Real-World Comparison of FFR and QFR: New Perspectives on the Functional Assessment of Coronary Stenoses
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. FFR Measurement
2.3. QFR Calculation
2.4. Statistical Analysis
3. Results
3.1. Causes of QFR Calculation Failure
3.2. Correlation and Agreement
3.3. Diagnostic Performance of QFR
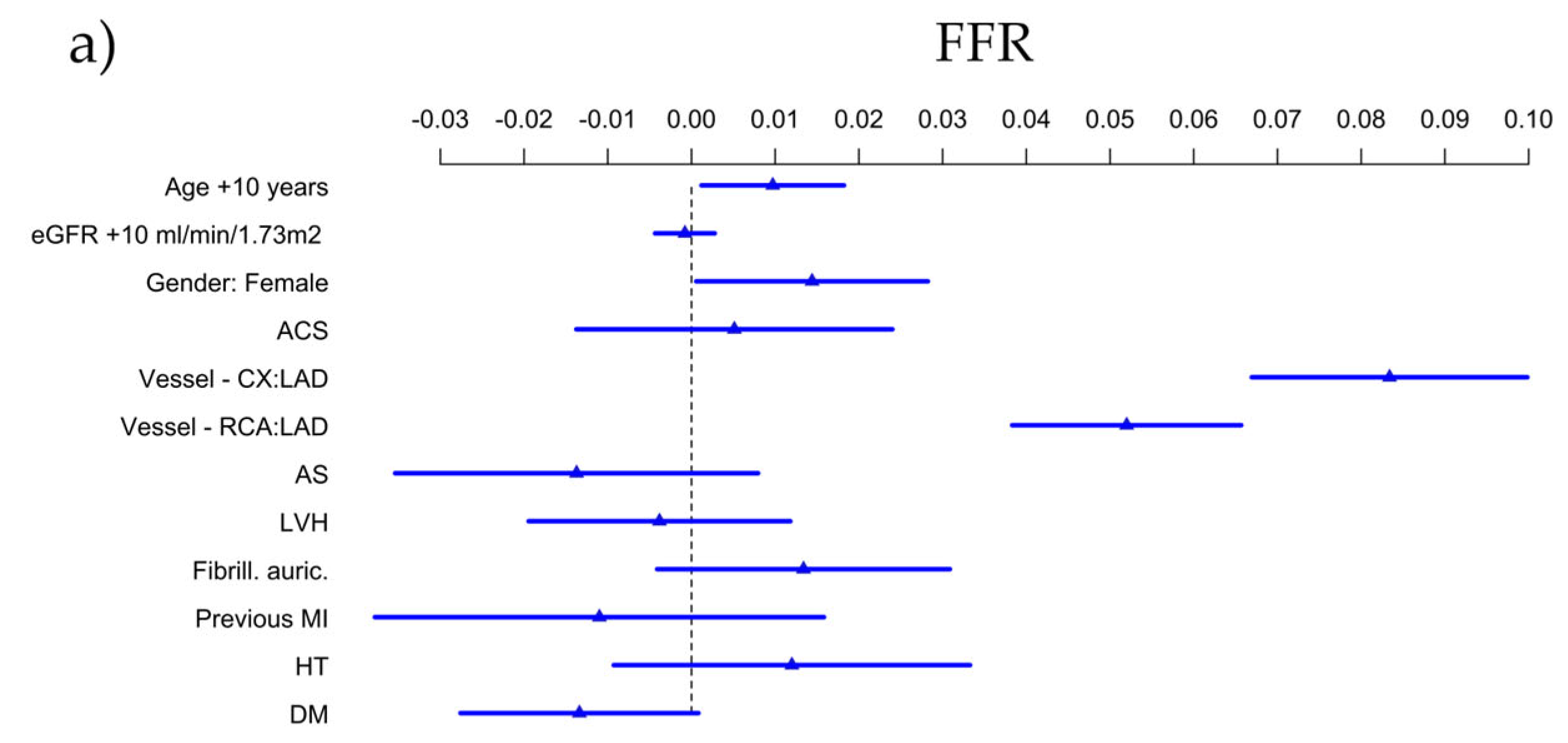
3.4. Predictors of FFR and QFR
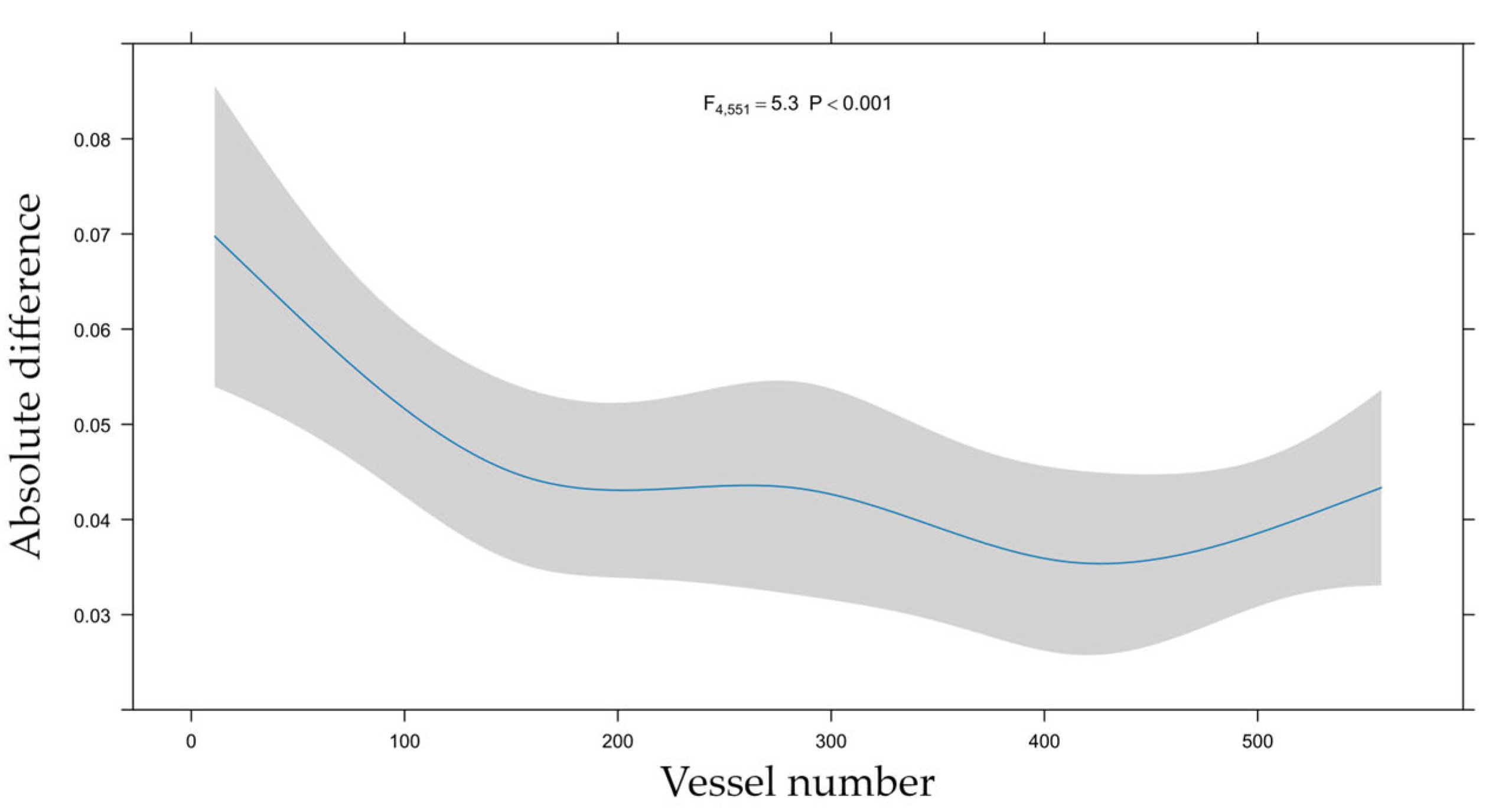
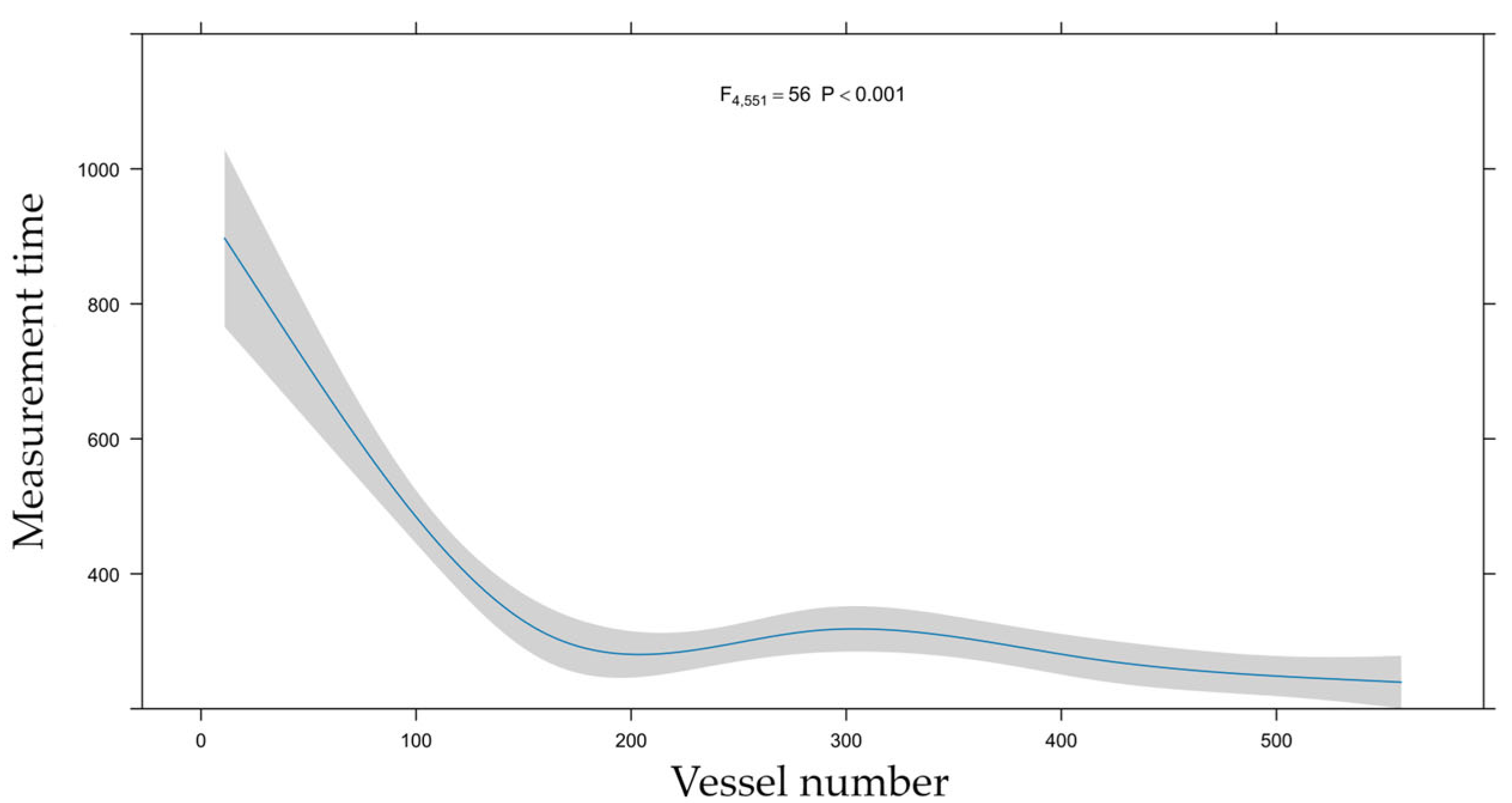
3.5. Learning Curve
4. Discussion
4.1. Comparison of QFR and FFR
4.2. Predictors of FFR, QFR and Their Difference
4.3. Learning Curve Analysis
4.4. Clinical Implications
4.5. Further Aspects of QFR Analysis
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| A-IMR | Angio-Based Index of Microcirculatory Resistance |
| angio-IMR | Angiography-derived hyperemic index of coronary microcirculatory resistance |
| AS | Aortic Stenosis |
| AUC | Area Under the Curve |
| CAD | Coronary Artery Disease |
| CCS | Chronic Coronary Syndrome |
| CFD | Computational Fluid Dynamics |
| CI | Confidence Interval |
| Cx | Left Circumflex Artery |
| DM | Diabetes Mellitus |
| eGFR | Estimated Glomerular Filtration Rate |
| ESC | European Society of Cardiology |
| FFR | Fractional Flow Reserve |
| HT | Hypertension |
| IMR | Index of Microcirculatory Resistance |
| IMRangio | Angiography-derived index of microcirculatory resistance |
| IQR | Interquartile Range |
| LAD | Left Anterior Descending Artery |
| LVH | Left Ventricular Hypertrophy |
| MI | Myocardial Infarction |
| µQFR | Murray law-based Quantitative Flow Ratio |
| QFR | Quantitative Flow Ratio |
| PCI | Percutaneous Coronary Intervention |
| Pd/Pa | Ratio of distal coronary pressure to aortic pressure |
| RCA | Right Coronary Artery |
| STEMI | ST-Elevation Myocardial Infarction |
References
- Fearon, W.F.; Nishi, T.; De Bruyne, B.; Boothroyd, D.B.; Barbato, E.; Tonino, P.; Jüni, P.; Pijls, N.H.; Hlatky, M.A. Clinical outcomes and cost-effectiveness of fractional flow reserve–guided percutaneous coronary intervention in patients with stable coronary artery disease: Three-year follow-up of the FAME 2 trial (fractional flow reserve versus angiography for multivessel evaluation). Circulation 2018, 137, 480–487. [Google Scholar]
- Zimmermann, F.M.; Ferrara, A.; Johnson, N.P.; Van Nunen, L.X.; Escaned, J.; Albertsson, P.; Erbel, R.; Legrand, V.; Gwon, H.-C.; Remkes, W.S.; et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur. Heart J. 2015, 36, 3182–3188. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, R.J.; Oldroyd, K.G. Pharmacological options for inducing maximal hyperaemia during studies of coronary physiology. Catheter. Cardiovasc. Interv. 2008, 71, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Lee, S.H.; Shin, D.; Choi, K.H.; Kim, H.K.; Park, T.K.; Yang, J.H.; Song, Y.B.; Hahn, J.-Y.; Choi, S.-H.; et al. Prognosis and medical cost of measuring fractional flow reserve in percutaneous coronary intervention. JACC Asia 2022, 2, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.D.; Narracott, A.; von Tengg-Kobligk, H.; Soto, D.A.S.; Hsiao, S.; Lungu, A.; Evans, P.; Bressloff, N.W.; Lawford, P.V.; Hose, D.R.; et al. Computational fluid dynamics modelling in cardiovascular medicine. Heart 2016, 102, 18–28. [Google Scholar] [CrossRef]
- Westra, J.; Sejr-Hansen, M.; Koltowski, L.; Mejía-Rentería, H.; Tu, S.; Kochman, J.; Zhang, Y.; Liu, T.; Campo, G.; Hjort, J.; et al. Reproducibility of quantitative flow ratio: The QREP study. EuroIntervention 2022, 17, 1252. [Google Scholar] [CrossRef]
- Tu, S.; Westra, J.; Yang, J.; von Birgelen, C.; Ferrara, A.; Pellicano, M.; Nef, H.; Tebaldi, M.; Murasato, Y.; Lansky, A.; et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: The international multicenter FAVOR pilot study. Cardiovasc. Interv. 2016, 9, 2024–2035. [Google Scholar]
- Xu, B.; Tu, S.; Qiao, S.; Qu, X.; Chen, Y.; Yang, J.; Guo, L.; Sun, Z.; Li, Z.; Tian, F.; et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J. Am. Coll. Cardiol. 2017, 70, 3077–3087. [Google Scholar] [CrossRef]
- Westra, J.; Andersen, B.K.; Campo, G.; Matsuo, H.; Koltowski, L.; Eftekhari, A.; Liu, T.; Di Serafino, L.; Di Girolamo, D.; Escaned, J.; et al. Diagnostic performance of in-procedure angiography-derived quantitative flow reserve compared to pressure-derived fractional flow reserve: The FAVOR II Europe-Japan study. J. Am. Heart Assoc. 2018, 7, e009603. [Google Scholar] [CrossRef]
- Xu, B.; Tu, S.; Song, L.; Jin, Z.; Yu, B.; Fu, G.; Zhou, Y.; Wang, J.; Chen, Y.; Pu, J.; et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): A multicentre, randomised, sham-controlled trial. Lancet 2021, 398, 2149–2159. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [PubMed]
- Andersen, B.K.; Sejr-Hansen, M.; Maillard, L.; Campo, G.; Råmunddal, T.; Stähli, B.E.; Guiducci, V.; Di Serafino, L.; Escaned, J.; Santos, I.A.; et al. Quantitative flow ratio versus fractional flow reserve for coronary revascularisation guidance (FAVOR III Europe): A multicentre, randomised, non-inferiority trial. Lancet 2024, 404, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Härle, T.; Luz, M.; Meyer, S.; Kronberg, K.; Nickau, B.; Escaned, J.; Davies, J.; Elsässer, A. Effect of coronary anatomy and hydrostatic pressure on intracoronary indices of stenosis severity. Cardiovasc. Interv. 2017, 10, 764–773. [Google Scholar] [CrossRef]
- White, N.K.; Edwards, J.E.; Dry, T.J. The relationship of the degree of coronary atherosclerosis with age, in men. Circulation 1950, 1, 645–654. [Google Scholar] [CrossRef]
- Fukunaga, M.; Fujii, K.; Mintz, G.S.; Kawasaki, D.; Nakata, T.; Miki, K.; Imanaka, T.; Tamaru, H.; Shibuya, M.; Masuyama, T. Distribution of pressure gradients along the left anterior descending artery in patients with angiographically normal arteries. Catheter. Cardiovasc. Interv. 2020, 96, E67–E74. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lim, H.S.; Doh, J.H.; Nam, C.W.; Shin, E.S.; Koo, B.K.; Yoon, M.-H.; Tahk, S.-J.; Kang, D.K.; Song, Y.B.; et al. Physiological severity of coronary artery stenosis depends on the amount of myocardial mass subtended by the coronary artery. JACC Cardiovasc. Interv. 2016, 9, 1548–1560. [Google Scholar] [CrossRef]
- Verdoia, M.; Gioscia, R.; Suryapranata, H.; Kedhi, E.; De Luca, G. Impact of aging on the effects of intracoronary adenosine, peak hyperemia and its duration during fractional flow reserve assessment. Coron. Artery Dis. 2021, 32, 625–631. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart disease and stroke statistics: A Report of US and Global Data from the American Heart Association. Circulation 2025, 15, e41–e660. [Google Scholar]
- Fineschi, M.; Guerrieri, G.; Orphal, D.; Palmerini, E.; Münzel, T.; Warnholtz, A.; Pierli, C.; Gori, T. The impact of gender on fractional flow reserve measurements. EuroIntervention 2013, 9, 360–366. [Google Scholar] [CrossRef]
- Engstrøm, T.; Kelbæk, H.; Helqvist, S.; Høfsten, D.E.; Kløvgaard, L.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamäki, K.; Clemmensen, P.; et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3–PRIMULTI): An open-label, randomised controlled trial. Lancet 2015, 386, 665–671. [Google Scholar] [CrossRef]
- Smits, P.C.; Abdel-Wahab, M.; Neumann, F.J.; Klerk, B.M.B.-D.; Lunde, K.; Schotborgh, C.E.; Piroth, Z.; Horak, D.; Wlodarczak, A.; Ong, P.J.; et al. For the Compare—Acute Investigators. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N. Engl. J. Med. 2017, 376, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Piróth, Z.; Boxma-de Klerk, B.M.; Omerovic, E.; Andréka, P.; Fontos, G.; Fülöp, G.; Abdel-Wahab, M.; Neumann, F.J.; Richardt, G.; Abdelghani, M.; et al. The natural history of nonculprit lesions in STEMI. An FFR substudy of the Compare-Acute Trial. J. Am. Coll. Cardiol. Interv. 2020, 13, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Hanratty, C.G.; Koyama, Y.; Rasmussen, H.H.; Nelson, G.I.; Hansen, P.S.; Ward, M.R. Exaggeration of nonculprit stenosis severity during acute myocardial infarction: Implications for immediate multivessel revascularization. J. Am. Coll. Cardiol. 2002, 40, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Sejr-Hansen, M.; Westra, J.; Thim, T.; Christiansen, E.H.; Eftekhari, A.; Kristensen, S.D.; Jakobsen, L.; Götberg, M.; Frøbert, O.; Hoeven, N.W.D.V.; et al. Quantitative flow ratio for immediate assessment of nonculprit lesions in patients with ST-segment elevation myocardial infarction-An iSTEMI substudy. Catheter. Cardiovasc. Interv. 2019, 94, 686–692. [Google Scholar] [CrossRef]
- Lauri, F.; Macaya, F.; Mejía-Rentería, H.; Goto, S.; Yeoh, J.; Nakayama, M.; Quirós, A.; Liontou, C.; Pareek, N.; Fernández-Ortíz, A.; et al. Collaborators. Angiography-derived functional assessment of non-culprit coronary stenoses in primary percutaneous coronary intervention. EuroIntervention 2020, 15, e1594–e1601. [Google Scholar] [CrossRef]
- Faria, D.; Hennessey, B.; Shabbir, A.; Mejía-Rentería, H.; Wang, L.; Lee, J.M.; Matsuo, H.; Biscaglia, S.; Koo, B.K.; Xu, B.; et al. Functional coronary angiography for the assessment of the epicardial vessels and the microcirculation. EuroIntervention 2023, 19, 203–221. [Google Scholar] [CrossRef]
- Tu, S.; Ding, D.; Chang, Y.; Li, C.; Wijns, W.; Xu, B. Diagnostic accuracy of quantitative flow ratio for assessment of coronary stenosis significance from a single angiographic view: A novel method based on bifurcation fractal law. Catheter. Cardiovasc. Interv. 2021, 97, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Salihu, A.; Zulauff, J.; Gadiri, M.A.; Metzinger, A.; Muller, J.; Skalidis, I.; Meier, D.; Noirclerc, N.; Mauler-Wittwer, S.; Zimmerli, A.; et al. Head-to-Head Comparison of Learning Curves Between QFR and FFRangio Software Users. Catheter. Cardiovasc. Interv. 2025, 105, 692–697. [Google Scholar] [CrossRef]
- Dobric, M.; Furtula, M.; Tešic, M.; Timčic, S.; Borzanovic, D.; Lazarevic, N.; Lipovac, M.; Farkic, M.; Ilic, I.; Boljevic, D.; et al. Current status and future perspectives of fractional flow reserve derived from invasive coronary angiography. Front. Cardiovasc. Med. 2023, 10, 1181803. [Google Scholar] [CrossRef]
- Biscaglia, S.; Tebaldi, M.; Brugaletta, S.; Cerrato, E.; Erriquez, A.; Passarini, G.; Ielasi, A.; Spitaleri, G.; Girolamo, D.D.; Mezzapelle, G.; et al. Prognostic value of QFR measured immediately after successful stent implantation: The international multicenter prospective HAWKEYE study. J. Am. Coll. Cardiol. Interv. 2019, 12, 2079–2088. [Google Scholar] [CrossRef]
- Csanádi, B.; Ferenci, T.; Gál, R.; Bora, N.; Piróth, Z. Correlation and Relative Prognostic Power of Post–Percutaneous Coronary Intervention Fractional Flow Reserve and Quantitative Flow Ratio. J. Am. Heart Assoc. 2025, 14, e040969. [Google Scholar] [CrossRef]
- Maria, G.L.D.; Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Oxford Acute Myocardial Infarction (OXAMI) Study Investigators; et al. Angiography-derived index of microcirculatory resistance as a novel, pressure-wire-free tool to assess coronary microcirculation in ST elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2020, 36, 1395–1406. [Google Scholar] [CrossRef]
- Tebaldi, M.; Biscaglia, S.; Di Girolamo, D.; Erriquez, A.; Penzo, C.; Tumscitz, C.; Campo, G. Angio-Based Index of Microcirculatory Resistance for the Assessment of the Coronary Resistance: A Proof of Concept Study. J. Interv. Cardiol. 2020, 1, 8887369. [Google Scholar] [CrossRef]
- Mejia-Renteria, H.; Lee, J.M.; Choi, K.H.; Lee, S.H.; Wang, L.; Kakuta, T.; Koo, B.K.; Escaned, J. Coronary microcirculation assessment using functional angiography: Development of a wire-free method applicable to conventional coronary angiograms. Catheter. Cardiovasc. Interv. 2021, 98, 1027–1037. [Google Scholar] [CrossRef]


| Patient Characteristics | Vessel Characteristics | ||||
|---|---|---|---|---|---|
| Number of patients; n | 435 | Number of vessels; n | 568 | ||
| Age; years (IQR) | 68.5 | (61–75) | Type of vessels | ||
| Female sex; n (%) | 146 | (34%) | LAD; n (%) | 302 | (53%) |
| Hypertension; n (%) | 363 | (83%) | Cx; n (%) | 120 | (21%) |
| Diabetes mellitus; n (%) | 170 | (39%) | RCA; n (%) | 146 | (26%) |
| Acute coronary syndrome; n (%) | 60 | (14%) | |||
| Significant aortic stenosis; n (%) | 48 | (11%) | Prior myocardial infarction in the territory; n (%) | 51 | (9%) |
| eGFR; ml/min/1.73 m2 (IQR) | 71 | (59–84) | |||
| Atrial fibrillation during exam; n (%) | 69 | (12%) | |||
| FFR | QFR | Absolute Difference | Measurement Time (s) | |
|---|---|---|---|---|
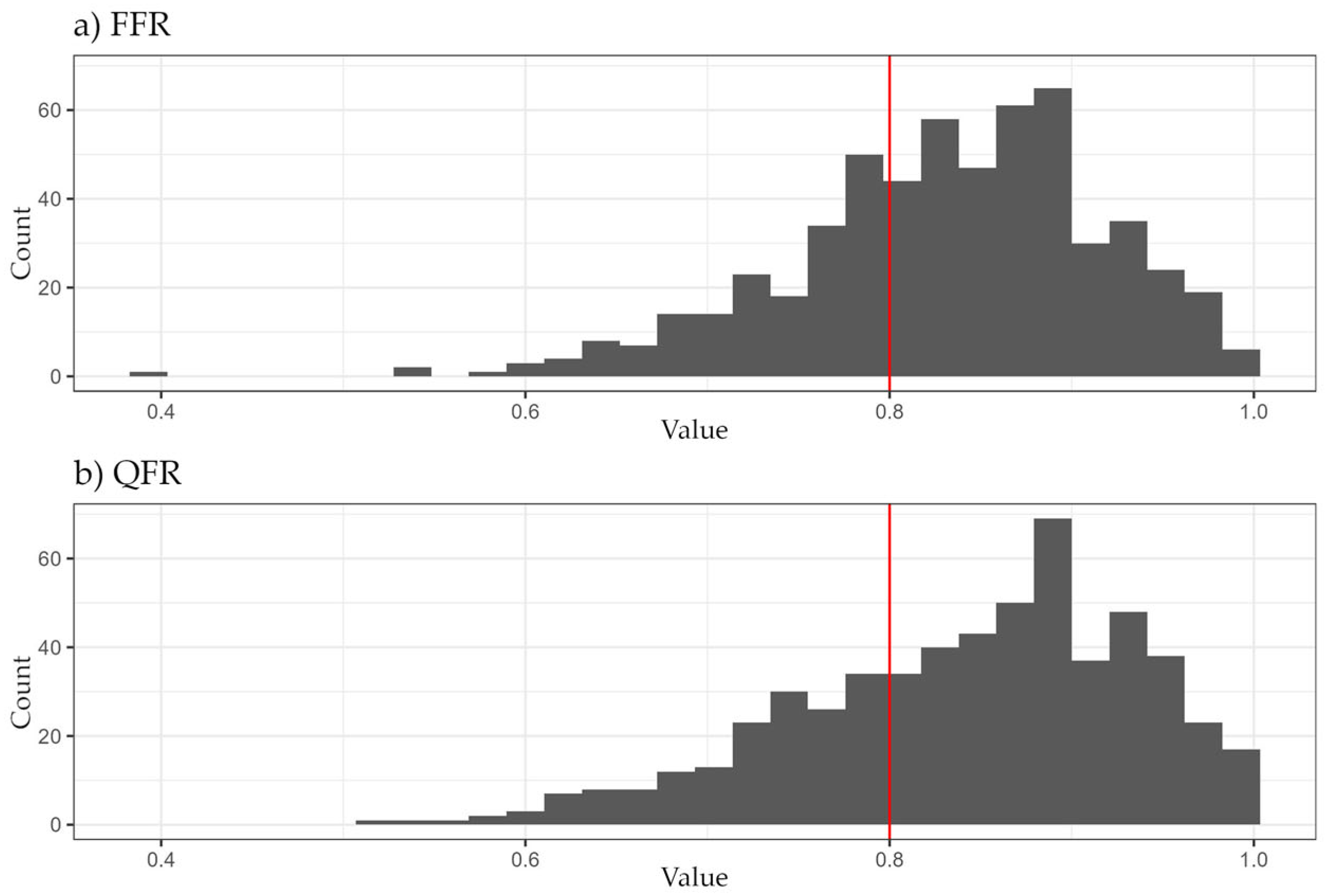
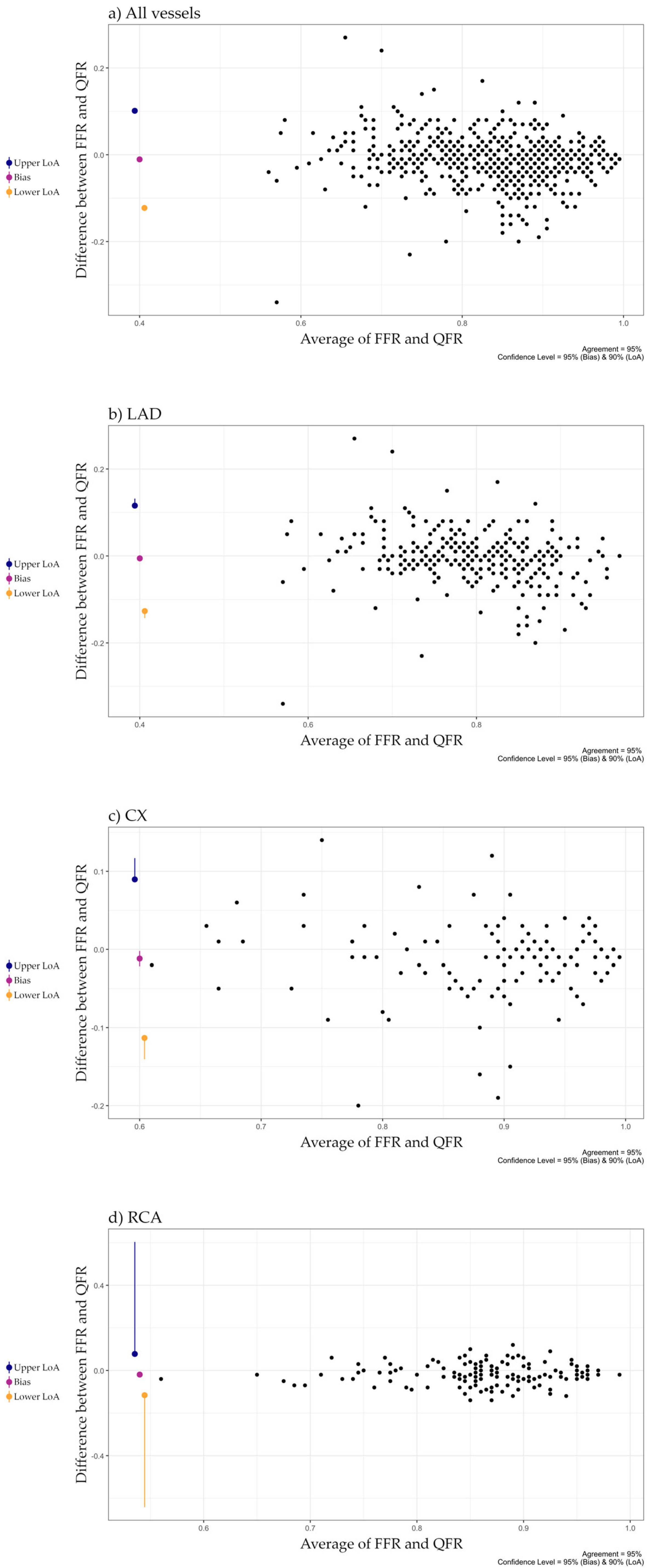
| All vessels | 0.84 (0.78, 0.89) | 0.85 (0.78, 0.92) | 0.03 (0.01, 0.06) | 271 (206, 374) |
| Type of vessels | ||||
| LAD | 0.81 (0.76, 0.86) | 0.81 (0.75, 0.87) | 0.03 (0.01, 0.06) | 302 (222, 437) |
| CX | 0.91 (0.83, 0.94) | 0.92 (0.85, 0.96) | 0.03 (0.01, 0.05) | 257 (211, 333) |
| RCA | 0.87 (0.82, 0.90) | 0.88 (0.84, 0.93) | 0.03 (0.02, 0.06) | 236 (191, 329) |
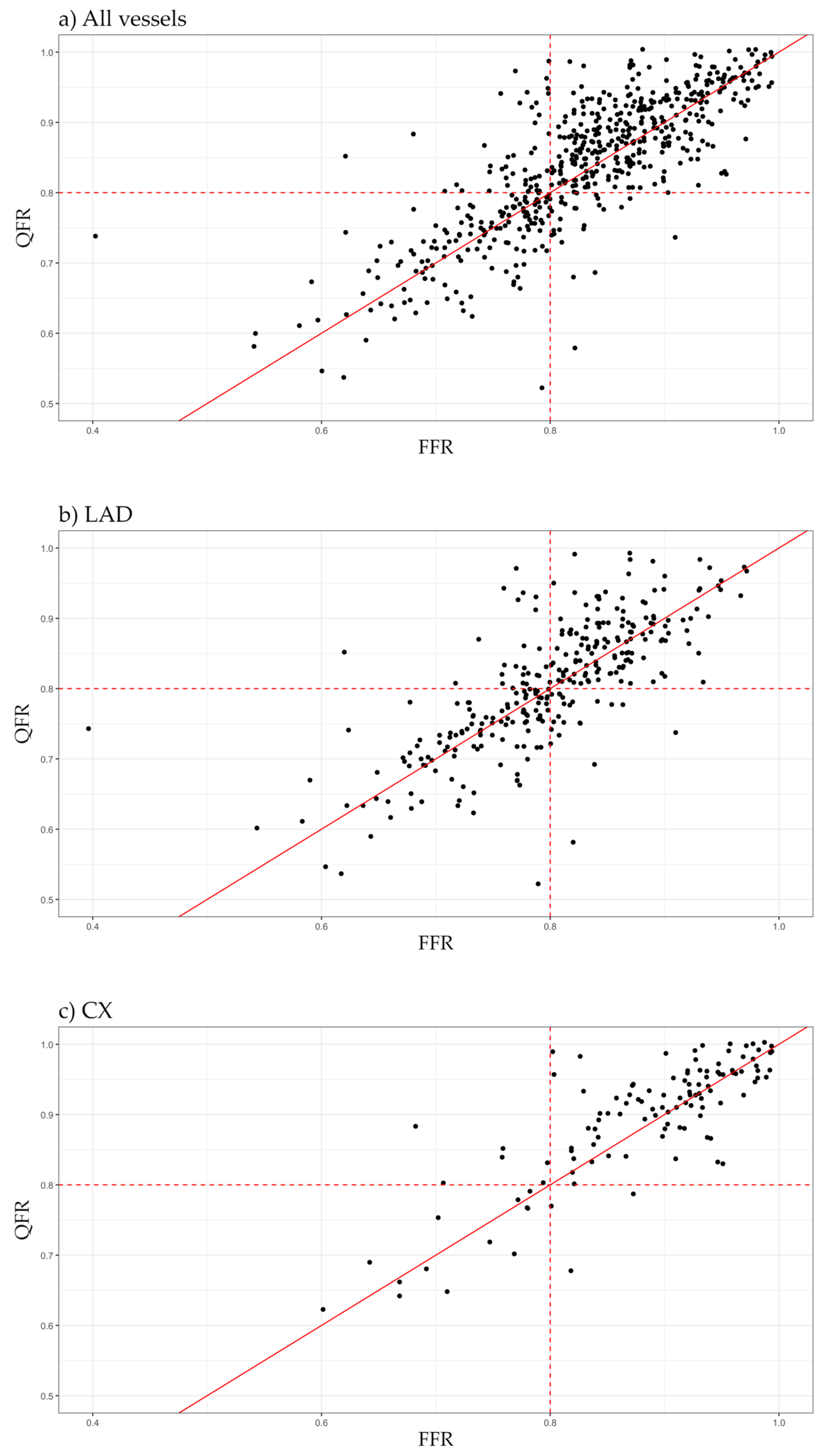
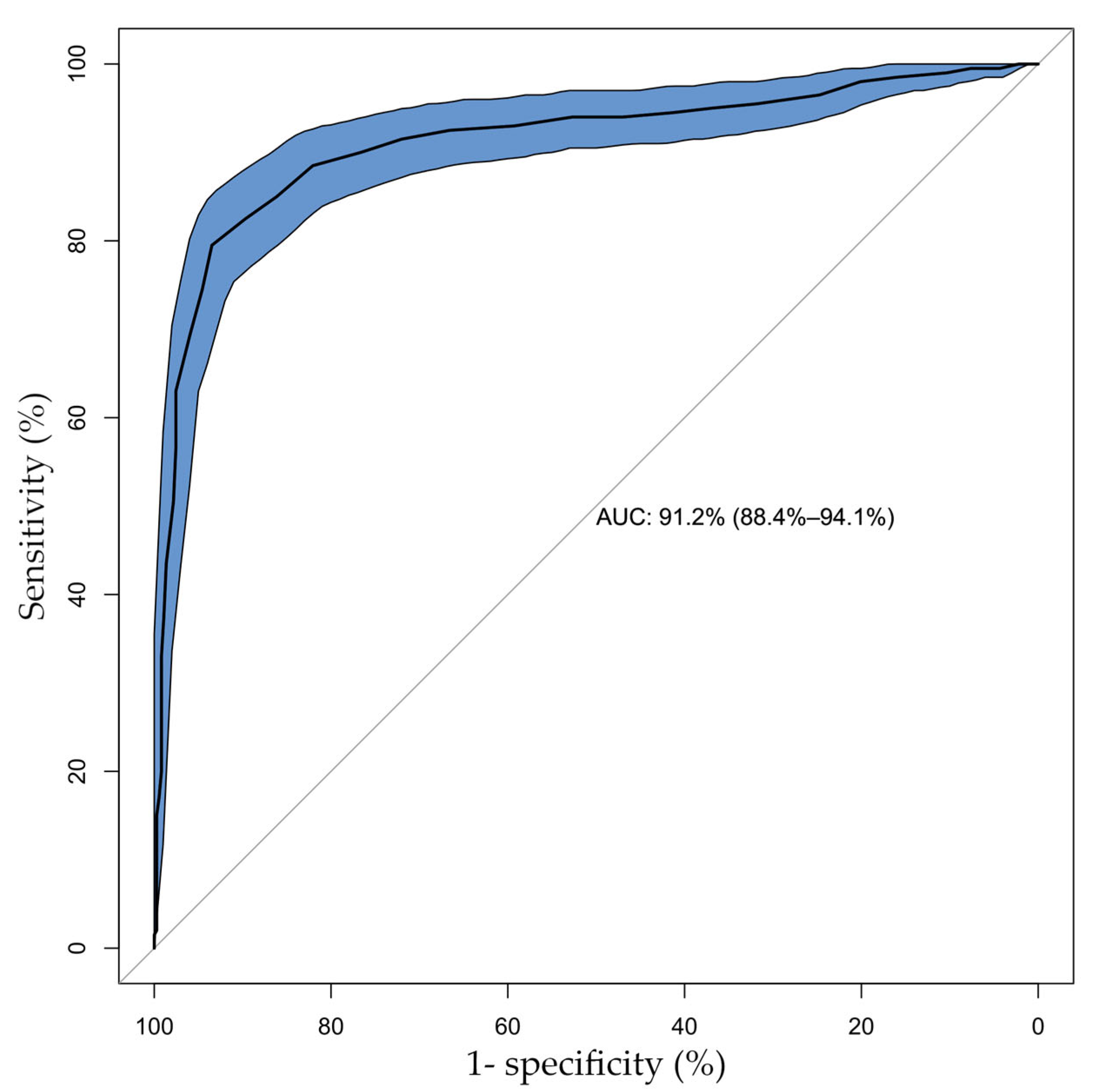
| FFR | QFR | Total | |
|---|---|---|---|
| ≤0.80 | >0.80 | ||
| ≤0.80 | 159 | 41 | 200 |
| >0.80 | 24 | 344 | 368 |
| Total | 183 | 385 | 568 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gál, R.; Csanádi, B.; Ferenci, T.; Bora, N.; Piróth, Z. Real-World Comparison of FFR and QFR: New Perspectives on the Functional Assessment of Coronary Stenoses. J. Clin. Med. 2025, 14, 5946. https://doi.org/10.3390/jcm14175946
Gál R, Csanádi B, Ferenci T, Bora N, Piróth Z. Real-World Comparison of FFR and QFR: New Perspectives on the Functional Assessment of Coronary Stenoses. Journal of Clinical Medicine. 2025; 14(17):5946. https://doi.org/10.3390/jcm14175946
Chicago/Turabian StyleGál, Róbert, Bettina Csanádi, Tamás Ferenci, Noémi Bora, and Zsolt Piróth. 2025. "Real-World Comparison of FFR and QFR: New Perspectives on the Functional Assessment of Coronary Stenoses" Journal of Clinical Medicine 14, no. 17: 5946. https://doi.org/10.3390/jcm14175946
APA StyleGál, R., Csanádi, B., Ferenci, T., Bora, N., & Piróth, Z. (2025). Real-World Comparison of FFR and QFR: New Perspectives on the Functional Assessment of Coronary Stenoses. Journal of Clinical Medicine, 14(17), 5946. https://doi.org/10.3390/jcm14175946






