholar] [CrossRef]
- Brentnall, T.A.; Crispin, D.A.; Rabinovitch, P.S.; Haggitt, R.C.; Rubin, C.E.; Stevens, A.C.; Burmer, G.C. Mutations in the p53 gene: An early marker of neoplastic progression in ulcerative colitis. Gastroenterology 1994, 107, 369–378. [Google Scholar] [CrossRef]
- Fujii, S.; Fujimori, T.; Chiba, T. Usefulness of analysis of p53 alteration and observation of surface microstructure for diagnosis of ulcerative colitis-associated colorectal neoplasia. J. Exp. Clin. Cancer Res. 2003, 22, 107–115. [Google Scholar] [PubMed]
- Hirsch, D.; Hardt, J.; Sauer, C.; Heselmeyer-Hadded, K.; Witt, S.H.; Kienle, P.; Ried, T.; Gaiser, T. Molecular characterization of ulcerative colitis-associated colorectal carcinomas. Mod. Pathol. 2021, 34, 1153–1166. [Google Scholar] [CrossRef]
- Xie, H.; Xiao, S.Y.; Pai, R.; Jiang, W.; Shadrach, B.; Carver, P.; Wang, Y.; Shen, B.; Zhou, W.; Liu, X. Diagnostic utility of TP53 and cytokeratin 7 immunohistochemistry in idiopathic inflammatory bowel disease-associated neoplasia. Mod. Pathol. 2014, 27, 303–313. [Google Scholar] [CrossRef]
- Singhi, A.D.; Waters, K.M.; Makhoul, E.P.; Parian, A.; Lazarev, M.G.; Proksell, S.S.; Dueker, J.M.; Schwartz, M.B.; Wald, A.I.; Nikiforova, M.N.; et al. Targeted next-generation sequencing supports serrated epithelial change as an early precursor to inflammatory bowel disease-associated colorectal neoplasia. Hum. Pathol. 2021, 112, 9–19. [Google Scholar] [CrossRef]
- Horvath, B.; Liu, G.; Wu, X.; Lai, K.K.; Shen, B.; Liu, X. Overexpression of p53 predicts colorectal neoplasia risk in patients with inflammatory bowel disease and mucosa changes indefinite for dysplasia. Gastroenterol. Rep. 2015, 3, 344–349. [Google Scholar] [CrossRef]
- Gerrits, M.M.; Chen, M.; Theeuwes, M.; van Dekken, H.; Sikkema, M.; Steyerberg, E.W.; Lingsma, H.F.; Siersema, P.D.; Xia, B.; Kusters, J.G.; et al. Biomarker-based prediction of inflammatory bowel disease-related colorectal cancer: A case-control study. Cell. Oncol. 2011, 34, 107–117. [Google Scholar] [CrossRef]
- Holzmann, M.; Weis-Klemm, B.; Klump, C.-J.; Hsieh, F.; Borchard, M.; Gregor, R.; Porschen, K. Comparison of flow cytometry and histology with mutational screening for p53 and Kiras mutations in surveillance of patients with long-standing ulcerative colitis. Scand. J. Gastroenterol. 2001, 36, 1320–1326. [Google Scholar] [CrossRef]
- Fujii, S.; Katsumata, D.; Fujimori, T. Limits of diagnosis and molecular markers for early detection of ulcerative colitis-associated colorectal neoplasia. Digestion 2008, 77 (Suppl. S1), 2–12. [Google Scholar] [CrossRef]
- Heinzlmann, M.; Lang, S.M.; Neynaber, S.; Reinshagen, M.; Emmrich, J.; Stratakis, D.F.; Heldwein, W.; Wiebecke, B.; Loeschke, K. Screening for p53 and K-ras mutations in whole-gut lavage in chronic inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2002, 14, 1061–1066. [Google Scholar] [CrossRef]
- Johnson, D.H.; Taylor, W.R.; Aboelsoud, M.M.; Foote, P.H.; Yab, T.C.; Cao, X.; Smyrk, T.C.; Loftus, E.V.; Mahoney, D.W.; Ahlquist, D.A.; et al. DNA Methylation and Mutation of Small Colonic Neoplasms in Ulcerative Colitis and Crohn’s Colitis: Implications for Surveillance. Inflamm. Bowel Dis. 2016, 22, 1559–1567. [Google Scholar] [CrossRef]
- Moriyama, T.; Matsumoto, T.; Nakamura, S.; Jo, Y.; Mibu, R.; Yao, T.; Iida, M. Hypermethylation of p14 (ARF) may be predictive of colitic cancer in patients with ulcerative colitis. Dis. Colon. Rectum 2007, 50, 1384–1392. [Google Scholar] [CrossRef]
- Ueda, Y.; Ando, T.; Nanjo, S.; Ushijima, T.; Sugiyama, T. DNA methylation of microRNA-124a is a potential risk marker of colitis-associated cancer in patients with ulcerative colitis. Dig. Dis. Sci. 2014, 59, 2444–2451. [Google Scholar] [CrossRef] [PubMed]
- Papadia, C.; Louwagie, J.; Del Rio, P.; Grooteclaes, M.; Coruzzi, A.; Montana, C.; Novelli, M.; Bordi, C.; Angelis, G.L.D.; Bassett, P.; et al. FOXE1 and SYNE1 genes hypermethylation panel as promising biomarker in colitis-associated colorectal neoplasia. Inflamm. Bowel Dis. 2014, 20, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Toiyama, Y.; Yamamoto, A.; Omura, Y.; Kusunoki, K.; Kusunoki, Y.; Iwata, T.; Fujikawa, H.; Yasuda, H.; Okita, Y.; et al. P1-001—MicroRNA-9 methylation reflect epigenetic drift and identify patients with risk for C-associated colorectal neoplasia. Ann. Oncol. 2019, 30 (Suppl. S6), vi118. [Google Scholar] [CrossRef]
- Beggs, A.D.; Mehta, S.; Deeks, J.J.; James, J.D.; Caldwell, G.M.; Dilworth, M.P.; Stockton, J.D.; Blakeway, D.; Pestinger, V.; Vince, A.; et al. Validation of epigenetic markers to identify colitis associated cancer: Results of module 1 of the ENDCAP-C study. eBioMedicine 2019, 39, 265–271. [Google Scholar] [CrossRef]
- Okayama, H.; Schetter, A.J.; Harris, C.C. MicroRNAs and inflammation in the pathogenesis and progression of colon cancer. Dig. Dis. 2012, 30 (Suppl. S2), 9–15. [Google Scholar] [CrossRef]
- Tan, Y.G.; Zhang, Y.F.; Guo, C.J.; Yang, M.; Chen, M.Y. Screening of differentially expressed microRNA in ulcerative colitis related colorectal cancer. Asian Pac. J. Trop. Med. 2013, 6, 972–976. [Google Scholar] [CrossRef]
- Barberio, B.; Borga, C.; Giada, M.; Zingone, F.; Fassan, M.; Savarino, E. P215 Mir-135b, an Early Biomarker of Sporadic and IBD-Related Colorectal Carcinogenetic Progression. J. Crohn’s Colitis 2024, 18, i540–i541. [Google Scholar] [CrossRef]
- Pekow, J.; Meckel, K.; Dougherty, U.; Haider, H.I.; Deng, Z.; Hart, J.; Rubin, D.T.; Bissonnette, M. Increased mucosal expression of miR-215 precedes the development of neoplasia in patients with long-standing ulcerative colitis. Oncotarget 2018, 9, 20709–20720. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, Z.; Rai, S.N.; Eichenberger, M.R.; Barnes, C.; Dworkin, A.M.; Weller, C.; Cohen, E.; Roberts, H.; Keskey, B.; Petras, R.E.; et al. Differential microRNA expression tracks neoplastic progression in inflammatory bowel disease-associated colorectal cancer. Hum. Mutat. 2012, 33, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Bovell, L.; Shanmugam, C.; Katkoori, V.R.; Zhang, B.; Vogtmann, E.; Grizzle, W.E.; Manne, U. miRNAs are stable in colorectal cancer archival tissue blocks. Front. Biosci. (Elite Ed.) 2012, 4, 1937–1940. [Google Scholar] [CrossRef]
- Salk, J.J.; Bansal, A.; Lai, L.A.; Crispin, D.A.; Ussakli, C.H.; Horwitz, M.S.; Bronner, M.P.; Brentnall, T.A.; Loeb, L.A.; Rabinovitch, P.S.; et al. Clonal expansions and short telomeres are associated with neoplasia in early-onset, but not late-onset, ulcerative colitis. Inflamm. Bowel Dis. 2013, 19, 2593–2602. [Google Scholar] [CrossRef]
- Shivakumar, B.M.; Chakrabarty, S.; Rotti, H.; Seenappa, V.; Rao, L.; Geetha, V.; Tantry, B.V.; Kini, H.; Dharamsi, R.; Pai, C.G.; et al. Comparative analysis of copy number variations in ulcerative colitis associated and sporadic colorectal neoplasia. BMC Cancer 2016, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Meling, G.I.; Clausen, O.P.; Bergan, A.; Schjølberg, A.; Rognum, T.O. Flow cytometric DNA ploidy pattern in dysplastic mucosa, and in primary and metastatic carcinomas in patients with longstanding ulcerative colitis. Br. J. Cancer 1991, 64, 339–344. [Google Scholar] [CrossRef]
- Rubin, C.E.; Haggitt, R.C.; Burmer, G.C.; Brentnall, T.A.; Stevens, A.C.; Levine, D.S.; Dean, P.J.; Kimmey, M.; Perera, D.R.; Rabinovitch, P.S. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology 1992, 103, 1611–1620. [Google Scholar] [CrossRef]
- Löfberg, R.; Tribukait, B.; Ost, A.; Broström, O.; Reichard, H. Flow cytometric DNA analysis in longstanding ulcerative colitis: A method of prediction of dysplasia and carcinoma development? Gut 1987, 28, 1100–1106. [Google Scholar] [CrossRef]
- Rebello, D.; Rebello, E.; Custodio, M.; Xu, X.; Gandhi, S.; Roy, H.K. Field carcinogenesis for risk stratification of colorectal cancer. Adv. Cancer Res. 2021, 151, 305–344. [Google Scholar] [CrossRef]
- Reznicek, E.; Arfeen, M.; Shen, B.; Ghouri, Y.A. Colorectal Dysplasia and Cancer Surveillance in Ulcerative Colitis. Diseases 2021, 9, 86. [Google Scholar] [CrossRef]
- Kornbluth, A.; Sachar, D.B. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 2010, 105, 501–524, Erratum in Am. J. Gastroenterol. 2010, 105, 500. [Google Scholar] [CrossRef]
- East, J.E.; Gordon, M.; Nigam, G.B.; Sinopoulou, V.; Bateman, A.C.; Din, S.; Iacucci, M.; Kabir, M.; Lamb, C.A.; Wilson, A.; et al. British Society of Gastroenterology guidelines on colorectal surveillance in inflammatory bowel disease. Gut. 2025. [Google Scholar] [CrossRef]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Al Sulais, E.; E Axelrad, J.; Balendran, K.; Burisch, J.; de Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohns Colitis 2023, 17, 827–854. [Google Scholar] [CrossRef]
- Iannone, A.; Ruospo, M.; Wong, G.; Principi, M.; Barone, M.; Strippoli, G.F.; Di Leo, A. Chromoendoscopy for Surveillance in Ulcerative Colitis and Crohn’s Disease: A Systematic Review of Randomized Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 1684–1697.e11. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.A.; Ward, B.A.; Mayberry, J.F. How gastroenterologists screen for colonic cancer in ulcerative colitis: An analysis of performance. Gastrointest. Endosc. 2000, 51, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Al Bakir, I.; Curtius, K.; Cresswell, G.D.; Grant, H.E.; Nasreddin, N.; Smith, K.; Nowinski, S.; Guo, Q.; Belnoue-Davis, H.L.; Fisher, J.; et al. Low-coverage whole genome sequencing of low-grade dysplasia strongly predicts advanced neoplasia risk in ulcerative colitis. Gut 2025, 74, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer current: Status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
| Analyte | Biomarker | Clinical Relevance | Reference |
|---|---|---|---|
| DNA | TP53 | TP53 mutations are prevalent in UC-associated dysplasia and cancer, detected in 71–100% of lesions, but absent in uninvolved mucosa. These mutations often occur in non-dysplastic tissue, supporting a field effect. TP53 overexpression on IHC correlates with dysplasia and neoplasia and improves diagnostic specificity when combined with CK7. While some mutations evade IHC detection, PCR analysis detects TP53 mutations in 93% of IHC-negative neoplastic lesions, indicating its value as a sensitive molecular marker. | [5,15,16,20,21,22,23,24,25,26,27,28,29,30,31,32,33] |
| KRAS | KRAS mutations are less frequent in UC-CRC than sporadic CRC, occur later in tumor progression, and often co-occur with TP53 mutations. Due to their low predictive value for dysplasia and poor specificity in stool and tissue samples, KRAS mutations likely have limited clinical utility in UC-CRC surveillance. | [20,34,35] | |
| Aneuploidy | In a prospective study of 25 high-risk UC patients, five of six individuals with aneuploidy developed dysplasia within 1–2.5 years, whereas none of the nineteen patients without aneuploidy progressed during the same period. Similar findings were reported by Lofberg et al., who observed that aneuploidy preceded, coincided with, or followed the development of dysplasia in a cohort of 59 patients [51]. | [10,12,18,48,49,50,51] | |
| 1-8U | IFN-inducible gene 1-8U was highly expressed in UC-associated cancers and chronically inflamed UC mucosa but absent in normal tissue. Its expression was independent of disease duration or extent. | [16] | |
| Telomere shortening | Shortened telomeres and clonal expansions were common in non-dysplastic mucosa of early-onset UC Progressors, distinguishing them from non-progressors with high sensitivity and specificity. These changes, absent in late-onset cases, suggest telomere shortening may serve as a biomarker for cancer risk in early-onset UC-associated colorectal cancer. | [5,47] | |
| Clonal expansions | Clonal expansions have been associated with proximity to dysplasia, and in one study, the mean percentage of clonally expanded mutations distinguished early-onset progressors from non-progressors with 100% sensitivity and 80% specificity. | [47] | |
| Copy number variations | Expression levels of amplified genes such as MYC, CCND1, and EGFR amplified in dysplastic and neoplastic tissues are correlated with disease progression. Low-pass whole genome sequencing (lpWGS) of low-grade dysplasia (LGD) lesions in UC patients can robustly predict progression to advanced neoplasia. A multivariate model achieved an AUC of 0.95 at 5 years. | [48,59] | |
| microRNA | miR-21 miR-135b | miR-21 is significantly upregulated in inflamed UC mucosa and even more elevated in UC-CRC, where it likely promotes inflammation-associated carcinogenesis by enhancing proliferative and anti-apoptotic pathways. miR-135b shows a stepwise increase in expression from non-dysplastic to dysplastic and finally to neoplastic tissues in UC, positioning it as a possible biomarker for tracking malignant progression. | [43] |
| miR-192 miR-194 miR-215 | Differential expression of miR-192, miR-194, and miR-215 has been observed between UC and UC-CRC tissues, highlighting their utility in distinguishing neoplastic from inflamed but non-cancerous tissue. In particular, miR-215 has been shown to be significantly upregulated in non-dysplastic mucosa 1 to 5 years prior to the onset of neoplasia in patients with long-standing UC. | [43,44] | |
| Methylation | p14ARF | In a prospective study, hypermethylation of p14ARF was present in all dysplastic tissues and 26% of non-dysplastic biopsies, suggesting it as an early, pre-dysplastic event. Its presence significantly predicted future dysplasia, supporting its potential role as a biomarker for neoplastic progression in ulcerative colitis. | [36] |
| miR-124a | Elevated methylation levels of miR-124a-3 were correlated with known risk factors such as pancolitis and long disease duration. Patients with both pancolitis and long-standing UC had 7.4-fold higher methylation levels than those without these risk factors. | [37] | |
| miR-9 | miR-9 methylation increases with age, disease duration, and proximity to cancer, and is significantly higher in rectal mucosa from UC-CRC patients compared to controls. Its methylation status has been used to distinguish cancer from non-neoplastic tissues with high accuracy (AUC: 0.94). | [39] | |
| SFRP2, SFRP4, WIF1, APC1A, APC2 | Accurate in detecting pre-cancerous and invasive neoplasia (AUC = 0.83) and dysplasia (AUC = 0.88). For non-neoplastic mucosa, a four-marker panel (APC1A, SFRP4, SFRP5, SOX7) had modest accuracy (AUC = 0.68; 95% CI: 0.62, 0.73) in predicting associated bowel neoplasia through the methylation signature of distant non-neoplastic colonic mucosa. | [40] | |
| SYNE1 FOXE1 ER BMP3 NDRG4 | Hypermethylation of SYNE1 and FOXE1 was detected in 80% and 60% of UC-CRC cases, respectively, but was absent in controls, correlating with disease severity. Additional hypermethylated genes (ER, BMP3, NDRG4) have also been linked to high UC-CRC risk. Single biopsy sampling may suffice due to widespread methylation patterns. | [33,38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kritzinger, J.; Kotrri, G.; Lakatos, P.L.; Bessissow, T.; Wild, G. The Role of Biomarkers in Surveillance of Ulcerative Colitis-Associated Colorectal Cancer: A Scoping Review. J. Clin. Med. 2025, 14, 5979. https://doi.org/10.3390/jcm14175979
Kritzinger J, Kotrri G, Lakatos PL, Bessissow T, Wild G. The Role of Biomarkers in Surveillance of Ulcerative Colitis-Associated Colorectal Cancer: A Scoping Review. Journal of Clinical Medicine. 2025; 14(17):5979. https://doi.org/10.3390/jcm14175979
Chicago/Turabian StyleKritzinger, Justin, Gynter Kotrri, Peter L. Lakatos, Talat Bessissow, and Gary Wild. 2025. "The Role of Biomarkers in Surveillance of Ulcerative Colitis-Associated Colorectal Cancer: A Scoping Review" Journal of Clinical Medicine 14, no. 17: 5979. https://doi.org/10.3390/jcm14175979
APA StyleKritzinger, J., Kotrri, G., Lakatos, P. L., Bessissow, T., & Wild, G. (2025). The Role of Biomarkers in Surveillance of Ulcerative Colitis-Associated Colorectal Cancer: A Scoping Review. Journal of Clinical Medicine, 14(17), 5979. https://doi.org/10.3390/jcm14175979








