Structural and Functional Assessment of the Macular Inner Retinal Layers in Multiple Sclerosis Eyes Without History of Optic Neuropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Optical Coherence Tomography
- (1)
- Ring analysis: Area 1 encloses the 0–1 mm central ring, Area 2 encloses the 1–3 mm annular area (a mean value of 4 averaged sectors), and Area 3 encloses the 3–6 mm annular area (a mean value of 4 averaged sectors)
- (2)
- ETDRS sector analysis: The GCL+-T was measured from the subfields within 1, 3, and 6 mm, respectively, defined by the ETDRS map. By considering ETDRS sectors between 1 and 3 mm (Area 2) and between 3 and 6 mm (Area 3), we obtained the averaged values of the superior (sup), nasal (nas), inferior (inf) and temporal (temp) sectors, each considered distinctly [23,24].
2.3. Multifocal Photopic Negative Responses Recordings
- (1)
- Ring analysis, as proposed in previous reports for mfERG responses [25,26,27,28]. The responses derived from five annular rings (R) centered on the fovea: Ring 1 (R1) enclosing a 5° radius circular area, Ring 2 (R2) as an annular area between 5 and 10°, Ring 3 (R3) as an annular area enclosed between 10 and 15°, Ring 4 (R4) as the more external annular area between 15 and 20°, and Ring 5 (R5) as the outermost annular area between 20 and 25° (Ring 5, R5).
- (2)
- ETDRS sector analysis, as previously evaluated by following the ETDRS OCT map [23,24]. We identified 9 sectors, where the central one corresponded to the R1 of the ring analysis (0–5°). Other sectors were the superior (sup), nasal (nas), inferior (inf) and temporal (temp) areas within 5–10° (R2). The outermost sectors analyzed the sup, nas, temp and inf areas within 10–20° (R3 + R4).
2.4. Statistical Analysis
3. Results
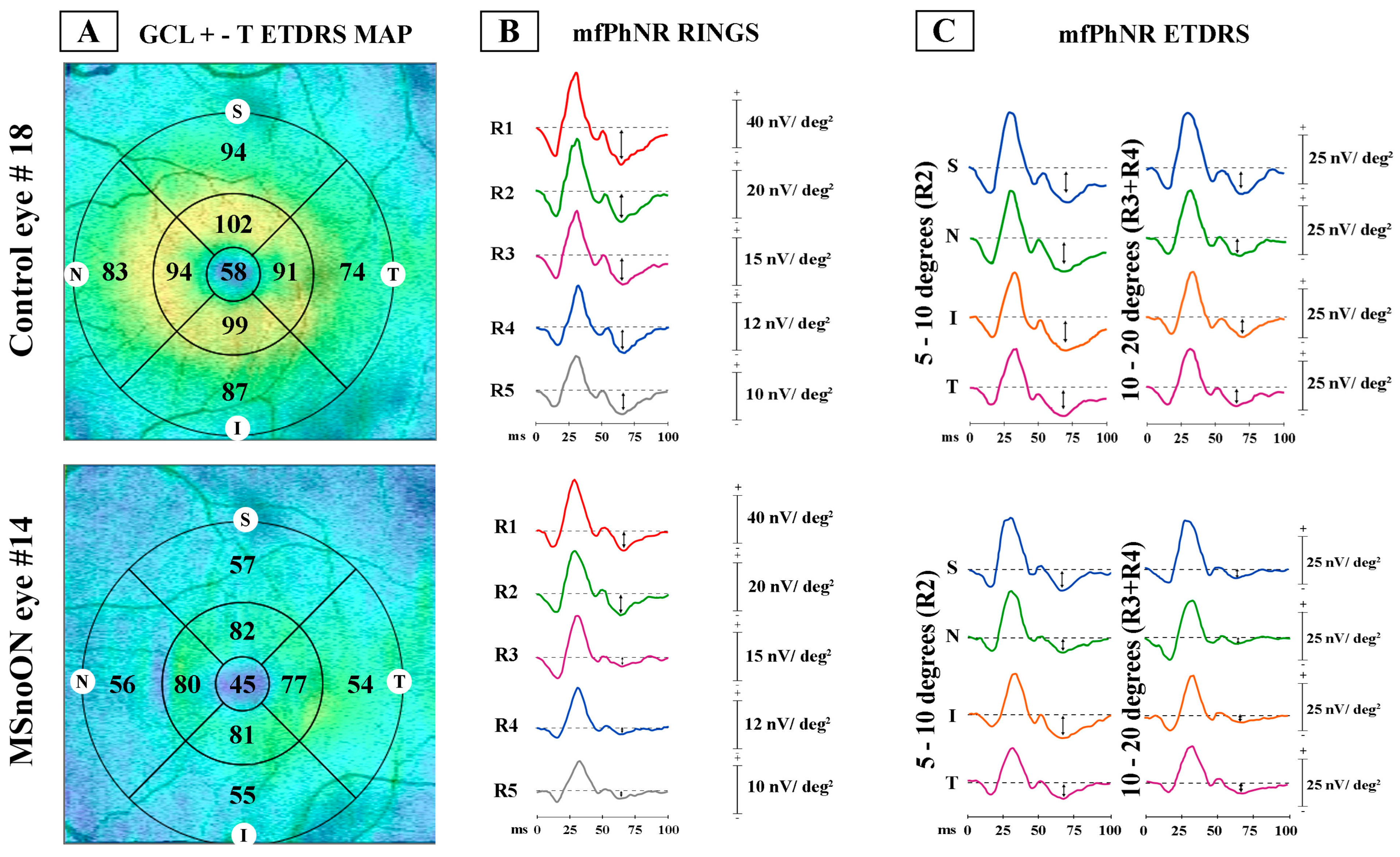
3.1. Ring Analysis of RGC Morphological Data
3.2. ETDRS Sector Analysis of RGC Morphological Data
3.3. Ring Analysis of RGC Functional Data
3.4. ETDRS Sector Analysis of RGC Functional Data
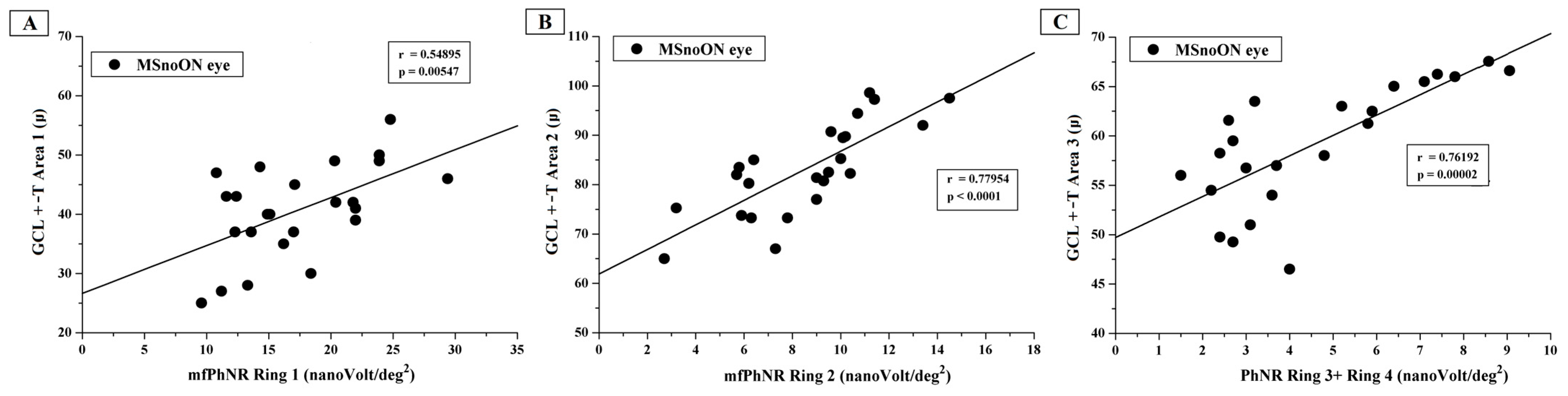
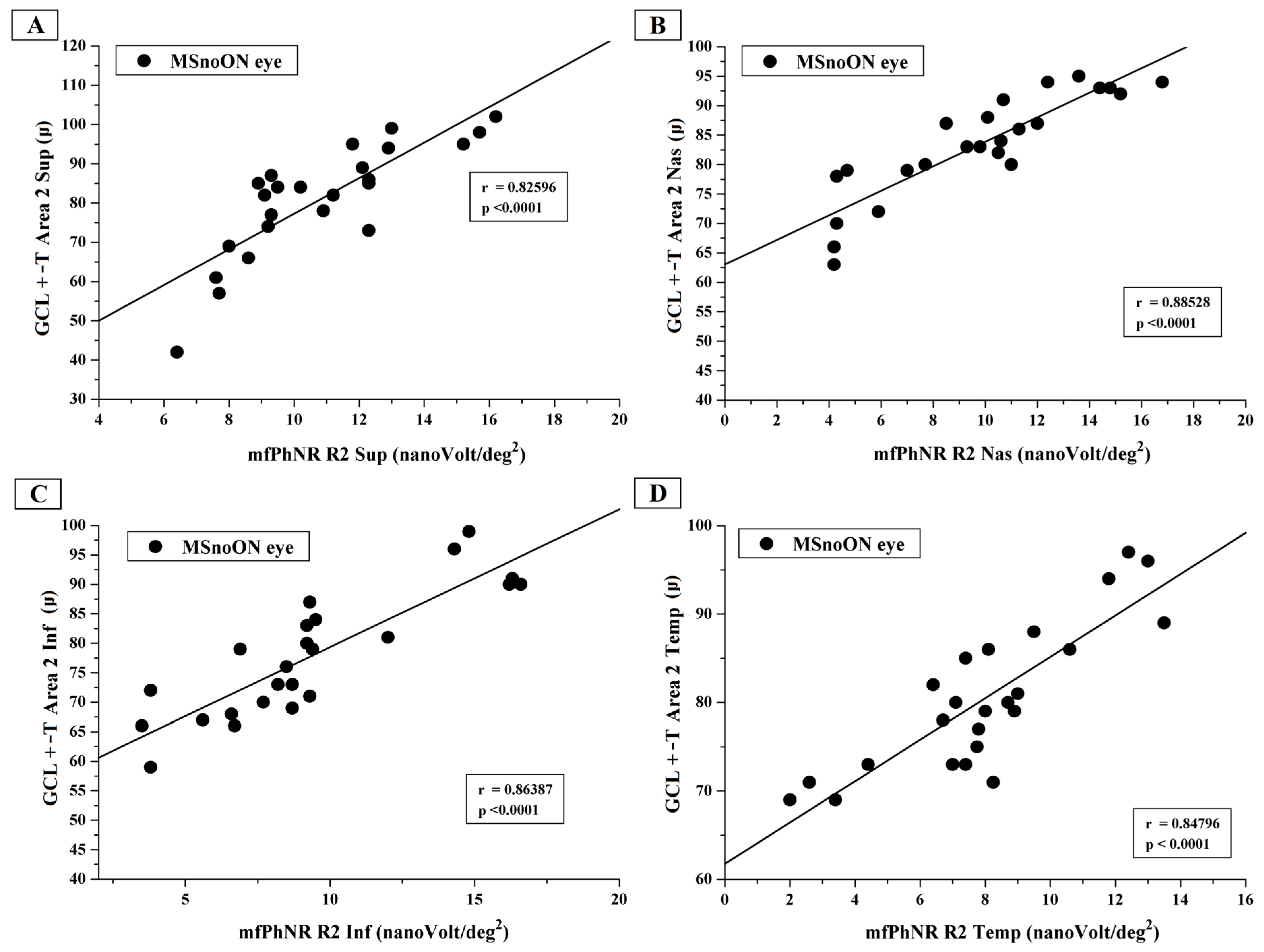
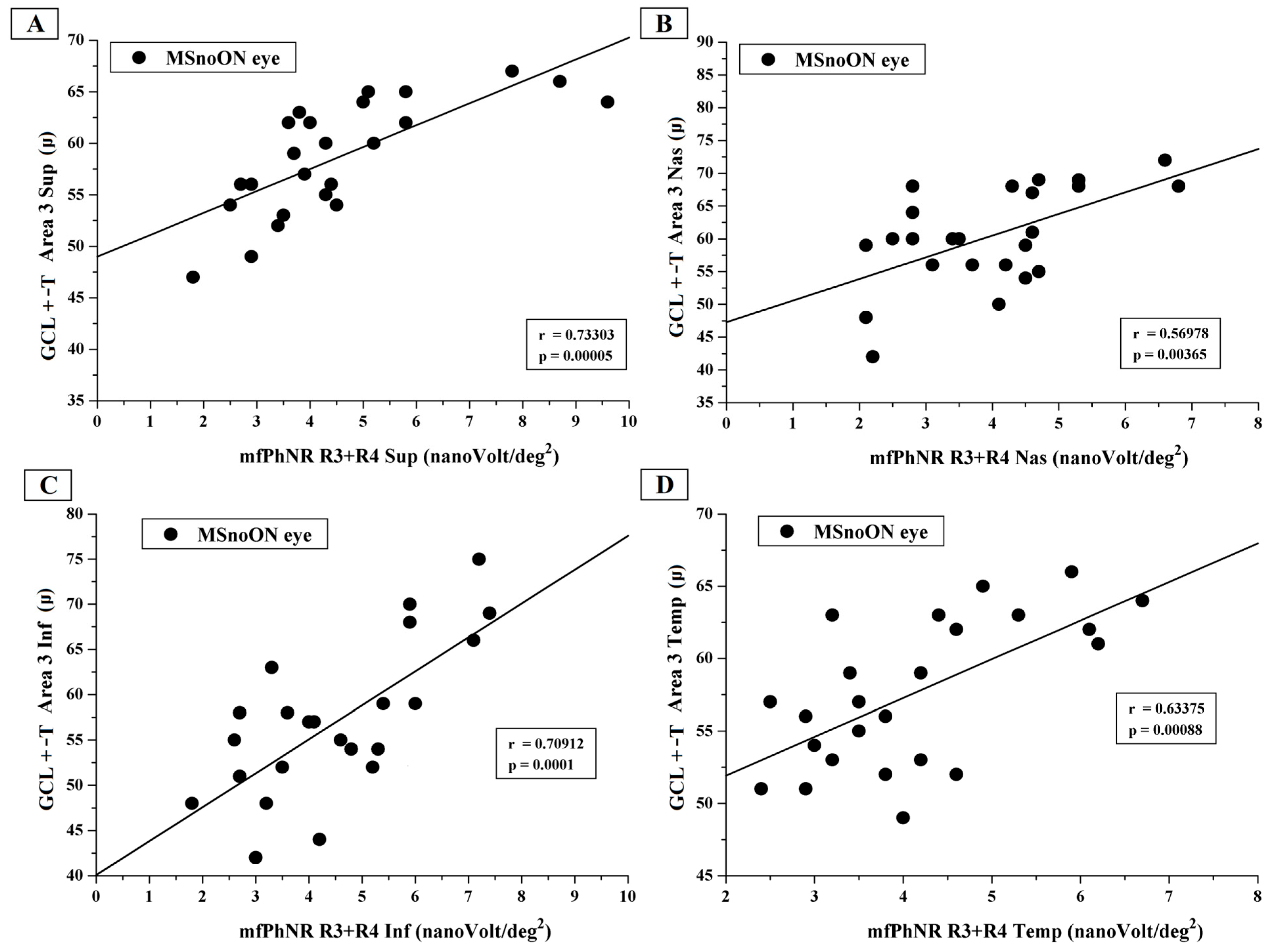
3.5. Correlations Between Structural and Functional RGC Changes in MSnoON Eyes
4. Discussion
4.1. Morphological Findings
4.2. Functional Findings
4.3. Morpho-Functional Relationships and Correlations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | multiple sclerosis |
| RGCs | retinal ganglion cells |
| ON | optic neuritis |
| OCT | optical coherence tomography |
| GCIPL | ganglion cell/inner plexiform layer |
| MSON | multiple sclerosis patients with optic neuritis |
| PERG | pattern electroretinogram |
| mfPhNR | multifocal Photopic Negative Response |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
| MSnoON | multiple sclerosis patients without history of optic neuritis |
| RNFL | retinal nerve fiber layer |
| ff-PhNR | full-field Photopic Negative Response |
| SS-OCT | swept-source optical coherence tomography |
| GCL+-T | ganglion cells layer thickness |
| IPL | inner plexiform layer |
| INL | inner nuclear layer |
| RAD | response amplitude density |
| R | Ring |
| sup | superior sector |
| nas | nasal sector |
| inf | inferior sector |
| temp | temporal sector |
| BCVA | best corrected visual acuity |
| SD | one standard deviation of the mean |
| N | number of eyes of each group |
| ANOVA | one-way analysis of variance |
| ISCEV | International Society for Clinical Electrophysiology of Vision |
References
- Graham, S.L.; Klistorner, A. Afferent visual pathways in multiple sclerosis: A review. Clin. Exp. Ophthalmol. 2017, 45, 62–72. [Google Scholar] [CrossRef]
- Britze, J.; Pihl-Jensen, G.; Frederiksen, J.L. Retinal ganglion cell analysis in multiple sclerosis and optic neuritis: A systematic review and meta-analysis. J. Neurol. 2017, 264, 1837–1853. [Google Scholar] [CrossRef]
- Petzold, A.; Balcer, L.J.; Calabresi, P.A.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Lapiscina, E.H.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017, 16, 797–812. [Google Scholar] [CrossRef]
- Al-Nosairy, K.O.; Horbrügger, M.; Schippling, S.; Wagner, M.; Haghikia, A.; Pawlitzki, M.; Hoffmann, M.B. Structure-Function Relationship of Retinal Ganglion Cells in Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 3419. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, O.; Nilsson, M.; Manouchehrinia, A.; Brautaset, R.; Kockum, I.; Venkataraman, A.P.; Dominguez-Vicent, A. Macular inner retinal layers in multiple sclerosis. Front. Neurol. 2025, 16, 1549091. [Google Scholar] [CrossRef] [PubMed]
- Bsteh, G.; Berek, K.; Hegen, H.; Altmann, P.; Wurth, S.; Auer, M.; Zinganell, A.; Di Pauli, F.; Rommer, P.; Leutmezer, F.; et al. Macular ganglion cell-inner plexiform layer thinning as a biomarker of disability progression in relapsing multiple sclerosis. Mult. Scler. 2021, 27, 684–694. [Google Scholar] [CrossRef]
- Del Palomar, A.P.; Cegoñino, J.; Montolío, A.; Orduna, E.; Vilades, E.; Sebastián, B.; Pablo, L.E.; Garcia-Martin, E. Swept source optical coherence tomography to early detect multiple sclerosis disease. The use of machine learning techniques. PLoS ONE 2019, 14, e0216410. [Google Scholar] [CrossRef]
- Ziccardi, L.; Barbano, L.; Boffa, L.; Albanese, M.; Grzybowski, A.; Centonze, D.; Parisi, V. Morphological Outer Retina Findings in Multiple Sclerosis Patients with or Without Optic Neuritis. Front. Neurol. 2020, 15, 858. [Google Scholar] [CrossRef] [PubMed]
- Wicki, C.A.; Hanson, J.V.M.; Schippling, S. Optical coherence tomography as a means to characterize visual pathway involvement in multiple sclerosis. Curr. Opin. Neurol. 2018, 31, 662–668. [Google Scholar] [CrossRef]
- Janáky, M.; Jánossy, Á.; Horváth, G.; Benedek, G.; Braunitzer, G. VEP and PERG in patients with multiple sclerosis, with and without a history of optic neuritis. Doc. Ophthalmol. 2017, 134, 185–193. [Google Scholar] [CrossRef]
- Monsalve, P. Decoding PERG: A neuro-ophthalmic retinal ganglion cell function review. Curr. Ophthalmol. Rep. 2019, 7, 51–58. [Google Scholar] [CrossRef]
- Falsini, B.; Bardocci, A.; Porciatti, V.; Bolzani, R.; Piccardi, M. Macular dysfunction in multiple sclerosis revealed by steady-state flicker and pattern ERGs. Electroencephalogr. Clin. Neurophysiol. 1992, 82, 53–59. [Google Scholar] [CrossRef]
- Machida, S. Clinical applications of the photopic negative response to optic nerve and retinal diseases. J. Ophthalmol. 2012, 2012, 397178. [Google Scholar] [CrossRef]
- Parisi, V.; Barbano, L.; Antonelli, G.; Nicoletti, C.G.; Landi, D.; Mataluni, G.; Di Renzo, A.; Buttari, F.; Marfia, G.A.; Centonze, D.; et al. Topographical Correlation between Structural and Functional Impairment of the Macular Inner Retinal Layers in Multiple Sclerosis Eyes with a History of Optic Neuropathy. J. Clin. Med. 2023, 12, 7175. [Google Scholar] [CrossRef]
- Albano, V.; Dammacco, R.; Manni, A.; Sisto, D.; Iaffaldano, A.; Mavilio, A.; Alessio, G.; Trojano, M.; Paolicelli, D. Macular ganglion cell-inner plexiform layer defect patterns in multiple sclerosis patients without optic neuritis: A Spectral-Domain-Optical Coherence Tomography Cross-Sectional, Case-Control, Pilot Study. Eur. J. Ophthalmol. 2023, 33, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, H.; Hu, Y.S.; Tang, R.A.; Frishman, L.J. The photopic negative response of the flash electroretinogram in multiple sclerosis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1315–1323. [Google Scholar] [CrossRef]
- Frishman, L.; Sustar, M.; Kremers, J.; McAnany, J.J.; Sarossy, M.; Tzekov, R.; Viswanathan, S. ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc. Ophthalmol. 2018, 136, 207–211. [Google Scholar] [CrossRef]
- Odom, J.V.; Michael Bach, M.; Brigell, M.; Graham E Holder, G.E.; McCulloch, D.L.; Atsushi Mizota, A.; Tormene, A.P. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc. Ophthalmol. 2016, 133, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Neurostatus.net. Available online: http://www.neurostatus.net/index.php?file=start (accessed on 6 March 2025).
- Williams, U.E.; Oparah, S.K.; Philip-Ephraim, E.E. Disease Modifying Therapy in Multiple Sclerosis. Int. Sch. Res. Not. 2014, 2014, 307064. [Google Scholar] [CrossRef]
- Cruz-Herranz, A.; Balk, L.J.; Oberwahrenbrock, T.; Saidha, S.; Martinez-Lapiscina, E.H.; Lagreze, W.A.; Schuman, J.S.; Villoslada, P.; Calabresi, P.; Balcer, L.; et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016, 86, 2303–2309. [Google Scholar] [CrossRef]
- Huang, J.; Liu, X.; Wu, Z.; Guo, X.; Xu, H.; Dustin, L.; Sadda, S. Macular and retinal nerve fiber layer thickness measurements in normal eyes with the Stratus OCT, the Cirrus HD-OCT, and the Topcon 3D OCT-1000. J. Glaucoma 2011, 20, 118–125. [Google Scholar] [CrossRef]
- Adhi, M.; Aziz, S.; Muhammad, K.; Adhi, M.I. Macular thickness by age and gender in healthy eyes using spectral domain optical coherence tomography. PLoS ONE 2012, 7, e37638. [Google Scholar] [CrossRef]
- Ziccardi, L.; Barbano, L.; Boffa, L.; Albanese, M.; Nicoletti, C.G.; Landi, D.; Grzybowski, A.; Falsini, B.; Marfia, G.A.; Centonze, D.; et al. Functional Assessment of Outer and Middle Macular Layers in Multiple Sclerosis. J. Clin. Med. 2020, 9, 3766. [Google Scholar] [CrossRef] [PubMed]
- Ziccardi, L.; Cioffi, E.; Barbano, L.; Gioiosa, V.; Falsini, B.; Casali, C.; Parisi, V. Macular Morpho-Functional and Visual Pathways Functional Assessment in Patients with Spinocerebellar Type 1 Ataxia with or without Neurological Signs. J. Clin. Med. 2021, 10, 5271. [Google Scholar] [CrossRef]
- Barbano, L.; Ziccardi, L.; Landi, D.; Nicoletti, C.G.; Mataluni, G.; Falsini, B.; Centonze, D.; Marfia, G.A.; Quaranta, L.; Parisi, V. Assessment of Macular Function by Multifocal Electroretinogram in Patients with Multiple Sclerosis Treated with Fingolimod. Adv. Ther. 2021, 38, 3986–3996. [Google Scholar] [CrossRef] [PubMed]
- Costello, F.; Burton, J.M. Retinal imaging with optical coherence tomography: A biomarker in multiple sclerosis? Eye Brain 2018, 10, 47–63. [Google Scholar] [CrossRef]
- Özbilen, K.T.; Gündüz, T.; Kartal, S.N.Ç.; Ceylan, N.A.; Eraksoy, M.; Kürtüncü, M. Detailed Evaluation of Macular Ganglion Cell Complex in Patients with Multiple Sclerosis. Noro Psikiyatr. Ars. 2021, 58, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Saidha, S.; Syc, S.B.; Durbin, M.K.; Eckstein, C.; Oakley, J.D.; Meyer, S.A.; Conger, A.; Frohman, T.C.; Newsome, S.; Ratchford, J.N.; et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult. Scler. 2011, 17, 1449–1463. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, H.; Gameiro, G.R.; Hernandez, J.; Delgado, S.; Wang, J. Focal thickness reduction of the ganglion cell-inner plexiform layer best discriminatesprior optic neuritis in patients with multiple sclerosis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4257–4426. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Sung, K.R.; Park, S.W. Patterns of Progressive Ganglion Cell-Inner Plexiform Layer Thinning in Glaucoma Detected by OCT. Ophthalmology 2018, 125, 1515–1525. [Google Scholar] [CrossRef]
- Balducci, N.; Savini, G.; Cascavilla, M.L.; La Morgia, C.; Triolo, G.; Giglio, R.; Carbonelli, M.; Parisi, V.; Sadun, A.A.; Bandello, F.; et al. Macular nerve fibre and ganglion cell layer changes in acute Leber’s hereditary optic neuropathy. Br. J. Ophthalmol. 2016, 100, 1232–1237. [Google Scholar] [CrossRef]
- Parisi, V.; Ziccardi, L.; Giammaria, S.; Barbano, L.; Tanga, L.; Michelessi, M.; Roberti, G.; Carnevale, C.; Dell’Aquila, C.; D’Andrea, M.; et al. Dysfunction and Morphological Involvement of Inner Macular Layers in Glaucoma. J. Clin. Med. 2024, 13, 6882. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, F.C.; Demirkaya, S.; Sobaci, G. Is optical coherence tomography really a new biomarker candidate in multiple sclerosis? A structural and functional evaluation. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5773–5781. [Google Scholar] [CrossRef] [PubMed]
| Controls (N c = 30) | MSnoON a (Nc = 24) | ANOVA b | ||||
|---|---|---|---|---|---|---|
| Mean | SD d | Mean | SD d | f (1.53) | p | |
| Area 1 GCL+-T e (µ f) | 48.467 | 8.080 | 40.667 | 7.800 | 12.81 | <0.001 |
| Area 2 GCL+-T e (µ f) | 93.408 | 5.484 | 83.481 | 9.866 | 21.96 | <0.001 |
| Area 3 GCL+-T e (µ f) | 65.375 | 4.504 | 59.479 | 6.315 | 16.01 | <0.001 |
| ETDRS g Area 2 sup h GCL+-T e (µ f) | 95.400 | 5.164 | 81.000 | 14.440 | 25.82 | <0.001 |
| ETDRS g Area 2 nas i GCL+-T e (µ f) | 93.767 | 6.426 | 83.292 | 8.971 | 24.96 | <0.001 |
| ETDRS g Area 2 inf j GCL+-T e (µ f) | 94.767 | 6.377 | 77.875 | 10.494 | 53.29 | <0.001 |
| ETDRS g Area 2 temp k GCL+-T e (µ f) | 89.700 | 6.052 | 80.458 | 8.272 | 22.47 | <0.001 |
| ETDRS g Area 3 sup h GCL+-T e (µ f) | 63.133 | 4.939 | 58.667 | 5.530 | 9.80 | =0.003 |
| ETDRS g Area 3 nas i GCL+-T e (µ f) | 68.833 | 4.624 | 60.375 | 7.529 | 20.05 | <0.001 |
| ETDRS g Area 3 inf j GCL+-T e (µ f) | 61.833 | 4.227 | 57.000 | 8.241 | 7.79 | 0.007 |
| ETDRS g Area 3 temp k GCL+-T e (µ f) | 67.700 | 5.633 | 57.625 | 5.106 | 46.31 | <0.001 |
| R1 l mfPhNR m RAD n (nV/deg2) o | 26.274 | 7.267 | 17.346 | 5.295 | 25.29 | 0.001 |
| R2 p mfPhNR m RAD n (nV/deg2) o | 11.404 | 4.163 | 8.567 | 2.913 | 8.00 | 0.007 |
| R3 q mfPhNR m RAD n (nV/deg2) o | 8.163 | 2.346 | 4.978 | 1.917 | 28.81 | <0.001 |
| R4 r mfPhNR m RAD n (nV/deg2) o | 4.323 | 1.082 | 2.865 | 0.854 | 29.06 | <0.001 |
| R5 s mfPhNR m RAD n (nV/deg2) o | 3.728 | 0.873 | 2.313 | 1.022 | 30.10 | <0.001 |
| ETDRS g R2 p sup h mfPhNR m RAD n (nV/deg2) o | 14.437 | 4.885 | 10.821 | 2.268 | 11.19 | =0.002 |
| ETDRS g R2 p nas i mfPhNR m RAD n (nV/deg2) o | 16.653 | 7.191 | 9.721 | 3.814 | 18.16 | <0.001 |
| ETDRS g R2 p inf j mfPhNR m RAD n (nV/deg2) o | 13.447 | 6.525 | 9.367 | 3.874 | 7.31 | 0.009 |
| ETDRS g R2 p temp k mfPhNR m RAD n (nV/deg2) o | 17.520 | 5.210 | 7.988 | 2.997 | 63.39 | <0.001 |
| ETDRS g R3 + R4 t sup h mfPhNR m RAD n (nV/deg2) o | 5.983 | 1.906 | 4.550 | 1.905 | 7.54 | =0.008 |
| ETDRS g R3 + R4 t nas i mfPhNR m RAD n (nV/deg2) o | 5.513 | 1.626 | 3.967 | 1.299 | 14.35 | <0.001 |
| ETDRS g R3 + R4 t inf j mfPhNR m RAD n (nV/deg2) o | 5.710 | 1.705 | 4.513 | 1.558 | 7.09 | =0.010 |
| ETDRS g R3 + R4 t temp k mfPhNR m RAD n (nV/deg2) o | 7.287 | 2.111 | 4.133 | 1.209 | 42.35 | <0.001 |
| Normal mfPhNR a and GCL+ b-T c Number of Eyes and (%) d | Reduced mfPhNR a and GCL+ b-T c Number of Eyes and (%) d | Normal mfPhNR a and Reduced GCL+ b-T c Number of Eyes and (%) d | Reduced mfPhNR a and Normal GCL+ b-T c Number of Eyes and (%) d | |
|---|---|---|---|---|
| mfPhNR a R1 e vs. GCL+ b-T c Area 1 | 4 (16.67) | 15 (62.50) | 0 (0.00) | 5 (20.83) |
| mfPhNR a R2 e vs. GCL+ b-T c Area 2 | 5 (20.83) | 19 (79.17) | 0 (0.00) | 0 (0.00) |
| mfPhNR a R3 + R4 e vs. GCL+ b-T c Area 3 | 5 (20.83) | 17 (70.83) | 0 (0.00) | 2 (8.33) |
| mfPhNR a ETDRS f R2 e Suprior vs. GCL+ b-T c ETDRS f Area 2 Superior | 5 (20.83) | 18 (75.00) | 0 (0.00) | 1 (4.17) |
| mfPhNR a ETDRS f R2 e Nasal vs. GCL+ b-T c ETDRS f Area 2 Nasal | 5 (20.83) | 18 (75.00) | 0 (0.00) | 1 (4.17) |
| mfPhNR a ETDRS f R2 e Inferior vs. GCL+ b-T c ETDRS f Area 2 Inferior | 5 (20.83) | 19 (79.17) | 0 (0.00) | 0 (0.00) |
| mfPhNR a ETDRS f R2 e Temporal vs. GCL+ b-T c ETDRS f Area 2 Temporal | 3 (12.50) | 20 (83.33) | 0 (0.00) | 1 (4.17) |
| mfPhNR a ETDRS f R3+ R4 e Suprior vs. GCL+ b-T c ETDRS f Area 3 Superior | 5 (20.83) | 14 (58.33) | 0 (0.00) | 5 (20.83) |
| mfPhNR a ETDRS f R3+ R4 e Nasal vs. GCL+ b-T c ETDRS f Area 3 Nasal | 4 (16.67) | 16 (66.67) | 0 (0.00) | 4 (16.67) |
| mfPhNR a ETDRS f R3+ R4 e Inferior vs. GCL+ b-T c ETDRS f Area 3 Inferior | 4 (16.67) | 19 (79.17) | 0 (0.00) | 1 (4.17) |
| mfPhNR a ETDRS f R3 + R4 e Temporal vs. GCL+ b-T c ETDRS f Area 3 Temporal | 5 (20.83) | 15 (62.50) | 0 (0.00) | 4 (16.67) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbano, L.; Ziccardi, L.; Dell’Aquila, C.; D’Andrea, M.; Gabri Nicoletti, C.; Landi, D.; Mataluni, G.; Di Renzo, A.; Buttari, F.; dell’Omo, R.; et al. Structural and Functional Assessment of the Macular Inner Retinal Layers in Multiple Sclerosis Eyes Without History of Optic Neuropathy. J. Clin. Med. 2025, 14, 5919. https://doi.org/10.3390/jcm14165919
Barbano L, Ziccardi L, Dell’Aquila C, D’Andrea M, Gabri Nicoletti C, Landi D, Mataluni G, Di Renzo A, Buttari F, dell’Omo R, et al. Structural and Functional Assessment of the Macular Inner Retinal Layers in Multiple Sclerosis Eyes Without History of Optic Neuropathy. Journal of Clinical Medicine. 2025; 14(16):5919. https://doi.org/10.3390/jcm14165919
Chicago/Turabian StyleBarbano, Lucilla, Lucia Ziccardi, Carmen Dell’Aquila, Mattia D’Andrea, Carolina Gabri Nicoletti, Doriana Landi, Giorgia Mataluni, Antonio Di Renzo, Fabio Buttari, Roberto dell’Omo, and et al. 2025. "Structural and Functional Assessment of the Macular Inner Retinal Layers in Multiple Sclerosis Eyes Without History of Optic Neuropathy" Journal of Clinical Medicine 14, no. 16: 5919. https://doi.org/10.3390/jcm14165919
APA StyleBarbano, L., Ziccardi, L., Dell’Aquila, C., D’Andrea, M., Gabri Nicoletti, C., Landi, D., Mataluni, G., Di Renzo, A., Buttari, F., dell’Omo, R., Marfia, G. A., Centonze, D., & Parisi, V. (2025). Structural and Functional Assessment of the Macular Inner Retinal Layers in Multiple Sclerosis Eyes Without History of Optic Neuropathy. Journal of Clinical Medicine, 14(16), 5919. https://doi.org/10.3390/jcm14165919







