1. Introduction
Heart failure (HF), particularly in advanced stages, contributes to a cascade of multi-organ failure (MOF), exerting a profound impact on disease progression and adversely influencing outcomes [
1,
2]. The intricate interplay between HF and the liver and kidneys gives rise to cardiohepatic and cardiorenal syndromes, further complicating the clinical landscape [
3].
The Model of End-Stage Liver Disease (MELD) was originally designed for prognostic assessment in advanced liver disease [
4,
5]. Over time, the MELD scale has undergone iterative updates and refinements. The MELD scoring system enables simultaneous assessment of both hepatic and renal function, offering a valuable tool for predicting outcomes in patients with HF. As a result, indicators of liver impairment and the MELD score have become key elements in evaluating risk among individuals with HF [
6,
7,
8]. The MELD XI is a scale that excludes the international normalized ratio (INR). It integrates parameters of liver and kidney function, which allows for the assessment of the severity of organ dysfunction, taking into account both of these important systems. Its utility has been demonstrated in various clinical settings. For instance, Amdani et al. found that both pre- and post-implant MELD-XI scores were significantly associated with survival in pediatric patients undergoing ventricular assist device implantation, highlighting the broader applicability of the scale [
9]. Additionally, it has been shown that the MELD score can serve as an indicator of MOF [
10]. The mechanism of MOF in advanced HF involves complex interactions between cardiac dysfunction and other organ systems. Elevated venous pressure, a hallmark of HF, negatively impacts both renal and hepatic function. In the kidneys, increased venous pressure leads to reduced glomerular filtration due to elevated pressures transmitted to the efferent arterioles, resulting in decreased filtration pressure and kidney damage [
11]. In the liver, increased venous pressure causes hepatocyte atrophy and perisinusoidal edema, impairing the diffusion of oxygen and nutrients to hepatocytes [
12]. Various biochemical markers have been explored in this context. A recent study by Qing et al. identified the direct-to-total bilirubin ratio as a predictor of right heart failure following LVAD implantation, underlining the prognostic relevance of liver function parameters in patients receiving mechanical circulatory support [
13]. Previous versions of the MELD have demonstrated its prognostic value, especially in the context of patients undergoing heart transplantation (HTx) [
8,
10,
14]. However, the independent prognostic role of MELD-XI in post-HTx and left ventricular assist device (LVAD) treatment remains underexplored. This study aims to elucidate the trajectory of MOF in individuals undergoing HTx. The gold standard of advanced HF or LVAD—HeartMate 3 implantation—is currently the best alternative to HTx, utilizing the MELD-XI scale over a 1-year follow-up period.
4. Discussion
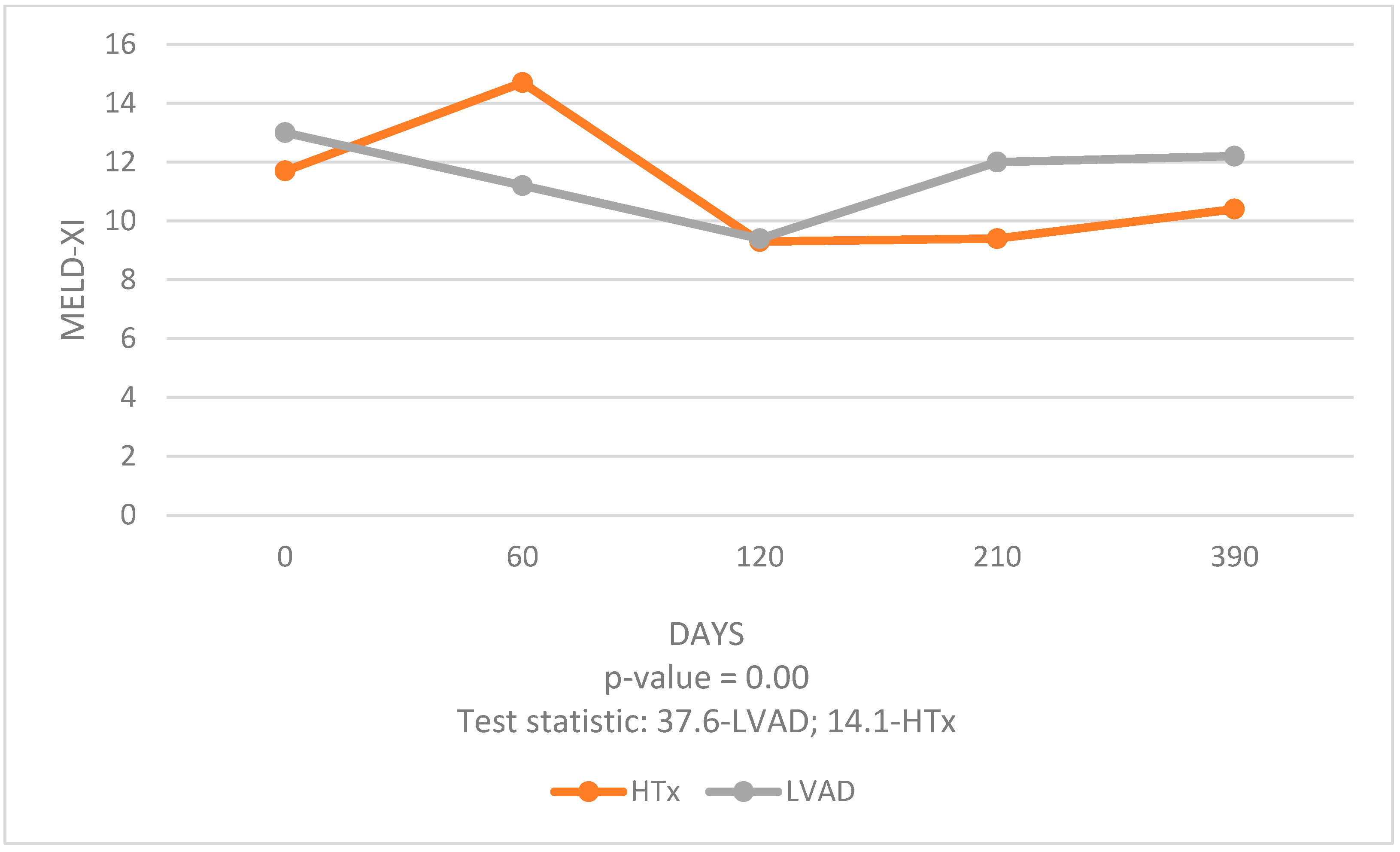
The analysis of data over a 1-year period post-procedure allowed us to compare the effects of these two different interventions on liver and kidney function. Our study revealed significant findings regarding the temporal changes in MELD-XI scores post-procedure. Both HTx and LVAD groups exhibited a significant early improvement in MELD-XI scores, indicative of enhanced liver and kidney function. However, the trajectory of these improvements differed between the two groups. LVAD patients experienced a significant decrease in MELD-XI scores at 3 months post-procedure compared to 1 month pre-procedure, suggesting an initial benefit. However, this improvement did not persist at 6 and 12 months, indicating less durable organ function stabilization. In contrast, HTx patients showed sustained improvements in MELD-XI scores at 3, 6, and 12 months post-procedure compared to 1 month pre-procedure. This consistent decline in MELD-XI scores highlights the long-term benefits of HTx in stabilizing and improving liver and kidney function.
The early improvement in the LVAD group can be attributed to the immediate hemodynamic stabilization provided by the device, which mechanically unloads the heart and enhances CO. However, the durability of these improvements may be compromised by complications associated with long-term LVAD use, such as infections, bleeding, and thromboembolic events, which can adversely affect organ function [
16,
17,
18,
19,
20].
HTx, despite its associated risks of early postoperative complications such as graft rejection and infections, offers more stable long-term benefits. The use of immunosuppressive therapy and close patient monitoring post-HTx contribute to the sustained improvement in organ function, as evidenced by the continuous decline in MELD-XI scores [
21]. It is commonly known that calcineurin inhibitors are known to induce nephrotoxicity. The immunosuppressive regimen administered to HTx recipients in our cohort followed a standard triple-therapy protocol. This included a calcineurin inhibitor—most commonly tacrolimus (98%), with target trough blood levels of 10–15 ng/mL, and less frequently cyclosporine (2%), with target levels of 200–300 ng/mL. In addition, patients received an antiproliferative agent, typically mycophenolate mofetil (94%), and glucocorticosteroids (100%), most often prednisone, which was tapered gradually and continued at reduced doses for up to 1 year post-transplant. Dosing adjustments for all immunosuppressants were individualized based on serum drug concentration monitoring during protocolized follow-up visits.
HTx remains the cornerstone treatment for selected patients with end-stage HF [
22]. Given the increasing number of patients with advanced HF and the stagnation in the availability of donor hearts, HTx is viable for a limited number of patients. Advanced heart assist devices like LVADs are emerging as a viable alternative, offering patients with advanced HF a chance for improved survival [
23,
24]. However, our study showed less lasting improvement in liver and kidney function in LVAD patients and higher early complications rates resulting in rehospitalizations compared with HTx patients. Nevertheless, we did not demonstrate statistically significant differences in MELD-XI scores between the LVAD and HTx groups in the long term.
It was also observed that liver functions improved more significantly than kidney functions, particularly in the HTx group. This distinction suggests that HTx has a more pronounced positive impact on hepatic function, possibly due to the liver’s greater ability to recover from the hemodynamic changes and venous congestion associated with advanced HF.
It is important to note that our study has some limitations—the number of patients in the LVAD group was smaller than in the HTx group, which may affect the overall statistical power of our analyses. Despite these limitations, our study provides valuable insights into the MOF trajectory post-HTx and LVAD implantation. Comparisons between the HTx and LVAD groups revealed no significant differences, except for body mass index (BMI), body mass, sex, CKD and NTproBNP levels. Differences in BMI and gender between the HTx and LVAD groups may be due to the difficulty in finding a suitable heart donor for patients with higher body weight and larger sizes. For men, who statistically have higher body mass and BMIs, there is a greater likelihood of being classified for LVAD therapy rather than HTx. This may explain the higher number of men and the higher BMI in the LVAD patient group compared to the HTx group. Although a higher percentage of patients with CKD was recorded in the LVAD group compared to the HTx group, it should be noted that glomerular filtration rate (GFR) rates and creatinine levels did not differ significantly between the groups. This suggests that the difference in the prevalence of CKD may be due to differences in medical documentation rather than an actual difference in kidney function between the groups. The LVAD group exhibited higher NTproBNP concentrations than the HTx group; however, both groups had values significantly above normal, highlighting the severity of the disease in the entire study cohort.
At the time of data collection for our study, LVAD implantation was not indicated as a destination therapy in our centre. Therefore, patients who received an LVAD were those who did not have suitable heart donors available and were considered for bridge-to-transplant therapy. Selection for LVAD was primarily driven by clinical urgency and the lack of a compatible donor, which was often due to factors such as rarer blood type or higher body mass index (BMI), making it more difficult to match with a donor organ. Consequently, while both groups had advanced heart failure, the HTx group consisted of patients for whom a suitable donor was available in a timely manner.
The interpretation of MELD-XI scores should consider the influence of age, comorbidities, and HF severity, as these factors may independently affect liver and kidney function. Their interplay can modulate MELD-XI trajectories over time and partially explain individual variability in response to both HTx and LVAD therapy.