Understanding Hemodialysis-Associated Pericarditis: Causes, Symptoms, and Management Strategies
Abstract
1. Introduction
2. Review of Literature
2.1. Epidemiology
2.2. Etiology and Classification
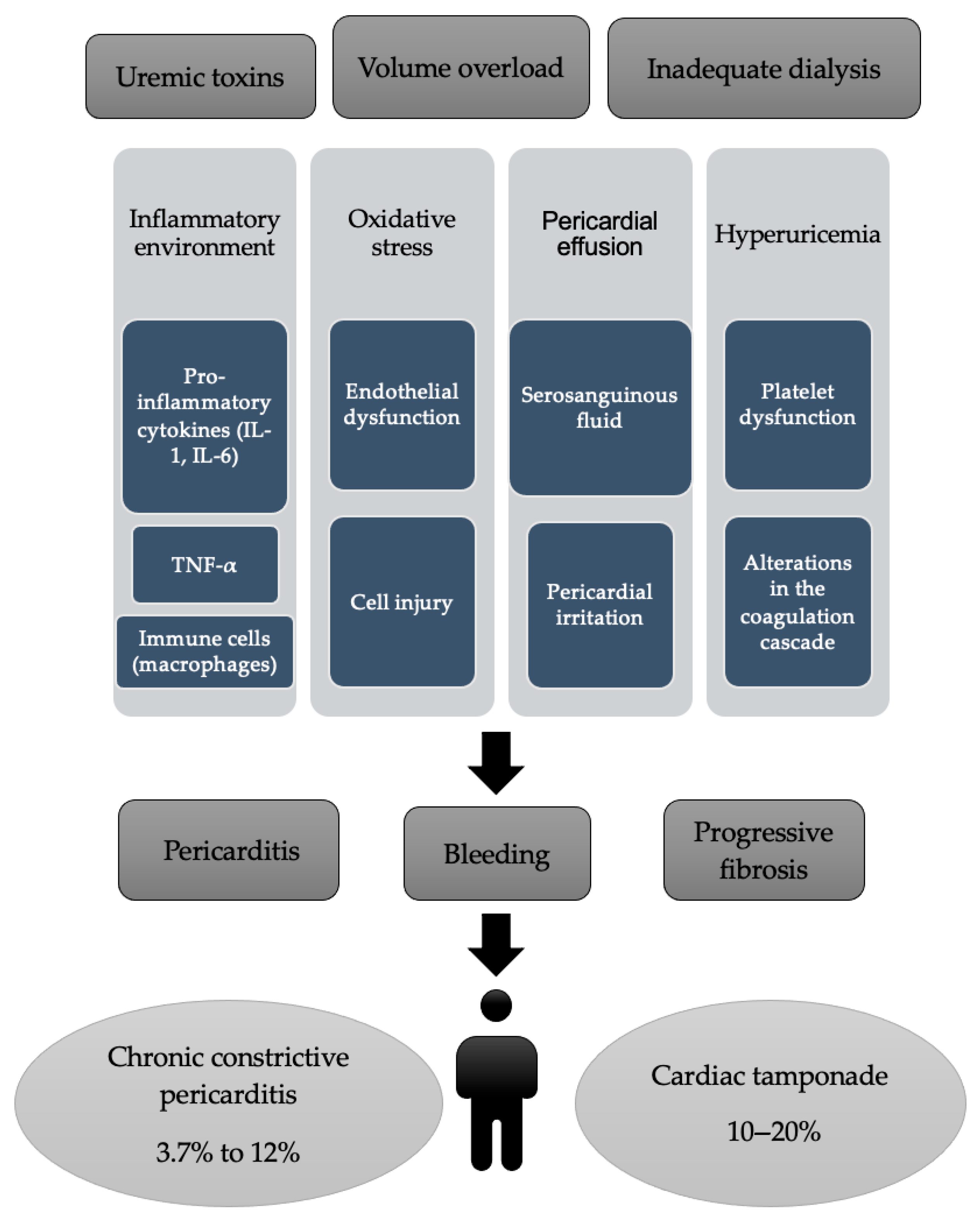
2.3. Pathogenesis
2.4. Diagnosis
- Stage I—diffuse modifications, such as ST-segment augmentation and PR-segment elevation in aVR-lead and V1 (differential diagnosis with myocardial infarction);
- Stage II—normalization of ST- and PR-segments, in the first week;
- Stage III—T-wave inversion;
- Stage IV—T-wave normalization.
2.5. Complications
2.6. Treatment
3. Case Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nesheiwat, Z.; Lee, J.J. Uremic Pericarditis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536920/#article-26961.r1 (accessed on 6 May 2025).
- Neves, P.D.M.M.; Lario, F.C.; Mohrbacher, S.; Ferreira, B.M.C.; Sato, V.A.H.; Oliveira, É.S.; Pereira, L.V.B.; Bales, A.M.; Nardotto, L.L.; Ferreira, J.N.; et al. Dialysis-related constrictive pericarditis: Old enemies may sometimes come back. J. Bras. Nefrol. 2022, 44, 602–606. [Google Scholar] [CrossRef]
- Javed, N.; Molina, M.; Nasr, R.; Diaz-Fuentes, G. Uremic Pericarditis with Cardiac Tamponade in a Patient on Hemodialysis. Case Rep. Cardiol. 2023, 2023, 5099005. [Google Scholar] [CrossRef] [PubMed]
- Dad, T.; Sarnak, M.J. Pericarditis and Pericardial Effusions in End-Stage Renal Disease. Semin. Dial. 2016, 29, 366–373. [Google Scholar] [CrossRef]
- Chugh, S.; Singh, J.; Kichloo, A.; Gupta, S.; Katchi, T.; Solanki, S. Uremic- and Dialysis-Associated Pericarditis. Cardiol. Rev. 2021, 29, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Davalos, J.; Sullivan, S.; McCool, H.; Fenner, B.; Islam, E. Uremic pericarditis: A case report of favorable outcomes with early detection and management. Southwest Respir. Crit. Care Chron. 2024, 12, 10–13. [Google Scholar] [CrossRef]
- Sadjadi, S.A.; Mashahdian, A. Uremic pericarditis: A report of 30 cases and review of the literature. Am. J. Case Rep. 2015, 16, 169–173. [Google Scholar] [CrossRef]
- Hajji, M.; Kheder, R.; Smaoui, W.; Jebali, H.; Beji, S.; Krid, M.; BenFatma, L.; Rais, L.; Zouaghi, K.; BenMoussa, F. Péricardite urémique en hémodialyse: Prévalence et facteurs prédictifs. Néphrologie Thérapeutique 2015, 11, 291–292. [Google Scholar] [CrossRef]
- Greenberg, K.I.; Choi, M.J. Hemodialysis Emergencies: Core Curriculum 2021. Am. J. Kidney Dis. 2021, 77, 796–809. [Google Scholar] [CrossRef]
- Bentata, Y.; Hamdi, F.; Chemlal, A.; Haddiya, I.; Ismaili, N.; El Ouafi, N. Uremic pericarditis in patients with End Stage Renal Disease: Prevalence, symptoms and outcome in 2017. Am. J. Emerg. Med. 2018, 36, 464–466. [Google Scholar] [CrossRef]
- Mermel, L.A.; Farr, B.M.; Sherertz, R.J.; Raad, I.I.; O’Grady, N.; Harris, J.S.; Craven, D.E.; Infectious Diseases Society of America; American College of Critical Care Medicine; Society for Healthcare Epidemiology of America. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 2001, 32, 1249–1272. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Taheri, H.; Bahiraie, P.; Rader, F.; Siegel, R.J.; Mandegar, M.H.; Hosseini, K.; Shahid, F. Incidence of secondary pericardial effusions associated with different etiologies: A comprehensive review of literature. J. Cardiothorac. Surg. 2025, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.; Zaidi, S.M.J.; Rana, A.S.; Haider, A.; Tahir, S. Post-cardiac injury syndrome: An evidence-based approach to diagnosis and treatment. Am. Heart. J. Plus 2021, 12, 100068. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.; Fried, M.; Jabareen, F.; Vardinon, N.; Turner, D.; Burke, M.; Yust, I. Anti-heart antibodies in postpericardiotomy syndrome: Cause or epiphenomenon? A prospective, longitudinal pilot study. Autoimmunity 2002, 35, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Hoit, B.D. Post-cardiac injury syndromes. An emerging cause of pericardial diseases. Int. J. Cardiol. 2013, 168, 648–652. [Google Scholar] [CrossRef]
- Gouriet, F.; Levy, P.Y.; Casalta, J.P.; Zandotti, C.; Collart, F.; Lepidi, H.; Cautela, J.; Bonnet, J.L.; Thuny, F.; Habib, G.; et al. Etiology of Pericarditis in a Prospective Cohort of 1162 Cases. Am. J. Med. 2015, 128, 784.e1–784.e8. [Google Scholar] [CrossRef]
- Imazio, M.; Negro, A.; Belli, R.; Beqaraj, F.; Forno, D.; Giammaria, M.; Trinchero, R.; Adler, Y.; Spodick, D. Frequency and prognostic significance of pericarditis following acute myocardial infarction treated by primary percutaneous coronary intervention. Am. J. Cardiol. 2009, 103, 1525–1529. [Google Scholar] [CrossRef]
- Campos, I.D.; Salgado, A.; Azevedo, P.; Vieira, C. Dressler’s syndrome: Are we underdiagnosing what we think to be rare? BMJ Case Rep. 2019, 12, e227772. [Google Scholar] [CrossRef]
- Aten, K.; Raney, K.; Alomar, A. Dressler Syndrome: Not Just a Relic of the Past. Cureus 2022, 14, e30670. [Google Scholar] [CrossRef]
- Lehto, J.; Kiviniemi, T. Postpericardiotomy syndrome after cardiac surgery. Ann. Med. 2020, 52, 243–264. [Google Scholar] [CrossRef]
- Alpert, M.A.; Ravenscraft, M.D. Pericardial involvement in end-stage renal disease. Am. J. Med. Sci. 2003, 325, 228–236. [Google Scholar] [CrossRef]
- Bastani, B.; Chu, N. Serum CA-125 level in end-stage renal disease patients maintained on chronic peritoneal dialysis or hemodialysis: The effect of continuous presence of peritoneal fluid, peritonitis, and peritoneal catheter implantation. Am. J. Nephrol. 1995, 15, 468–472. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Fatehi Hassanabad, A.; Zarzycki, A.; Deniset, J.F.; Fedak, P.W. An overview of human pericardial space and pericardial fluid. Cardiovasc. Pathol. 2021, 53, 107346. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.W.; Aisenberg, G.M. Pericardial Effusion in Patients with End-Stage Renal Disease. Tex. Heart Inst. J. 2015, 42, 596. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.A.; Betancor, J.; Xu, B.; Kumar, A.; Rivas, C.G.; Sato, K.; Wong, L.P.; Asher, C.R.; Klein, A.L. Uremic pericarditis, pericardial effusion, and constrictive pericarditis in end-stage renal disease: Insights and pathophysiology. Clin. Cardiol. 2017, 40, 839–846. [Google Scholar] [CrossRef]
- de Araujo Antunes, A.; Caramori, J.C.; Vannini, F.D.; Zanati, S.G.; Barretti, P.; Matsubara, B.B.; da Silva Franco, R.J.; Martin, L.C. Markers of uremia and pericardial effusion in peritoneal dialysis. Int. Urol. Nephrol. 2012, 44, 923–927. [Google Scholar] [CrossRef]
- Eslami, V.; Mousavi, S.; Irilouzadian, R.; Baghsheikhi, H.; Fesharaki, M.J.; Samavat, S. Pericardial effusion in patients with chronic kidney disease: A two-center study. PLoS ONE 2024, 19, e0302200. [Google Scholar] [CrossRef]
- Gomchok, D.; Ge, R.L.; Wuren, T. Platelets in Renal Disease. Int. J. Mol. Sci. 2023, 24, 14724. [Google Scholar] [CrossRef]
- Baaten, C.C.F.M.J.; Sternkopf, M.; Henning, T.; Marx, N.; Jankowski, J.; Noels, H. Platelet Function in CKD: A Systematic Review and Meta-Analysis. J. Am. Soc. Nephrol. 2021, 32, 1583–1598. [Google Scholar] [CrossRef]
- Patel, M.; Rao, S.J.; Chittal, A.R.; Al-Talib, K.; Padmanabhan, S. Uremic Pericarditis and Cardiac Tamponade Resolving With Intensive Hemodialysis. J. Community Hosp. Intern. Med. Perspect. 2024, 14, 67–71. [Google Scholar] [CrossRef]
- McGuire, S.; Horton, E.J.; Renshaw, D.; Jimenez, A.; Krishnan, N.; McGregor, G. Hemodynamic Instability during Dialysis: The Potential Role of Intradialytic Exercise. Biomed. Res. Int. 2018, 2018, 8276912. [Google Scholar] [CrossRef]
- Yarrarapu, S.N.S.; Shah, P.; Arty, F.; Ravilla, J.; Ghose, M.; Khan, M.A.; Anwar, D. Pericardial Tamponade and Berger’s Disease: An Unusual Association. Cureus 2023, 15, e41281. [Google Scholar] [CrossRef]
- Rahman, A.; Saraswat, A. Pericarditis. Aust. Fam. Physician 2017, 46, 810–814. [Google Scholar]
- de Albuquerque Suassuna, P.G.; Sanders-Pinheiro, H.; de Paula, R.B. Uremic Cardiomyopathy: A New Piece in the Chronic Kidney Disease-Mineral and Bone Disorder Puzzle. Front. Med. 2018, 5, 206. [Google Scholar] [CrossRef]
- Ravi, V.; Iskander, F.; Saini, A.; Brecklin, C.; Doukky, R. Clinical predictors and outcomes of patients with pericardial effusion in chronic kidney disease. Clin. Cardiol. 2018, 41, 660–665. [Google Scholar] [CrossRef]
- Ito, T.; Akamatsu, K. Echocardiographic manifestations in end-stage renal disease. Heart Fail. Rev. 2024, 29, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Dababneh, E.; Siddique, M.S. Pericarditis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431080/ (accessed on 8 May 2025).
- Hassan, M.; Rizk, R.; Essam, H.; Abouelnour, A. Validation of equations for pleural effusion volume estimation by ultrasonography. J. Ultrasound 2017, 20, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Watase, H.; Oka, K.; Yamane, F.; Sano, C.; Ohta, R. Hemodialysis-Related Pericarditis With Cardiac Tamponade. Cureus 2022, 14, e24748. [Google Scholar] [CrossRef] [PubMed]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- Rutsky, E.A.; Rostand, S.G. Treatment of uremic pericarditis and pericardial effusion. Am. J. Kidney Dis. 1987, 10, 2–8. [Google Scholar] [CrossRef]
- Rosen, R.J.; Valeri, A.M. Management of Patients with Kidney Failure and Pericarditis. Clin. J. Am. Soc. Nephrol. 2023, 18, 270–272. [Google Scholar] [CrossRef]
- Imazio, M.; Gaita, F. Diagnosis and treatment of pericarditis. Heart 2015, 101, 1159–1168. [Google Scholar] [CrossRef]
- Imazio, M.; Spodick, D.H.; Brucato, A.; Trinchero, R.; Adler, Y. Controversial issues in the management of pericardial diseases. Circulation 2010, 121, 916–928. [Google Scholar] [CrossRef]
- Lotrionte, M.; Biondi-Zoccai, G.; Imazio, M.; Castagno, D.; Moretti, C.; Abbate, A.; Agostoni, P.; Brucato, A.L.; Di Pasquale, P.; Raatikka, M.; et al. International collaborative systematic review of controlled clinical trials on pharmacologic treatments for acute pericarditis and its recurrences. Am. Heart J. 2010, 160, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Brucato, A.; Trinchero, R.; Spodick, D.; Adler, Y. Individualized therapy for pericarditis. Expert Rev. Cardiovasc. Ther. 2009, 7, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Bobbio, M.; Cecchi, E.; Demarie, D.; Demichelis, B.; Pomari, F.; Moratti, M.; Gaschino, G.; Giammaria, M.; Ghisio, A.; et al. Colchicine in addition to conventional therapy for acute pericarditis: Results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 2005, 112, 2012–2016. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Brucato, A.; Adler, Y. A randomized trial of colchicine for acute pericarditis. N. Engl. J. Med. 2014, 370, 780–781. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Cumetti, D.; Brambilla, G.; Demichelis, B.; Ferro, S.; Maestroni, S.; Cecchi, E.; Belli, R.; Palmieri, G.; et al. Corticosteroids for recurrent pericarditis: High versus low doses: A nonrandomized observation. Circulation 2008, 118, 667–671. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Maestroni, S.; Cumetti, D.; Dominelli, A.; Natale, G.; Trinchero, R. Prevalence of C-reactive protein elevation and time course of normalization in acute pericarditis: Implications for the diagnosis, therapy, and prognosis of pericarditis. Circulation 2011, 123, 1092–1097. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Trinchero, R.; Spodick, D.; Adler, Y. Colchicine for pericarditis: Hype or hope? Eur. Heart J. 2009, 30, 532–539. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Belli, R.; Forno, D.; Ferro, S.; Trinchero, R.; Adler, Y. Colchicine for the prevention of pericarditis: What we know and what we do not know in 2014—Systematic review and meta-analysis. J. Cardiovasc. Med. 2014, 15, 840–846. [Google Scholar] [CrossRef]
- Alabed, S.; Cabello, J.B.; Irving, G.J.; Qintar, M.; Burls, A. Colchicine for pericarditis. Cochrane Database Syst. Rev. 2014, 2014, CD010652. [Google Scholar] [CrossRef]
- Imazio, M.; Adler, Y. Management of pericardial effusion. Eur. Heart J. 2013, 34, 1186–1197. [Google Scholar] [CrossRef]
- Sagristà-Sauleda, J.; Mercé, J.; Permanyer-Miralda, G.; Soler-Soler, J. Clinical clues to the causes of large pericardial effusions. Am. J. Med. 2000, 109, 95–101. [Google Scholar] [CrossRef]
- Imazio, M.; Mayosi, B.M.; Brucato, A.; Markel, G.; Trinchero, R.; Spodick, D.H.; Adler, Y. Triage and management of pericardial effusion. J. Cardiovasc. Med. 2010, 11, 928–935. [Google Scholar] [CrossRef]
- Ristić, A.D.; Imazio, M.; Adler, Y.; Anastasakis, A.; Badano, L.P.; Brucato, A.; Caforio, A.L.; Dubourg, O.; Elliott, P.; Gimeno, J.; et al. Triage strategy for urgent management of cardiac tamponade: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2014, 35, 2279–2284. [Google Scholar] [CrossRef]
- Ramesh, P.; Kanagalingam, S.; Zargham Ul Haq, F.; Victory Srinivasan, N.; Khan, A.I.; Mashat, G.D.; Hazique, M.; Khan, K.I.; Khan, S. Acquired Von Willebrand Deficiency in Adults With Aortic Stenosis: A Systematic Review. Cureus 2022, 14, e28879. [Google Scholar] [CrossRef]
- Badwan, O.; Skoza, W.; Braghieri, L.; Persits, I.; Klein, A.L. When should pharmacologic therapies be used for uremic pericarditis? Clevel. Clin. J. Med. 2023, 90, 549–554. [Google Scholar] [CrossRef]
- Mauro, A.G.; Bonaventura, A.; Vecchié, A.; Mezzaroma, E.; Carbone, S.; Narayan, P.; Potere, N.; Cannatà, A.; Paolini, J.F.; Bussani, R.; et al. The Role of NLRP3 Inflammasome in Pericarditis: Potential for Therapeutic Approaches. JACC Basic Transl. Sci. 2021, 6, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Aghsaeifard, Z.; Firouzi, R.; Alizadeh, R. Predisposing factors and uremic pericardial effusion among ESRD patients undergoing dialysis. Ann. Med. Surg. 2022, 77, 103579. [Google Scholar] [CrossRef]
- Abu-Abaa, M.; Hassan, M.; Mousa, A.; Arshad, H.; Shah, S. Cardiac Tamponade Risk Associated with Anticoagulation for Atrial Fibrillation in Dialysis-Associated Pericarditis: A Case Report. Cureus 2023, 15, e39072. [Google Scholar] [CrossRef]
- Imazio, M.; Cecchi, E.; Demichelis, B.; Ierna, S.; Demarie, D.; Ghisio, A.; Pomari, F.; Coda, L.; Belli, R.; Trinchero, R. Indicators of poor prognosis of acute pericarditis. Circulation 2007, 115, 2739–2744. [Google Scholar] [CrossRef]
- Imazio, M.; Cecchi, E.; Demichelis, B.; Chinaglia, A.; Ierna, S.; Demarie, D.; Ghisio, A.; Pomari, F.; Belli, R.; Trinchero, R. Myopericarditis versus viral or idiopathic acute pericarditis. Heart 2008, 94, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]





| Morphology Type | Causes |
|---|---|
| Acute forms | |
| Serous | Viral infections, early stages of tuberculosis pericarditis etc. |
| Fibrinous | Uremia, systemic lupus erythematosus, rheumatic fever etc. |
| Purulent | Pyogenic infections |
| Hemorrhagic | Neoplasia, tuberculosis pericarditis, sepsis etc. |
| Chronic forms | |
| Chronic effusive | Idiopathic or secondary to any acute forms |
| Chronic adhesive | Stage of organization of different forms of acute pericarditis |
| Chronic constrictive | Tuberculosis pericarditis, secondary to cardiac surgery or radiotherapy, purulent pericarditis etc. |
| Caseous | Tuberculosis, fungal infections |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peride, I.; Nechita, A.-M.; Dumitrache, B.; Tiglis, M.; Neagu, T.P.; Checherita, I.A.; Niculae, A. Understanding Hemodialysis-Associated Pericarditis: Causes, Symptoms, and Management Strategies. J. Clin. Med. 2025, 14, 5944. https://doi.org/10.3390/jcm14175944
Peride I, Nechita A-M, Dumitrache B, Tiglis M, Neagu TP, Checherita IA, Niculae A. Understanding Hemodialysis-Associated Pericarditis: Causes, Symptoms, and Management Strategies. Journal of Clinical Medicine. 2025; 14(17):5944. https://doi.org/10.3390/jcm14175944
Chicago/Turabian StylePeride, Ileana, Ana-Maria Nechita, Bianca Dumitrache, Mirela Tiglis, Tiberiu Paul Neagu, Ionel Alexandru Checherita, and Andrei Niculae. 2025. "Understanding Hemodialysis-Associated Pericarditis: Causes, Symptoms, and Management Strategies" Journal of Clinical Medicine 14, no. 17: 5944. https://doi.org/10.3390/jcm14175944
APA StylePeride, I., Nechita, A.-M., Dumitrache, B., Tiglis, M., Neagu, T. P., Checherita, I. A., & Niculae, A. (2025). Understanding Hemodialysis-Associated Pericarditis: Causes, Symptoms, and Management Strategies. Journal of Clinical Medicine, 14(17), 5944. https://doi.org/10.3390/jcm14175944





