Anatomical Predictors of Clinical Improvement After Profundoplasty in Patients with an Occluded Superficial Artery: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- -
- Group one: Patients with stenosis of the CFA and/or DFA and in whom (multiple) peripheral bypass procedures already had been performed and further peripheral bypass procedures were not possible. All these patients had an occluded SFA (TASC II D) [3]. Profundoplasty was performed as last resort for limb salvation.
- -
- Group two: Patients with an occluded SFA (TASC II D) [3] and CFA and/or DFA pathology. In these patients femoropopliteal, -crural, or -pedal bypass could be performed if profundoplasty was unsatisfactory.
2.2. Procedure
2.3. Predictive Factors
2.4. Postoperative Surveillance
2.5. Outcome Parameters
- Markedly improved (ankle brachial index essentially normalized to more than 0.90);
- Moderately improved (no open foot lesions, still symptomatic but only with exercise and improved in at least one category, increased by more than 0.10);
- Minimally improved (greater than 0.10 increase, but no categorical improvement or vice versa);
- No change (no categorical shift and less than 0.10 change);
- Mildly worse (no categorical shift, but decrease of more than 0.10 or downward categorical shift with a decrease less than 0.10);
- Moderately worse (one category worse or unexpected minor amputation);
- Markedly worse (more than one category worse or unexpected major amputation).
2.6. Statistical Analysis
3. Results
3.1. Demographics
3.2. Outcome
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| CFA | Common femoral artery |
| DFA | Deep femoral artery |
| MACE | Major adverse cardiac event |
| MALE | Major adverse limb event |
| PAD | Peripheral artery disease |
| SFA | Superficial femoral artery |
References
- Kruse, R.R.; Doomernik, D.E.; Maltha, K.V.; Kooloos, J.G.M.; Kozicz, T.L.; Reijnen, M.M.P.J. Collateral artery pathways of the femoral and popliteal artery. J. Surg. Res. 2017, 211, 45–52. [Google Scholar] [CrossRef]
- Papon, X.; Pasco, A.; Fournier, H.D.; Baron, A.; Cronier, P.; Mercier, P. Descriptive anatomic study of the fourth perforating artery of the femoral system. Surg. Radiol. Anat. 1999, 21, 277–281. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R.; Tasc II Working Group. Inter-Society Consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef]
- Berguer, R.; Higgings, R.F.; Cotton, L.T. Geometry, blood flow, and reconstruction of the deep femoral artery. Am. J. Surg. 1975, 130, 68–73. [Google Scholar] [CrossRef]
- Stoner, M.C.; Calligaro, K.D.; Chaer, R.A.; Dietzek, A.M.; Farber, A.; Guzman, R.J.; Hamdan, A.D.; Landry, G.J.; Yamaguchi, D.J. Reporting standards of the society for vascular surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J. Vasc. Surg. 2016, 61, e1–e21. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Baker, J.D.; Ernst, C.; Johnston, K.W.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef]
- Mils, J.L.; Conte, M.S.; Armstrong, D.G.; Pomposelli, F.B.; Schanzer, A.S.; Sidawy, A.N.; Andros, G. Society for Vascular Surgery Lower Extremity Guidelines Committee. The society for vascular surgery lower extremity threatened limb classification system: Risk stratification based on wound, ischemia, and foot infection (WIfI). J. Vasc. Surg. 2014, 59, 220–234. [Google Scholar] [CrossRef]
- Wright, C.J. Effect of femoral profundaplasty on blood flow. Can. J. Surg. 1983, 26, 325–327. [Google Scholar] [PubMed]
- Natali, J. By-pass using the deep femoral artery (technique and indications). J. Chir. 1962, 83, 565–580. [Google Scholar]
- Taurino, M.; Persiani, F.; FIcarelli, R.; Filippi, F.; Dito, R.; Rizzo, L. The role of the profundoplasty in the modern management of patient with peripheral vascular disease. Ann. Vasc. Surg. 2017, 45, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawai, M.; Elkassaby, M.; McVeigh, N.; Dowdall, J.; Barry, M.; Sheehan, S. Clinical outcomes from profundoplasty performed as a sole procedure for revascularization in critically ischaemic limbs. Vascular 2021, 29, 396–403. [Google Scholar] [CrossRef]
- Rollins, D.L.; Towne, J.B.; Bernhard, V.M.; Baum, P.L. Isolated profundaplasty for limb salvage. J. Vasc. Surg. 1985, 2, 585–590. [Google Scholar] [CrossRef]
- Savolainen, H.; Hansen, A.; Diehm, N.; Baumgartner, I.; Dick, F.; Heller, G.; Gahl, B.; Schmidli, J. Small is beautiful: Why profundaplasty should not be forgotten. World J. Surg. 2007, 31, 2058–2061. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.; Mangipudi, S.; Pomy, B.J.; Nguyen, B.N.; Sidawy, A.N.; Amdur, R.L.; Lala, S.; Macsata, R. A novel angiography scoring system predicts improvement after isolated common femoral endarterectomy with profundaplasty. Ann. Vasc. Surg. 2022, 81, 308–315. [Google Scholar] [CrossRef]
- Graham, A.M.; Gewertz, B.L.; Zarins, C.K. Efficacy of isolated profundaplasty. Can. J. Surg. 1986, 29, 330. [Google Scholar]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.; Bjórck, M.G.; Brodmann, M.; Cohnert, T.U.; Collet, J.P.; Czerny, M.; De Carlo, M.D.; Debus, S.E.; et al. Editor’s Choice—2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vasc Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 305–368. [Google Scholar] [CrossRef]
- Gerhard-herman, M.D.; Gornik, H.L.; Barrett, R.N. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 69, e71–e126. [Google Scholar] [CrossRef]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.A.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threathening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S. [Google Scholar] [CrossRef] [PubMed]
- Koscielny, A.; Pütz, U.; Willinek, W.; Hirner, A.; Mommertz, G. Case-control comparison of profundaplasty and femoropopliteal supragenicual bypass for peripheral arterial disease. Br. J. Surg. 2010, 97, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Hamad, H.A.A.; Allah, R.A.B.D.; Jararaa, S.; Elshikhawoda, M.S.; Omer, O.K.; Zafar, M.A.; Ibrahim, Y.; Okaz, M.; Mohammed, M.J.; Barakat, T. Evaluating the efficiacy of common femoral endarterectomy with profundoplasty: A three-year single-center perspective. Cureus 2024, 16, e74809. [Google Scholar]
- Torsello, G.B.; Gouveia e Melo, R.; Zeller, T.; Böhme, T.; Korosoglou, G.; Coscas, R.; Stavroulakis, K.; Kapetanios, D.; Torsello, G.F.; Nasr, B. Atherectomy followed by drug-coated balloon angioplasty versus surgery for symptomatic deep femoral artery arteriosclerotic disease. J. Endovasc. Ther. 2024; ahead of print. [Google Scholar]
- de Athayde Soares, R.; Fernando Matielo, M.; Cardoso Brochado Neto, F.; Cury, M.V.M.; Chacon, A.C.M.; Nakamura, E.T.; Sacilotto, R. The importance of the superficial and profunda femoris arteries in limb salvage following endovascular treatment of chronic aortoiliac occlusive disease. J. Vasc. Surg. 2018, 68, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
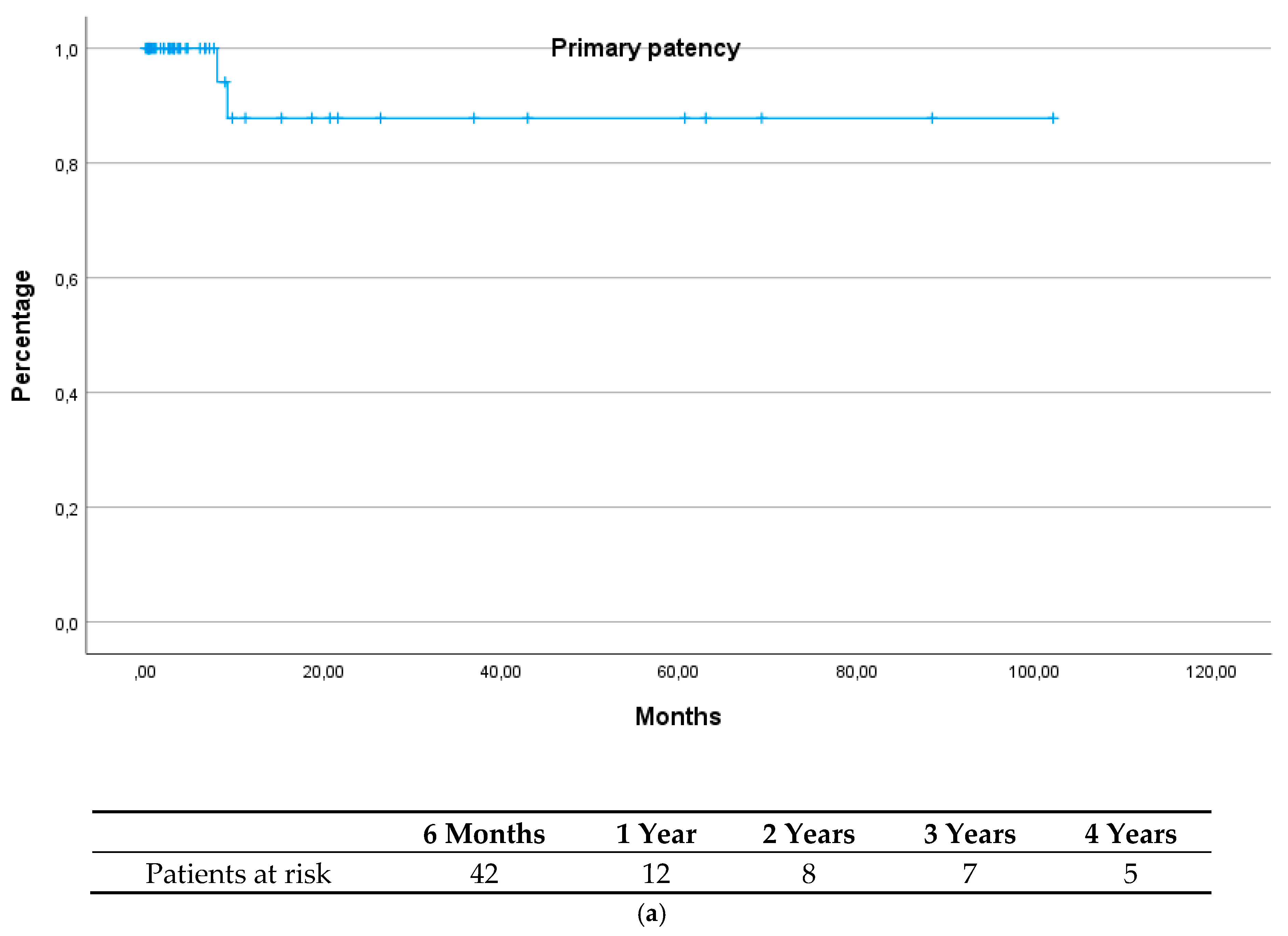
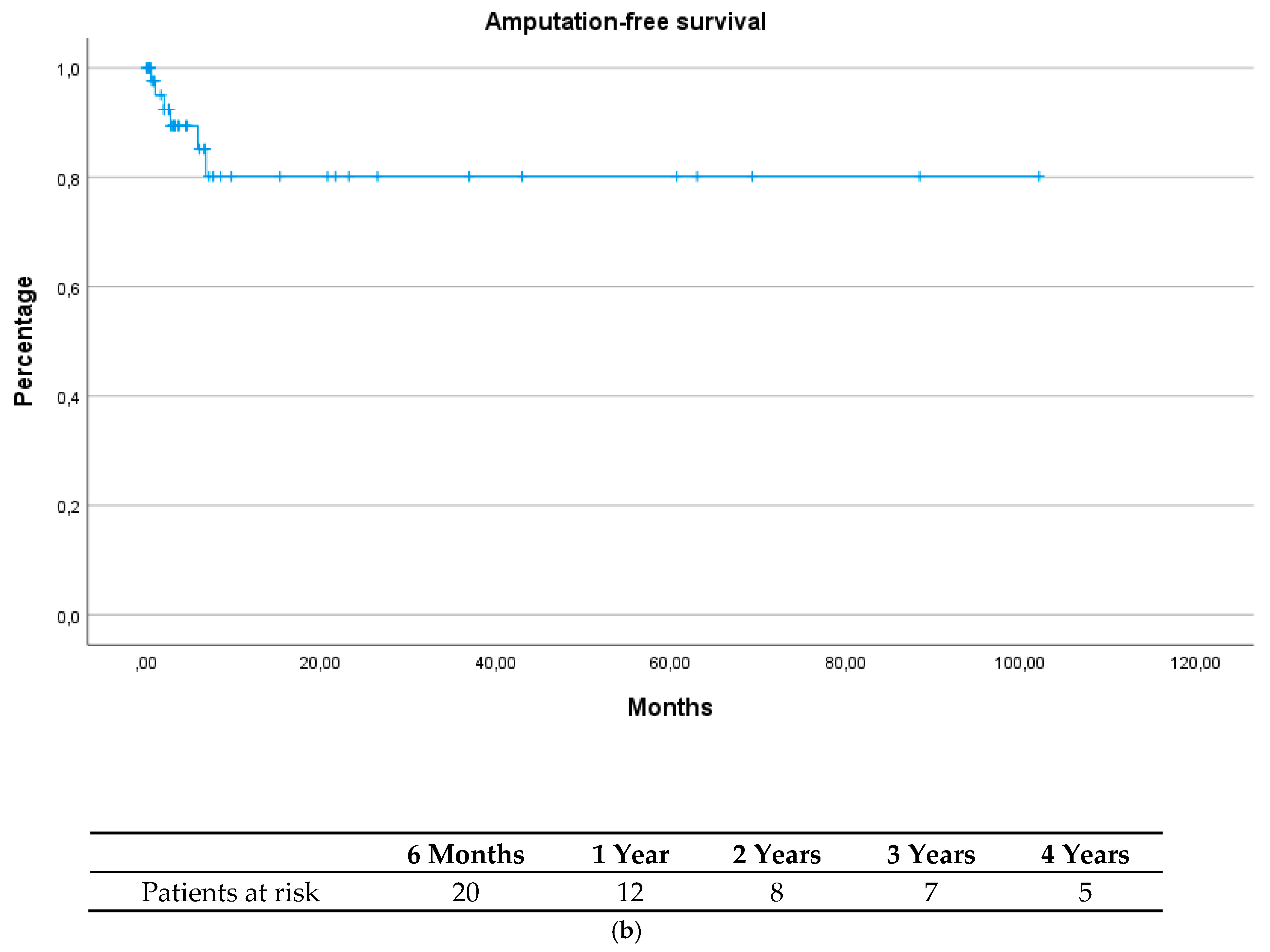
| Total (n = 49) | Group 1 (n = 15) | Group 2 (n = 34) | p-Value | |
|---|---|---|---|---|
| Age (years) | 73.9 ± 8.5 | 72.9 ± 7.6 | 74.4 ± 9.0 | 0.55 |
| Male gender | 33 (67.3%) | 9 (60.0%) | 24 (70.6%) | 0.47 |
| Cardiovascular risk factors | ||||
| Current or former smoker | 31 (63.3%) | 11 (73.3%) | 20 (58.8%) | 0.33 |
| Hypertension | 43 (87.8%) | 14 (93.3%) | 29 (85.3%) | 0.43 |
| Hyperlipidemia | 31 (63.3%) | 7 (46.7%) | 24 (70.6%) | 0.11 |
| Diabetes mellitus | 16 (32.7%) | 4 (26.7%) | 12 (35.3%) | 0.55 |
| Diabetes mellitus, insulin dependent | 7 (14.3%) | 3 (20.0%) | 4 (11.8%) | 0.45 |
| Comorbidities | ||||
| Coronary artery disease | 18 (36.7%) | 5 (33.3%) | 13 (38.2%) | 0.74 |
| Cerebrovascular disease | 4 (8.2%) | 0 (0.0%) | 4 (11.8%) | 0.17 |
| Chronic kidney insufficiency | 23 (46.9%) | 9 (60.0%) | 14 (41.2%) | 0.22 |
| Rutherford classification * | ||||
| Rutherford 1 | 1 (2.1%) | 0 (0.0%) | 1 (2.9%) | 0.25 |
| Rutherford 2 | 4 (8.3%) | 2 (14.3%) | 2 (5.9%) | 0.36 |
| Rutherford 3 | 13 (27.1%) | 5 (35.7%) | 8 (23.5%) | 0.35 |
| Rutherford 4 | 15 (31.3%) | 6 (42.9%) | 9 (26.5%) | 0.34 |
| Rutherford 5 | 8 (16.7%) | 0 (0.0%) | 8 (23.5%) | 0.04 |
| Rutherford 6 | 7 (14.6%) | 1 (7.1%) | 6 (17.6%) | 0.30 |
| Ankle brachial index † | 0.30 (IQR 0.00–0.50) | 0.10 (IQR 0.00–0.36) | 0.34 (IQR 0.00–0.50) | 0.13 |
| Left side | 23 (46.9%) | 6 (40.0%) | 17 (50.0%) | 0.52 |
| Medication | ||||
| ASA | 36 (73.5%) | 11 (73.3%) | 25 (73.5%) | 0.99 |
| Clopidogrel | 6 (12.2%) | 3 (20.0%) | 3 (8.8%) | 0.27 |
| DOAC | 12 (24.5%) | 6 (40.0%) | 6 (17.6%) | 0.09 |
| DOAC low dose | 1 (2.0%) | 0 (0.0%) | 1 (2.9%) | 0.50 |
| Vitamin K antagonist | 4 (8.2%) | 1 (6.7%) | 3 (8.8%) | 0.80 |
| Cilostazol | 1 (2.0%) | 1 (6.7%) | 0 (0.0%) | 0.13 |
| Number of patent tibial arteries | 1.8 ± 1.0 | 1.7 ± 1.0 | 1.9 ± 1.0 | 0.27 |
| WIfI | 4.7 ± 2.0 | ** | 4.7 ± 2.5 |
| Total (n = 49) | Group 1 (n = 15) | Group 2 (n = 34) | p-Value | |
|---|---|---|---|---|
| CT angiography measurements | ||||
| Maximal stenosis of the CFA (%) † | 70 (IQR 40–100) | 70 (IQR 50–100) | 90 (IQR 28–100) | 0.94 |
| Maximal stenosis of the DFA (%) | 61 ± 49 | 80 ± 41 | 53 ± 51 | 0.04 |
| Maximal diameter of the proximal DFA (mm) † | 5.1 ± 0.5 | 4.7 ± 0.5 | 5.5 ± 0.5 | 0.35 |
| Length of DFA stenosis (mm) | 18 (IQR 9–30) | 23 (IQR 15–42) | 15.5 (IQR 7.5–22.5) | 0.02 |
| Number of side branches of the DFA | 5.1 ± 0.5 | 3.3 ± 0.5 | 5.7 ± 0.5 | 0.11 |
| Number of side branches > 25% of the SFA | 4.9 ± 0.5 | 3.3 ± 0.5 | 5.6 ± 0.5 | 0.08 |
| Runoff score | 12.2 ± 5.1 | 11.1 ± 7.2 | 13.0 ± 3.7 | <0.01 |
| Length of hospital stay (days) † | 7 (IQR 5–18) | 5 IQR 21 | 8 (IQR 5–17) | 0.34 |
| Concomitant endovascular treatment of iliac arteries | 18 (36.7%) | 2 (13.3%) | 16 (47.1%) | 0.02 |
| 30-day outcome | ||||
| Hematoma or major bleeding, resolved with surgical evacuation | 1 (2.0%) | 1 (6.7%) | 0 (0.0%) | 0.31 |
| Cardiac grade 2 | 2 (4.1%) | 0 (0.0%) | 2 (5.9%) | 0.34 |
| Respiratory grade 3 | 1 (2.0%) | 1 (6.7%) | 0 (0.0%) | 0.31 |
| Wound complications, prompt recovery without surgery | 2 (4.1%) | 0 (0.0%) | 2 (5.9%) | 0.34 |
| Wound complications, resolved with redo surgery | 6 (12.2%) | 5 (33.3%) | 1 (2.9%) | <0.01 |
| Pulmonary embolism, hemodynamic instability | 1 (2.0%) | 1 (6.7%) | 0 (0.0%) | 0.31 |
| Thrombosis, resolved with redo surgery | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | -- |
| Ankle brachial index † | 0.27 (IQR 0.00–0.53) | 0.20 (IQR 0.00–0.36) | 0.25 (IQR 0.00–0.56) | 0.23 |
| Mortality | 2 (4.1%) | 1 (6.7%) | 1 (2.9%) | 0.54 |
| Midterm outcomes | ||||
| Thrombosis, resolved with redo surgery | 2 (4.1%) | 1 (6.7%) | 1 (2.9%) | 0.54 |
| Femoropopliteal or -crural bypass | 10 (20.4%) | 0 (0.0%) | 10 (29.4%) | 0.02 |
| Major amputation | 6 (12.2%) | 3 (20.0%) | 3 (8.8%) | 0.27 |
| MALE | 8 (16.3%) | 4 (26.7%) | 4 (11.8%) | 0.23 |
| MACE | 5 (10.2%) | 1 (6.7%) | 4 (11.8%) | 0.59 |
| Rutherford classification at last follow-up * | ||||
| Rutherford 0 | 5 (10.9%) | 1 (7.7%) | 4 (12.1%) | 0.51 |
| Rutherford 1 | 9 (19.6%) | 4 (30.8%) | 5 (15.2%) | 0.27 |
| Rutherford 2 | 12 (26.1%) | 4 (30.8%) | 8 (24.2%) | 0.54 |
| Rutherford 3 | 5 (10.9%) | 1 (7.7%) | 4 (12.1%) | 0.51 |
| Rutherford 4 | 3 (6.5%) | 0 (0.0%) | 3 (9.1%) | 0.33 |
| Rutherford 5 | 3 (6.5%) | 0 (0.0%) | 3 (9.1%) | 0.33 |
| Rutherford 6 | 9 (19.6%) | 3 (23.1%) | 6 (18.2%) | 0.57 |
| Markedly improved, moderately improved, or minimally improved clinical status | 31 (63.3%) | 11 (73.3%) | 20 (60.1%) | 0.33 |
| WIfI | 3.5 ± 2.5 | ** | 3.6 ± 2.8 | |
| Ankle brachial index at last follow-up † | 0.30 (IQR 0.00–0.62) | 0.24 (IQR 0.00–0.58) | 0.31 (IQR 0.00–0.65) | 0.07 |
| No Major Amputation | Major Amputation | p-Value | |
|---|---|---|---|
| Stenosis of the CFA (%) | 90 (IQR 40–100) | 60 (IQR 30–90) | 0.33 |
| Diameter of the DFA (mm) | 5.4 ± 0.1 | 5.8 ± 0.8 | 0.18 |
| % Stenosis of the DFA (%) | 74.2 ± 35.5 | 45.0 ± 40.4 | 0.04 |
| Length of DFA stenosis (mm) | 17 (IQR 8–28) | 20 (IQR 13.5–50) | 0.10 |
| Number of side branches of the DFA | 6.8 ± 2.0 | 5.6 ± 1.6 | 0.47 |
| Number of side branches > 25% of the SFA | 5.3 ± 1.5 | 5.2 ± 1.5 | 0.42 |
| Number of patent tibial arteries | 1.9 ± 0.9 | 1.2 ± 1.2 | 0.05 |
| Runoff score | 12.0 ± 5.2 | 15.4 ± 2.2 | 0.07 |
| Need for peripheral bypass * | No peripheral bypass * | ||
| Stenosis of the CFA (%) | 90 (IQR 15–100) | 90 (IQR 40–100) | 0.42 |
| Diameter of the DFA | 5.3 ± 0.2 | 5.6 ± 0.1 | 0.20 |
| Stenosis of the DFA (%) | 62.0 ± 32.6 | 66.7 ± 41.2 | 0.11 |
| Length of DFA stenosis | 14.5 (IQR 7–20) | 16.5 (IQR 7–30) | 0.05 |
| Number of side branches of the DFA | 7.2 ± 1.8 | 8.2 ± 1.7 | 0.47 |
| Number of side branches > 25% of the SFA | 4.5 ± 1.4 | 5.9 ± 1.4 | 0.77 |
| Number of patent tibial arteries | 1.4 ± 1.1 | 2.0 ± 0.9 | 0.37 |
| Runoff score | 15.0 ± 2.9 | 12.2 ± 3.7 | 0.32 |
| Markedly improved, moderately improved, or minimally improved ankle brachial index | No change, mildly worse, moderately worse, or markedly worse ankle brachial index | p-value † | |
| Stenosis of the CFA (%) | 90 (IQR 30–100) | 60 (IQR 45–100) | 0.32 |
| Diameter of the DFA | 5.4 ± 0.6 | 5.6 ± 0.2 | 0.38 |
| Stenosis of the DFA (%) | 80.5 ± 37.2 | 49.8 ± 37.5 | 0.01 |
| Length of DFA stenosis | 19.6 ± 15.3 | 24.4 ± 19.8 | 0.25 |
| Number of side branches of the DFA | 7.8 ± 1.8 | 7.7 ± 2.2 | 0.95 |
| Number of side branches > 25% of the SFA | 5.5 ± 1.5 | 4.7 ± 1.3 | 0.61 |
| Number of patent tibial arteries | 1.9 ± 1.0 | 1.4 ± 0.9 | 0.81 |
| Runoff score | 11.4 ± 5.5 | 14.8 ± 2.8 | 0.02 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | |
| Need for major amputation | ||||
| % Stenosis of the CFA | 1.0 (0.97–1.02) | 0.64 | 1.04 (0.97–1.12) | 0.29 |
| Diameter of the DFA | 1.03 (0.96–1.10) | 0.46 | 1.07 (0.91–1.23) | 0.45 |
| % Stenosis of the DFA | 0.98 (0.96–0.99) | 0.04 | 0.94 (0.87–1.00) | 0.06 |
| Length of the DFA stenosis | 1.03 (0.98–1.08) | 0.24 | 1.09 (0.99–1.12) | 0.08 |
| Number of side branches of the DFA | 1.02 (0.65–1.59) | 0.94 | 0.93 (0.46–1.87) | 0.83 |
| Number of side branches > 25% of the SFA | 0.94 (0.52–1.69) | 0.83 | 0.45 (0.11–1.86) | 0.27 |
| Number of patent tibial arteries | 0.44 (0.16–1.02) | 0.07 | 1.64 (0.13–20.61) | 0.70 |
| Runoff score | 1.26 (0.97–1.71) | 0.09 | 1.65 (0.60–4.53) | 0.33 |
| Concomitant endovascular treatment of iliac arteries | 0.00 (0.00–19.76) | 0.99 | 0.00 (0.00–25.74) | 0.34 |
| Need for peripheral bypass | ||||
| % Stenosis of the CFA | 0.99 (0.98–1.01) | 0.71 | 0.99 (0.95–1.04) | 0.77 |
| Diameter of the DFA | 0.98 (0.93–1.04) | 0.51 | 0.98 (0.89–1.08) | 0.66 |
| % Stenosis of the DFA | 0.99 (0.98–1.02) | 0.74 | 0.99 (0.95–1.05) | 0.94 |
| Length of the DFA stenosis | 0.97 (0.92–1.03) | 0.30 | 1.01 (0.91–1.13) | 0.82 |
| Number of side branches of the DFA | 0.69 (0.43–1.12) | 0.14 | 0.88 (0.45–1.72) | 0.70 |
| Number of side branches > 25% of the SFA | 0.46 (0.23–0.90) | 0.02 | 0.38 (0.15–0.99) | 0.05 |
| Number of patent tibial arteries | 0.46 (0.19–1.10) | 0.08 | 0.33 (0.07–1.61) | 0.17 |
| Runoff score | 1.31 (0.99–1.73) | 0.05 | 1.19 (0.77–1.84) | 0.43 |
| Concomitant endovascular treatment of iliac arteries | 0.36 (0.08–1.75) | 0.21 | 0.43 (0.04–5.12) | 0.50 |
| Clinical improvement as defined by Rutherford et al. [6] | ||||
| % Stenosis of the CFA | 0.99 (0.98–1.01) | 0.28 | 0.99 (0.96–1.01) | 0.29 |
| Diameter of the DFA | 1.02 (0.97–1.07) | 0.54 | 1.04 (0.96–1.11) | 0.34 |
| % Stenosis of the DFA | 1.05 (1.00–1.05) | 0.05 | 0.95 (0.93–1.01) | 0.09 |
| Length of the DFA stenosis | 1.02 (0.98–1.01) | 0.37 | 1.05 (0.99–1.11) | 0.09 |
| Number of side branches of the DFA | 0.98 (0.70–1.36) | 0.89 | 0.95 (0.62–1.46) | 0.82 |
| Number of side branches > 25% of the SFA | 0.67 (0.42–1.07) | 0.09 | 0.56 (0.30–1.05) | 0.07 |
| Number of patent tibial arteries | 0.57 (0.29–1.12) | 0.10 | 0.53 (0.20–1.43) | 0.21 |
| Runoff score | 0.98 (0.85–1.00) | 0.05 | 1.18 (0.95–1.45) | 0.13 |
| Concomitant endovascular treatment of iliac arteries | 0.60 (0.16–2.23) | 0.46 | 2.02 (0.32–12.8) | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir-van Brunschot, D.M.D.; Holzhey, D. Anatomical Predictors of Clinical Improvement After Profundoplasty in Patients with an Occluded Superficial Artery: A Pilot Study. J. Clin. Med. 2025, 14, 5938. https://doi.org/10.3390/jcm14175938
Özdemir-van Brunschot DMD, Holzhey D. Anatomical Predictors of Clinical Improvement After Profundoplasty in Patients with an Occluded Superficial Artery: A Pilot Study. Journal of Clinical Medicine. 2025; 14(17):5938. https://doi.org/10.3390/jcm14175938
Chicago/Turabian StyleÖzdemir-van Brunschot, Denise Michelle Danielle, and David Holzhey. 2025. "Anatomical Predictors of Clinical Improvement After Profundoplasty in Patients with an Occluded Superficial Artery: A Pilot Study" Journal of Clinical Medicine 14, no. 17: 5938. https://doi.org/10.3390/jcm14175938
APA StyleÖzdemir-van Brunschot, D. M. D., & Holzhey, D. (2025). Anatomical Predictors of Clinical Improvement After Profundoplasty in Patients with an Occluded Superficial Artery: A Pilot Study. Journal of Clinical Medicine, 14(17), 5938. https://doi.org/10.3390/jcm14175938






