Evaluating the Prognostic Significance of Circulating Biomarkers of End Organ Damage in Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Searches and Inclusion Criteria
2.2. Selection of Studies for Inclusion in the Review
2.3. Data Collection and Management
3. Results
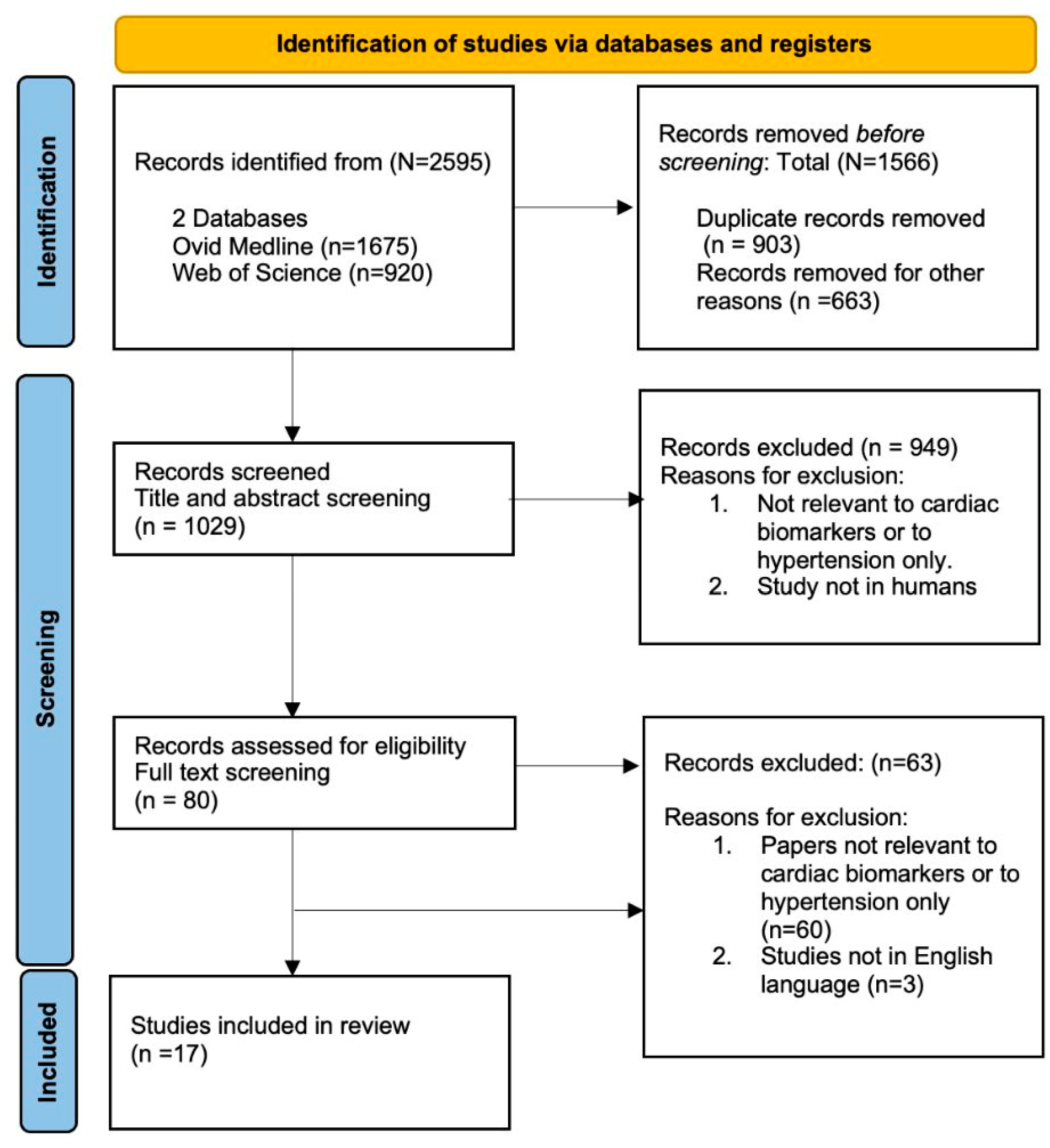
3.1. Search Strategy
3.2. Quality Appraisal of Included Studies
3.3. Demographics and Study Design
3.4. Prognostic Utility of BNP and NTpro BNP
3.5. Prognostic Utility of Cardiac Troponins
4. Discussion
4.1. Limitations
4.2. Clinical Implications
4.3. Future Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Angeli, F.; Reboldi, G. Hypertension and Atrial Fibrillation: Doubts and Certainties From Basic and Clinical Studies. Circ. Res. 2018, 122, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Rapsomaniki, E.; Timmis, A.; George, J.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; White, I.R.; Caulfield, M.J.; Deanfield, J.E.; Smeeth, L.; et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 2014, 383, 1899–1911. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Hypertension. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 15 November 2024).
- Calas, L.; Subiros, M.; Ruello, M.; Hassani, Y.; Gabet, A.; Angue, M.; Pointeau, O.; Olié, V.; Grave, C. Hypertension prevalence, awareness, treatment and control in 2019 in the adult population of Mayotte. Eur. J. Public Health 2022, 32, 408–414. [Google Scholar] [CrossRef]
- Schutte, A.E.; Venkateshmurthy, N.S.; Mohan, S.; Prabhakaran, D. Hypertension in Low- and Middle-Income Countries. Circ. Res. 2021, 128, 808–826. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef]
- Beilin, L.J.; Puddey, I.B.; Burke, V. Lifestyle and hypertension. Am. J. Hypertens. 1999, 12, 934–945. [Google Scholar] [CrossRef]
- Jeemon, P.; Séverin, T.; Amodeo, C.; Balabanova, D.; Campbell, N.R.C.; Gaita, D.; Kario, K.; Khan, T.; Melifonwu, R.; Moran, A.; et al. World Heart Federation Roadmap for Hypertension—A 2021 Update. Glob. Heart 2021, 16, 63. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. NICE. Quality Statement 1: Full Formal Risk Assessment Using QRISK3. 2023. Available online: https://www.nice.org.uk/guidance/qs100/chapter/Quality-statement-1-Full-formal-risk-assessment-using-QRISK3#definitions-of-terms-used-in-this-quality-statement (accessed on 15 November 2024).
- SCORE2 Working Group and ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.E.; Liu, X.; Collister, J.A.; Clifton, D.A.; Cairns, B.J.; Clifton, L. Independent external validation of the QRISK3 cardiovascular disease risk prediction model using UK Biobank. Heart 2023, 109, 1690–1697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amegadzie, J.E.; Gao, Z.; Quint, J.K.; Russell, R.; Hurst, J.R.; Lee, T.Y.; Sin, D.D.; Chen, W.; Bafadhel, M.; Sadatsafavi, M. QRISK3 underestimates the risk of cardiovascular events in patients with COPD. Thorax 2024, 79, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Bodinier, B.; Whitaker, M.; Wada, R.; Cooke, G.; Ward, H.; Tzoulaki, I.; Elliott, P.; Chadeau-Hyam, M. Sex inequalities in cardiovascular risk prediction. Cardiovasc. Res. 2024, 120, 1327–1335. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Januzzi, J.L.; van Kimmenade, R.; Lainchbury, J.; Bayes-Genis, A.; Ordonez-Llanos, J.; Santalo-Bel, M.; Pinto, Y.M.; Richards, M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP Study. Eur. Heart J. 2005, 27, 330–337. [Google Scholar] [CrossRef]
- Apple, F.S.; Sandoval, Y.; Jaffe, A.S.; Ordonez-Llanos, J.; IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clin. Chem. 2017, 63, 73–81. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Chronic Heart Failure in Adults: Diagnosis and Management; National Institute for Health and Care Excellence: London, UK, 2018. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- PROSPERO. International Prospective Register of Systematic Reviews. 2024. Available online: https://www.crd.york.ac.uk/prospero (accessed on 8 June 2024).
- The EndNote Team. EndNote; Clarivate: Philadelphia, PA, USA, 2013. [Google Scholar]
- Ouzzani, M.H.H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.5 (Updated August 2024); Cochrane: London, UK, 2024. [Google Scholar]
- Ali, N.; Aftab, U.; Soomar, S.M.; Tareen, H.; Khan, U.R.; Khan, B.A.; Razzak, J.A. Clinical utility of routine investigations and risk factors of end-organ damage in asymptomatic severe hypertension. Intern. Emerg. Med. 2023, 18, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Ballo, P.; Betti, I.; Barchielli, A.; Balzi, D.; Castelli, G.; De Luca, L.; Gheorghiade, M.; Zuppiroli, A. Prognostic role of N-terminal pro-brain natriuretic peptide in asymptomatic hypertensive and diabetic patients in primary care: Impact of age and gender : Results from the PROBE-HF study. Clin. Res. Cardiol. 2016, 105, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.D.; Chen, H.; Nambi, V.; Ambrosius, W.T.; Ascher, S.B.; Shlipak, M.G.; Ix, J.H.; Gupta, R.; Killeen, A.; Toto, R.D.; et al. Effect of Intensive Blood Pressure Control on Troponin and Natriuretic Peptide Levels: Findings from SPRINT. Circulation 2023, 147, 310–323. [Google Scholar] [CrossRef]
- Jia, X.; Nambi, V.; Berry, J.D.; Dalmacy, D.; Ascher, S.B.; Taylor, A.A.; Hoogeveen, R.C.; de Lemos, J.A.; Ballantyne, C.M. High-Sensitivity Cardiac Troponins I and T and Cardiovascular Outcomes: Findings from the Systolic Blood Pressure Intervention Trial (SPRINT). Clin. Chem. 2023, 70, 414–424. [Google Scholar] [CrossRef]
- Berry, J.D.; Nambi, V.; Ambrosius, W.T.; Chen, H.; Killeen, A.A.; Taylor, A.; Toto, R.D.; Soliman, E.Z.; McEvoy, J.W.; Pandey, A.; et al. Associations of High-Sensitivity Troponin and Natriuretic Peptide Levels with Outcomes After Intensive Blood Pressure Lowering: Findings from the SPRINT Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 1397–1405. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Gluba-Brzózka, A.; Michalska-Kasiczak, M.; Misztal, M.; Rysz, J.; Banach, M. The multi-biomarker approach for heart failure in patients with hypertension. Int. J. Mol. Sci. 2015, 16, 10715–10733. [Google Scholar] [CrossRef]
- Conti, A.; Alesi, A.; Trausi, F.; Scorpiniti, M.; Angeli, E.; Bigiarini, S.; Bianchi, S.; Donnini, C.; Lazzeretti, D.; Padeletti, L. Hypertension and atrial fibrillation: Prognostic aspects of troponin elevations in clinical practice. Crit. Pathw. Cardiol. 2014, 13, 141–146. [Google Scholar] [CrossRef]
- Daya, N.R.; McEvoy, J.W.; Christenson, R.H.; Tang, O.; Foti, K.; Juraschek, S.P.; Selvin, E.; Echouffo-Tcheugui, J.B. Prevalence of Elevated NT-proBNP and its Prognostic Value by Blood Pressure Treatment and Control. Am. J. Hypertens. 2023, 36, 602–611. [Google Scholar] [CrossRef]
- Everett, B.M.; Zeller, T.; Glynn, R.J.; Ridker, P.M.; Blankenberg, S. High-Sensitivity Cardiac Troponin I and B-Type Natriuretic Peptide as Predictors of Vascular Events in Primary Prevention. Circulation 2015, 131, 1851–1860. [Google Scholar] [CrossRef]
- de Hartog-Keyzer, J.M.; Pop, V.J.; Rodwell, L.; Nijveldt, R.; Messaoudi, S.E. Prediction of cardiovascular events in older patients with hypertension in primary care: A cohort study. Br. J. Gen. Pract. 2024, 74, e219–e226. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.; Watson, C.; Zhou, S.; Ryan, F.; Ledwidge, M.; McDonald, K. B-Type Natriuretic Peptide and Ventricular Dysfunction in the Prediction of Cardiovascular Events and Death in Hypertension. Am. J. Hypertens. 2018, 31, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, G.; Kossyvakis, C.; Angelidis, C.; Efremidis, M.; Panagopoulou, V.; Letsas, K.; Bouras, G.; Vassilikos, V.P.; Goudevenos, J.; Tousoulis, D.; et al. Amino-terminal B-natriuretic peptide levels and postablation recurrence in hypertensive patients with paroxysmal atrial fibrillation. Heart Rhythm 2015, 12, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lee, Y.; Lim, Y.-H.; Shin, J.; Shin, J.-H. Association between B-type natriuretic peptide and long-term mortality in patients with acute severe hypertension visiting the emergency department. Sci. Rep. 2022, 12, 21001. [Google Scholar] [CrossRef]
- McGrady, M.; Reid, C.M.; Shiel, L.; Wolfe, R.; Boffa, U.; Liew, D.; Campbell, D.J.; Prior, D.; Stewart, S.; Krum, H. NT-proB natriuretic peptide, risk factors and asymptomatic left ventricular dysfunction: Results of the SCReening Evaluation of the Evolution of New Heart Failure study (SCREEN-HF). Int. J. Cardiol. 2013, 169, 133–138. [Google Scholar] [CrossRef]
- Okuyama, R.; Ishii, J.; Takahashi, H.; Kawai, H.; Muramatsu, T.; Harada, M.; Yamada, A.; Motoyama, S.; Matsui, S.; Naruse, H.; et al. Combination of high-sensitivity troponin I and N-terminal pro-B-type natriuretic peptide predicts future hospital admission for heart failure in high-risk hypertensive patients with preserved left ventricular ejection fraction. Heart Vessel. 2017, 32, 880–892. [Google Scholar] [CrossRef]
- Philippsen, T.J.; Christensen, L.S.; Nybo, M.; Hansen, M.S.; Dahl, J.S.; Brandes, A. Circulating biomarkers, echocardiographic parameters, and incident subclinical atrial fibrillation in patients with diabetes and hypertension. Pacing Clin. Electrophysiol. 2022, 45, 35–42. [Google Scholar] [CrossRef]
- Pokharel, Y.; Sun, W.; de Lemos, J.A.; Taffet, G.E.; Virani, S.S.; Ndumele, C.E.; Mosley, T.H.; Hoogeveen, R.C.; Coresh, J.; Wright, J.D.; et al. High-Sensitivity Troponin T and Cardiovascular Events in Systolic Blood Pressure Categories. Hypertension 2015, 65, 78–84. [Google Scholar] [CrossRef]
- Schmalstieg-Bahr, K.; Gladstone, D.J.; Hummers, E.; Suerbaum, J.; Healey, J.S.; Zapf, A.; Köster, D.; Werhahn, S.M.; Wachter, R. Biomarkers for predicting atrial fibrillation: An explorative sub-analysis of the randomised SCREEN-AF trial. Eur. J. Gen. Pract. 2024, 30, 2327367. [Google Scholar] [CrossRef]
- Shafi, T.; Zager, P.G.; Sozio, S.M.; Grams, M.E.; Jaar, B.G.; Christenson, R.H.; Boulware, L.E.; Parekh, R.S.; Powe, N.R.; Coresh, J. Troponin I and NT-proBNP and the association of systolic blood pressure with outcomes in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. Am. J. Kidney Dis. 2014, 64, 443–451. [Google Scholar] [CrossRef]
- CASP. Critical Appraisal Skills Programme Checklists. 2023. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 10 March 2025).
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Shemisa, K.; Bhatt, A.; Cheeran, D.; Neeland, I.J. Novel Biomarkers of Subclinical Cardiac Dysfunction in the General Population. Curr. Heart Fail. Rep. 2017, 14, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Malachias, M.V.B.; Wijkman, M.O.; Bertoluci, M.C. NT-proBNP as a predictor of death and cardiovascular events in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2022, 14, 64. [Google Scholar] [CrossRef]
- Rubattu, S.; Forte, M.; Marchitti, S.; Volpe, M. Molecular Implications of Natriuretic Peptides in the Protection from Hypertension and Target Organ Damage Development. Int. J. Mol. Sci. 2019, 20, 798. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720. [Google Scholar] [CrossRef]
- Michaels, A.; Aurora, L.; Peterson, E.; Liu, B.; Pinto, Y.M.; Sabbah, H.N.; Williams, K.; Lanfear, D.E. Risk Prediction in Transition: MAGGIC Score Performance at Discharge and Incremental Utility of Natriuretic Peptides. J. Card. Fail. 2020, 26, 52–60. [Google Scholar] [CrossRef]
- Jehn, S.; Mahabadi, A.A.; Pfohl, C.; Vogel, L.; Al-Rashid, F.; Luedike, P.; Totzeck, M.; Rassaf, T.; Dykun, I. BNP and NT-proBNP Thresholds for the Assessment of Prognosis in Patients Without Heart Failure. JACC Adv. 2023, 2, 100688. [Google Scholar] [CrossRef]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; Boer, R.A.d.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Chauin, A. The Main Causes and Mechanisms of Increase in Cardiac Troponin Concentrations Other Than Acute Myocardial Infarction (Part 1): Physical Exertion, Inflammatory Heart Disease, Pulmonary Embolism, Renal Failure, Sepsis. Vasc. Health Risk Manag. 2021, 17, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.N.; Ken, B.; Daniel, H.; Alla, I.; Mrkobrada, M. Prognostic significance of elevated troponin in non-cardiac hospitalized patients: A systematic review and meta-analysis. Ann. Med. 2014, 46, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.B. Release of cardiac troponin from healthy and damaged myocardium. Front. Lab. Med. 2017, 1, 144–150. [Google Scholar] [CrossRef]
- Wang, T.J.; Wollert, K.C.; Larson, M.G.; Coglianese, E.; McCabe, E.L.; Cheng, S.; Ho, J.E.; Fradley, M.G.; Ghorbani, A.; Xanthakis, V.; et al. Prognostic utility of novel biomarkers of cardiovascular stress: The Framingham Heart Study. Circulation 2012, 126, 1596–1604. [Google Scholar] [CrossRef]
- Hamo, C.E.; Echouffo-Tcheugui, J.B.; Zhang, S.; Florido, R.; Pankow, J.S.; Michos, E.D.; Goldberg, R.; Nambi, V.; Gerstenblith, G.; Post, W.S.; et al. Diabetes Duration and Subclinical Myocardial Injury: The Atherosclerosis Risk in Communities Study (ARIC). Clin. Chem. 2022, 68, 1272–1280. [Google Scholar] [CrossRef]
- Khan, M.S.; Januzzi, J.L.; Liu, Y.; Xu, J.; Shaw, W.; Sattar, N.; Mahaffey, K.W.; Neal, B.; Hansen, M.K.; Butler, J. Natriuretic Peptides and Prognosis in Patients with Type 2 Diabetes Mellitus and High Risk for Cardiovascular Events. J. Card. Fail. 2024, 30, 1544–1551. [Google Scholar] [CrossRef]
- Di Angelantonio, E.; Chowdhury, R.; Sarwar, N.; Ray, K.K.; Gobin, R.; Saleheen, D.; Thompson, A.; Gudnason, V.; Sattar, N.; Danesh, J. B-Type Natriuretic Peptides and Cardiovascular Risk. Circulation 2009, 120, 2177–2187. [Google Scholar] [CrossRef]
- Geng, Z.; Huang, L.; Song, M.; Song, Y. N-terminal pro-brain natriuretic peptide and cardiovascular or all-cause mortality in the general population: A meta-analysis. Sci. Rep. 2017, 7, 41504. [Google Scholar] [CrossRef]
- Zhu, Q.; Wenkai, X.; Yongyi, B.; Ping, Y.; Leiming, L.; Peng, G.; Hongmei, W.; Bai, J. The prognostic value of the plasma N-terminal pro-brain natriuretic peptide level on all-cause death and major cardiovascular events in a community-based population. Clin. Interv. Aging 2016, 11, 245–253. [Google Scholar] [CrossRef]
- Pareek, M.; Bhatt, D.L.; Vaduganathan, M.; Biering-Sørensen, T.; Qamar, A.; Diederichsen, A.C.; Møller, J.E.; Hindersson, P.; Leósdóttir, M.; Magnusson, M.; et al. Single and multiple cardiovascular biomarkers in subjects without a previous cardiovascular event. Eur. J. Prev. Cardiol. 2020, 24, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.W.; Abdullah, S.M.; Drazner, M.H.; Das, S.R.; Khera, A.; McGuire, D.K.; Wians, F.; Sabatine, M.S.; Morrow, D.A.; de Lemos, J.A. Prevalence and Determinants of Troponin T Elevation in the General Population. Circulation 2006, 113, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Alhejily, W.A. High sensitivity Troponins In Patients with elevated prohormone of beta natriuretic peptide and acute heart failure (HIGH TRIP Trial). Sci. Rep. 2022, 12, 1838. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E. Circulating Biomarkers in Heart Failure. In Heart Failure: From Research to Clinical Practice: Volume 3; Islam, M.S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 89–108. [Google Scholar]
- Bhatty, A.; Wilkinson, C.; Sydes, M.; Gale, C.P. Defining the need for cardiovascular event definitions. Eur. Heart J.-Qual. Care Clin. Outcomes 2024, 10, 105–107. [Google Scholar] [CrossRef]
- Bosco, E.; Hsueh, L.; McConeghy, K.W.; Gravenstein, S.; Saade, E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: A systematic review. BMC Med. Res. Methodol. 2021, 21, 241. [Google Scholar] [CrossRef]
| Population | Participants Were > 18 Years, Any Sex and Ethnicity Who Had a Diagnosis of Hypertension or High Blood Pressure. Studies Reporting on Pregnant Women were Excluded. |
|---|---|
| Exposure | Circulating biomarkers (Natriuretic peptides—BNP/NT-proBNP and cardiac troponin (T or I)). Studies reporting on atrial natriuretic peptides were excluded |
| Outcomes | End organ damage was the outcome and was defined as a record/diagnosis of one of the following: Primary outcome: Incident major adverse cardiac event (MACE)—defined as a composite outcome of myocardial infarction; heart failure; atrial fibrillation; heart failure hospitalisation; stroke; and all-cause mortality Secondary outcome: Any component of the composite primary outcome |
| Time frame | Studies included in this narrative review were from 2013 up to February 2025: Ovid Medline and Web of Science |
| Setting | The study applies to any clinical settings involved in the management of hypertension, including but not limited to primary care, hospital-based care (e.g., emergency departments, cardiology units, general medicine wards, intensive care units, and coronary care units). |
| Study Title | Country Journal Published | Study Design | Sample Size | Age Range Mean Age, Sex % | Length of Follow Up | Biomarkers Tested Threshold/Cutoff | Clinical Outcomes | Main Results/Findings with HR/OR/with 95% CI |
|---|---|---|---|---|---|---|---|---|
| Ali et al., 2023 | Pakistan Internal and Emergency Medicine | Retrospective study | 180 | Participants were aged 18 years and above, with a mean age of 54.9 ± 13.4 years. Females accounted for 56.1% | 2 years | High sensitivity Troponin I | End organ damage Kidney Heart | Out of 180 patients, 164 (91%) had troponin administered. In total, 34 (18.9%) patients had abnormal values New onset EOD was diagnosed in 15 (8.3%) patients. In total, 73.3% developed EOD in form of kidney followed by (26.6%) in heart (HR 1.27) cTnI Median IQR was 0.012 (0.006–0.054) for end organ damage, significant difference from no end organ damage (p = 0.001) |
| Ballo et al., 2015 | Italy Clin Res Cardiol | Prospective cohort study | 1012 | Participants mean age was 66.6 years with males comprising 48.1% | Over median follow up of 49.8 ± 6.7 months | NT-proBNP threshold: 141.4 pg/mL for women (sensitivity 70.0%, specificity 71.3%) and 128.9 pg/mL for men (sensitivity 50.0%, specificity 79.3%) | Cardiovascular mortality Heart failure hospitalization Non-fatal myocardial infarction | NT proBNP correlated with age and was higher in women than men 91.9 (51.2–173.9) vs. 65.7 (35.9–119.9) pg/mL, p < 0.0001] In 72 patients, 128 had events (4 cardiac deaths, 45 hospitalizations for HF, and 79 acute coronary syndromes) NT-proBNP plasma concentration as a continuous variable was a predictor of events [HR 2.7 (1.9–3.6), p < 0.0001] Elevated NT proBNP analysed as a dichotomous variable was associated with a threefold higher event rate compared with normal levels (RR 3.0, 95% CI 1.9–4.6: p < 0.0001) |
| Jarret et al., 2023 | U.S.A and Peurto Rico Circulation (AHA) | RCT | 9361 patients | Participants were older than 50 years, with a mean age of 68.7 years. The cohort included 35.0% females and 65.0% males | Follow-up period of 1 year | High sensitivity- cardiac troponin T > 6 ng/L NT-proBNP > 125 pg/mL | Primary outcome was composite of all-cause mortality and HF Secondary outcomes included all-cause mortality, the composite CVD end point | Higher hs-cTnT levels were associated with older age, male sex, higher systolic and lower diastolic blood pressure, and impaired renal function, reflected by lower eGFR and elevated urinary albumin. Similarly, elevated NT-proBNP levels were linked to older age, white race, reduced renal function, and lower systolic and diastolic blood pressure. The hazard ratio for the composite outcome of CVD and mortality was 1.85 (95% CI 1.04–3.28). For all-cause mortality alone, the HR was 2.63 (95% CI, 1.11–6.26). The combined outcome of heart failure and all-cause mortality showed an HR of 3.47 (95% CI, 1.54–7.82). For NT-proBNP: The HR for all-cause mortality was 2.40 (95% CI, 1.06–5.43). For the combined outcome of heart failure and all-cause mortality, the HR was 3.72 (95% CI, 1.68–8.23) |
| Xiaoming et al., 2024 | U.S.A and Puerto Rico Clinical chemistry. | RCT | 8796 | Participants were older than 50 years, with a median age of 67 years (IQR: 61–76). The cohort included 3238 females (36.8%) and 5558 males (63.2%) | Follow-up period of 1 year | High-sensitivity cardiac troponin T and I NT proBNP | Heart failure All cause death primary composite CVD (myocardial infarction, other acute coronary syndrome, stroke, HF, and cardiovascular death) ASCVD (myocardial infarction, other acute coronary syndrome, stroke, and cardiovascular death) | Increases in NT-proBNP and hs-cTnT levels from baseline to one year were independently associated with a higher risk of major cardiovascular outcomes. For NT-proBNP, primary composite CVD (HR 1.24, 95% CI: 1.11–1.37; p < 0.001), ASCVD (HR 1.16, 95% CI: 1.03–1.30; p = 0.02), heart failure (HR 1.66, 95% CI: 1.32–2.08; p < 0.001), and all-cause mortality (HR 1.15, 95% CI: 1.00–1.32; p = 0.05). Similarly, elevated hs-cTnT composite CVD (HR 1.19, 95% CI: 1.02–1.40; p = 0.03), heart failure (HR 1.44, 95% CI: 1.04–1.98; p = 0.03), and all-cause death (HR 1.46, 95% CI: 1.18–1.80; p < 0.001), though not significantly with ASCVD (HR 1.11, 95% CI: 0.93–1.32; p = 0.23) |
| Jarett et al., 2021 | U.S.A. JAMA Cardiol. | RCT | 9361 patients enrolled in SPRINT | Participants were older than 50 years with a mean age of 68.0 years (SD 9.5); 5915 (63.2%) were male and 3446 (36.8%) were female | Follow-up period of 4 years | The threshold values of NTproBNP were 125 pg/mL or more and hscTnT of 14 ng/L or more | The primary outcome was composite of all-cause mortality and HF Secondary outcomes included all-cause mortality and the composite CVD endpoint | The median hscTnT concentration was 9.4 ng/L, with 25.6% of patients exhibiting levels ≥ 14 ng/L. The median NT-proBNP concentration was 86 pg/mL, with higher values observed in women (112 pg/mL) compared with men (73 pg/mL) In fully adjusted models, elevated hscTnT was associated with a higher risk of both primary and secondary outcomes: -composite of all-cause mortality and HF (421 events; [HR], 1.60 [95% CI, 1.26–2.04]), -all-cause mortality (339 events; HR, 1.56 [95% CI, 1.19–2.05]), -composite CVD end point (531 events; HR, 1.26 [95% CI, 1.02–1.57]), -combined composite CVD end point and all-cause mortality (713 events; HR, 1.39 [95% CI, 1.15–1.67]) Similarly, NT-proBNP levels ≥ 125 pg/mL were linked to greater risk of the all-cause mortality and HF composite (HR 2.26, 95% CI 1.76–2.89), all-cause mortality (HR 1.98, 95% CI 1.51–2.60), composite CVD endpoint (HR 1.81, 95% CI 1.47–2.23), and the combined CVD and mortality endpoint (HR 1.82, 95% CI 1.52–2.19) Participants with both hs-cTnT ≥ 14 ng/L and NT-proBNP ≥ 125 pg/mL had significantly higher risks compared with those with lower biomarker levels: all-cause mortality and HF composite (HR 4.75, 95% CI 3.48–6.81), all-cause mortality (HR 3.78, 95% CI 2.68–5.32), composite CVD endpoint (HR 2.82, 95% CI 2.17–3.68), and the combined CVD plus mortality endpoint (HR 3.04, 95% CI 2.42–3.83). |
| Agata et al., 2015 | Poland International Journal of Molecular Sciences | Prospective, observational, cohort study | 120 | Participants in the non–HF group had a mean age of 61.8 ± 11 years and were 45% male. Those in the HF group were older, with a mean age of 64.5 ± 11 years, and predominantly male (86%) | Follow-up period of 8 years | NT-proBNP | Heart failure | NT-proBNP > 332 pg/mL was a significant predictor of heart failure, with an odds ratio (OR) of 3.08 (95% CI, 1.54–6.14). |
| Conti et al., 2014 | Italy Critical Pathways in Cardiology | prospective study | 1299 | Participants had a mean age of 76 ± 10 years, including 653 females (50.3%) and 646 males (49.7% | Follow-up period of 6 months | Troponin I Cut off/measurement not given | Composite endpoint of ischemic vascular events (stroke, acute coronary syndrome, revascularization, and mortality) | Among 113 patients with elevated troponin I, 15 reached the composite endpoint compared with 43 patients without e-TnI (p < 0.001). In the elevated TnI group, outcomes included 3 strokes (5%), 8 cases of coronary heart disease (14%), and 2 deaths (4%). In the non-elevated TnI group, there were 3 strokes (5%), 2 cases of coronary heart disease (4%), and 2 deaths (2%). Both univariate and multivariate analyses identified cTnI as a significant predictor of the primary endpoint, with odds ratios of 4.07 (95% CI, 2.2–7.6; p < 0.001) and 3.21 (95% CI, 1.7–6.1; p < 0.001), respectively. |
| Natalie et al., 2023 | U.S.A. American Journal of Hypertension | Retrospective study | 10,382 participants | Participants were aged 20 years and above with a mean age 44.3 years, 52.3% women, and 71.5% non-Hispanic White) | Over a median follow up of 17.3 years | NT-proBNP- threshold ≥ 125 pg/mL, | All-cause mortality and CVD mortality | Elevated NT-proBNP all-cause mortality (HR 2.29, 95% CI 1.79, 2.95) and cardiovascular mortality (HR 3.83, 95% CI 2.34, 6.29) |
| Everett et al., 2015 | U.S.A. American Heart Association journals/Circulation | Multinational RCT conducted in 26 countries | 12, 956 participants | Participants had a mean age of 65.7 years, with 36.2% females and 63.8% males | 2.0 years (quartile1–3 [Q1–Q3], 1.5–2.5 years) | A total of 12,956 samples were analyzed for high-sensitivity cardiac troponin I and 11,057 samples for BNP. The tertile cut points for hsTnI were 3.0 and 4.6 ng/L in men, and 2.6 and 3.9 ng/L in women. For BNP, tertile cut points were 20 and 28.6 ng/L in men, and 20 and 44.4 ng/L in women | Study outcomes were major vascular event composite of nonfatal MI, nonfatal stroke, hospitalization for unstable angina, arterial revascularization, or death and all-cause mortality | hsTnI concentrations in the highest tertile (men ≥ 4.6 ng/L; women ≥ 3.9 ng/L) were associated with a first major cardiovascular event (adjusted hazard ratio [aHR], 2.19; 95% CI, 1.56–3.06; p < 0.001). BNP levels in the highest tertile (men ≥ 28.6 ng/L; women ≥ 44.4 ng/L) were also linked to a first cardiovascular event (aHR, 1.94; 95% CI, 1.41–2.68; p < 0.001). Baseline cardiac troponin I and BNP were associated with the risk of vascular events and all-cause mortality. hsTnI and all-cause mortality (HR, 2.61; 95% CI, 1.81–3.78; p < 0.0001), as well as between the composite of the primary endpoint plus all-cause mortality (HR, 2.42; 95% CI, 1.86–3.15; p < 0.0001). |
| Josephine et al., 2024 | Netherlands 2024 British Journal of General Practice | Prospective cohort study in 5 Dutch general practices btn 2010–2012 and 2020 | 530 patients | Participants were aged 60 to 85 years, with a mean age of 70 (±6.5) years. Females accounted for 301 (56.8%) and males 229 (43.2%) | 9 years of follow up | BNP levels had a median of 10.0 pmol/L (IQR, 5.7–18.0). Elevated BNP, defined as ≥10 pmol/L, was observed in 257 participants (48.5%) | All-cause mortality (ACM) Cardiovascular events (CVEs) Heart failure | Among 530 participants, 31 (5.8%) developed a coronary event, 44 (8.3%) a cerebrovascular accident, 53 (10.0%) atrial fibrillation, 23 (4.3%) heart failure, and 66 (12.5%) died Elevated BNP increased the risk of ACM, CVEs, and HF independently by 44% HR 1.44 (95% CI, 1.07, 1.94), p = −0.017), 45% HR 1.45, (95% CI, 1.15, 1.82), p < 0.002), and 288% (HR 3.88, 95% CI, 2.13, 7.10), p < 0.001), respectively |
| Gallagher et al., 2018 | Ireland American Journal of Hypertension, | Prospective study-Screening to prevent heart failure (STOP-HF) cohort | 572 patients | Participants had a mean age of 64.7 years (SD 9.9), with 309 males (54.0%) and 263 females (46.0% | Median follow up 4.0 years | BNP | MACE-I or 2 of (arrhythmia, transient ischemic attack, stroke, myocardial infarction, peripheral or pulmonary thrombosis/embolus, or heart failure) death, MACE + death | Among 572 patients with uncomplicated and complicated hypertension, there were 33 (5.77%) events of MACE plus death. BNP predicted future MACE/death with an odds ratio (OR) of 2.06 (95% CI, 1.50–2.83; p < 0.001). There were 16 MACE events (2.80%) and 17 deaths (2.97%), totaling 33 events (5.77%; p = 0.011). Among 427/572 uncomplicated hypertension patients, BNP had OR 2.08, 95% CI 1.35, 3.19) p < 0.001 of predicting MACE Among 145/572 complicated hypertension patients, BNP had a 1.75 (1.05, 2.91) p = 0.032 of predicting MACE |
| Giannopoulos et al., 2015 | Greece Journal of Heart Rhythm society | Post hoc analysis of a prospective RCT study | 296 patients | Participants were aged between 54 and 66 years, with a mean age of 60 years. There were 207 males (70%) and 89 females (30%) | Over median follow up of 13.7 months | NT-proBNP- cut off point of ≥125 pg/mL was used; however, thresholds of ≥300 pg/mL and ≥450 pg/mL were assessed as well | Atrial Fibrillation (AF recurrence) | NT-proBNP showed a significant univariate correlation with AF recurrence, with each higher quartile of NT-proBNP corresponding to a 47%, 95% CI 21.5–77.9%) (p < 0.001) increase in the risk of recurrence All patients with NT-proBNP at baseline btn (155–211–338 pg/mL) had a HR 1.29, 95% CI 0.98–1.68 p = 0.66 while patients (n = 190) with normal LVEF had 1.31 95% CI 0.96–1.80, p = 0.94 |
| Kim et al., 2022 | Korea Scientific Reports Journal | Retrospective cohort study | 3099 patients | Participants were aged over 18 years, with a median age of 68 years (IQR: 53–79). Females comprised 46.3% (n = 1435) | Follow-up period of 5.2 years | BNP tertiles were defined as follows: Tertile 1 (BNP ≤ 37 pg/mL), Tertile 2 (BNP > 37 pg/mL and <167 pg/mL), and Tertile 3 (BNP ≥ 167 pg/mL) | Long term mortality | Within a 3-year follow-up period, all-cause mortality occurred in 6.4% of patients in the first tertile of BNP, 24.8% in the second tertile, and 44.4% in the third (highest) tertile Compared with patients in the first tertile of BNP, those in the second tertile had a significantly higher risk of 3-year all-cause mortality (adjusted HR 2.64; 95% CI, 1.96–3.55), as did those in the third tertile (adjusted HR 4.18; 95% CI, 3.09–5.64) |
| Okuyama et al., 2017 | Japan Heart and Vessels Journal | Prospective study | 493 patients | Participants were older adults with a mean age of 68.5 years (SD ± 10.2), comprising 355 males (72%) and 138 females (28%) | Mean follow up 86.1 months | Troponin I (hs-TnI) levels were stratified into 3 categories: Lowest: <5.0 pg/mL Middle: 5.0–10.6 pg/mL Highest: ≥10.6 pg/mL NT-proBNP levels were categorized as follows: Lowest: <74.1 pg/mL Middle: 74.1–239.7 pg/mL Highest: ≥239.7 pg/mL | Incident heart failure | During a mean follow-up period of 86.1 months, 44 heart failure (HF) admissions were recorded, 31 due to HF with preserved ejection fraction (HFpEF) and 13 due to HF with reduced ejection fraction (HFrEF; LVEF < 50%). Both high-sensitivity troponin I (hsTnI) and NT-proBNP levels were found to be independent predictors of HF admission, whether analyzed as continuous or categorical variables: as continuous variables: hsTnI: HR 2.56 (95% CI, 1.31–5.00; p = 0.005) NT-proBNP: HR 3.55 (95% CI, 1.82–6.91; p = 0.0002), as categorical variables (≥highest tertile): hsTnI: HR 3.10 (95% CI, 1.53–6.28; p = 0.002) NT-proBNP: HR 3.17 (95% CI, 1.45–6.90; p = 0.004) The combined elevation of both hsTnI and NT-proBNP was strongly associated with an increased risk of HFpEF admission, with a hazard ratio of 9.45 (95% CI, 2.47–35.4) when compared with participants with neither biomarker elevated |
| Philippsen et al., 2022 | Denmark Pacing and Clin Electrophysiology Journal | Prospective, single-center observational study | 82 Patients | Participants were aged ≥65 years, with a mean age of 71.3 years (IQR: 67.4–75.1). A total of 52 participants (63%) were male | Median follow-up of 588 days (IQR: 453–712 days) | Cardiac troponin I (cTnI) Brain natriuretic peptide (BNP) | Incident subclinical Atrial Fibrillation | After a median follow up of 588 days, 20.7% (17 patients out of 82) developed incident sub clinical atrial fibrillation. Multivariate analysis, both biomarkers, troponin I and BNP, were not associated with incident subclinical AF: (BNP OR 1.00 95% CI 0.99–1.02) cTnI (OR 0.99, 95% CI 0.96–1.05) |
| Pokharel et al., 2015 | U.S.A. Hypertension-AHA Journal | Population-based observational study | 11,191 participants | Participants had a mean age of 63 years (SD 6), with females comprising 6263 (55.9%) of the cohort | Median follow up of 12 years | Cardiac troponin T (cTnT): Mean level was 7.5 ng/L (SD 17) with cutoff categories defined as <3, 3–5, 6–8, 9–13, and >14 ng/L. NT-proBNP: Median concentration was 68 pg/mL (IQR 33–134) | Incident CV disease (CHD, stroke and HF hospitalization) | Approximately 53% of cardiovascular events occurred in patients with cTnT levels ≥ 3 ng/L Among patients with SBP 140–149 mmHg and cTnT ≥ 14 ng/L, the risks were elevated as follows: incident heart failure hospitalization (HR 4.3; 95% CI, 2.7–6.8; p = 0.002), coronary heart disease (HR 2.1; 95% CI, 1.4–3.2; p = 0.092), hard CHD (HR 2.0; 95% CI, 1.2–3.3; p = 0.006), and stroke (HR 3.0; 95% CI, 1.6–5.6; p = 0.173 |
| Bahr et al., 2024 | Germany European Journal of GP | Explorative sub-analysis of randomised clinical trial- SCREEN-AF | 291 patients | Participants were 75 years and above had a mean age of 80 ± 3 years, with 171 females (59%) and 120 males (41%) | Follow-up period up to 6 months | BNP NT-proBNP high sensitivity troponin I | Atrial fibrillation | At 6 months, 8 of 291 patients developed incident atrial fibrillation (AF). Compared with those without AF (n = 283), patients with AF (n = 8) had significantly higher median levels of BNP [78 (IQR 64.5–112) vs. 41 (27–77) ng/L, p = 0.0121], NT-proBNP [273 (201.05–587.1) vs. 186 (111.1–319) ng/L, p = 0.0293], and hs-cTnI [7.4 (4.15–16.2) vs. 3.9 (3.1–5.9) ng/L, p = 0.0129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbeta, E.; Williams, K.; Yates, J.; Sankaranarayanan, R.; Penson, P.; Lip, G.Y.H.; McDowell, G. Evaluating the Prognostic Significance of Circulating Biomarkers of End Organ Damage in Hypertension. J. Clin. Med. 2025, 14, 5935. https://doi.org/10.3390/jcm14175935
Mbeta E, Williams K, Yates J, Sankaranarayanan R, Penson P, Lip GYH, McDowell G. Evaluating the Prognostic Significance of Circulating Biomarkers of End Organ Damage in Hypertension. Journal of Clinical Medicine. 2025; 14(17):5935. https://doi.org/10.3390/jcm14175935
Chicago/Turabian StyleMbeta, Elliot, Katie Williams, James Yates, Rajiv Sankaranarayanan, Peter Penson, Gregory Y. H. Lip, and Garry McDowell. 2025. "Evaluating the Prognostic Significance of Circulating Biomarkers of End Organ Damage in Hypertension" Journal of Clinical Medicine 14, no. 17: 5935. https://doi.org/10.3390/jcm14175935
APA StyleMbeta, E., Williams, K., Yates, J., Sankaranarayanan, R., Penson, P., Lip, G. Y. H., & McDowell, G. (2025). Evaluating the Prognostic Significance of Circulating Biomarkers of End Organ Damage in Hypertension. Journal of Clinical Medicine, 14(17), 5935. https://doi.org/10.3390/jcm14175935








