Temporary Peripheral Nerve Stimulation (PNS) of the Cervical Medial Branch Nerve (CMBN) for Chronic Axial Neck Pain—A Literature Review and Case Series
Abstract
1. Introduction
2. Methods
3. Results
3.1. Literature Review
3.2. Case Series
3.3. Procedure Description
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- GBD 2021 Neck Pain Collaborators. Global, regional, and national burden of neck pain, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2024, 6, e142–e155. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Atkinson, C.; Bhalla, K.; Birbeck, G.; Burstein, R.; Chou, D.; Dellavalle, R.; Danaei, G.; Ezzati, M.; Fahimi, A.; et al. The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Borghouts, J.A.J.; Koes, B.W.; Vondeling, H.; Bouter, L.M. Cost-of-illness of neck pain in The Netherlands in 1996. Pain 1999, 80, 629–636. [Google Scholar] [CrossRef]
- Alexander, E.P. History, physical examination, and differential diagnosis of neck pain. Phys. Med. Rehabil. Clin. N. Am. 2011, 22, 383‐vii. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.; Shah, N.; Padalia, D. Facet Joint Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kirpalani, D.; Mitra, R. Cervical facet joint dysfunction: A review. Arch. Phys. Med. Rehabil. 2008, 89, 770–774. [Google Scholar] [CrossRef]
- van Eerd, M.; Patijn, J.; Lataster, A.; Rosenquist, R.W.; van Kleef, M.; Mekhail, N.; Van Zundert, J. 5. Cervical facet pain. Pain Pract. 2010, 10, 113–123. [Google Scholar] [CrossRef]
- Engel, A.; Rappard, G.; King, W.; Kennedy, D.J.; Standards Division of the International Spine Intervention Society. The Effectiveness and Risks of Fluoroscopically-Guided Cervical Medial Branch Thermal Radiofrequency Neurotomy: A Systematic Review with Comprehensive Analysis of the Published Data. Pain Med. 2016, 17, 658–669. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.; D’Souza, R.S. Peripheral Nerve Stimulation: The Evolution in Pain Medicine. Biomedicines 2021, 10, 18. [Google Scholar] [CrossRef]
- A Gilmore, C.; Ilfeld, B.M.; Rosenow, J.M.; Li, S.; Desai, M.J.; Hunter, C.W.; Rauck, R.L.; Nader, A.; Mak, J.; Cohen, S.P.; et al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg. Anesth. Pain Med. 2019, 45, 44–51. [Google Scholar] [CrossRef]
- Tieppo Francio, V.; Westerhaus, B.D.; Rupp, A.; Sayed, D. Non-Spinal Neuromodulation of the Lumbar Medial Branch Nerve for Chronic Axial Low Back Pain: A Narrative Review. Front. Pain Res. 2022, 3, 835519. [Google Scholar] [CrossRef]
- Strand, N.; D’SOuza, R.S.; Hagedorn, J.M.; Pritzlaff, S.; Sayed, D.; Azeem, N.; Abd-Elsayed, A.; Escobar, A.; A Huntoon, M.; Lam, C.M.; et al. Evidence-Based Clinical Guidelines from the American Society of Pain and Neuroscience for the Use of Implantable Peripheral Nerve Stimulation in the Treatment of Chronic Pain. J. Pain Res. 2022, 15, 2483–2504. [Google Scholar] [CrossRef]
- Char, S.; Jin, M.Y.; Francio, V.T.; Hussain, N.; Wang, E.J.; Morsi, M.; Orhurhu, V.; Prokop, L.J.; Fink, A.; D’souza, R.S. Implantable Peripheral Nerve Stimulation for Peripheral Neuropathic Pain: A Systematic Review of Prospective Studies. Biomedicines 2022, 10, 2606. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Jin, M.Y.; Abd-Elsayed, A. Peripheral Nerve Stimulation for Low Back Pain: A Systematic Review. Curr. Pain Headache Rep. 2023, 27, 117–128. [Google Scholar] [CrossRef]
- Farrar, J.T.; Young, J.P., Jr.; LaMoreaux, L.; Werth, J.L.; Poole, M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Ostelo, R.W.J.G.; Deyo, R.A.; Stratford, P.; Waddell, G.; Croft, P.; Von Korff, M.; Bouter, L.M.; de Vet, H.C. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Lipov, E.G.; Joshi, J.R.; Sanders, S.; Slavin, K.V. Use of peripheral subcutaneous field stimulation for the treatment of axial neck pain: A case report. Neuromodulation 2009, 12, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Burgher, A.H.; Huntoon, M.A.; Turley, T.W.; Doust, M.W.; Stearns, L.J. Subcutaneous peripheral nerve stimulation with inter-lead stimulation for axial neck and low back pain: Case series and review of the literature. Neuromodulation 2012, 15, 100–107. [Google Scholar] [CrossRef]
- Owada, H.; Sumitani, M.; Inoue, R.; Kawashima, M.; Ishii, K.; Shin, M.; Uchida, K. Peripheral nerve field stimulation successfully manages axial pain after posterior cervical spine surgery: Case report. Ann. Palliat. Med. 2021, 10, 5792–5796. [Google Scholar] [CrossRef]
- Haider, N.; Gargya, A. Management of Osteoarthritic Axial Neck Pain with Cervical Neuromodulation. Cureus 2023, 15, e46890. [Google Scholar] [CrossRef]
- Deer, T.R.; Eldabe, S.; Falowski, S.M.; Huntoon, M.A.; Staats, P.S.; Cassar, I.R.; Crosby, N.D.; Boggs, J.W. Peripherally Induced Reconditioning of the Central Nervous System: A Proposed Mechanistic Theory for Sustained Relief of Chronic Pain with Percutaneous Peripheral Nerve Stimulation. J. Pain Res. 2021, 14, 721–736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deer, T.; Shah, A.; Slavin, K.; Vorenkamp, E.K.; Shah, S.; Leong, M.; McRoberts, W.P. Birds of a Feather Redux: Defining Ways to Stimulate the Peripheral Nervous System. J. Pain Res. 2023, 16, 1219–1224. [Google Scholar] [CrossRef]
- Huntoon, M.A.; Slavin, K.V.; Hagedorn, J.M.; Crosby, N.D.; Boggs, J.W. A Retrospective Review of Real-world Outcomes Following 60-day Peripheral Nerve Stimulation for the Treatment of Chronic Pain. Pain Physician 2023, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Mainkar, O.; Singh, H.; Gargya, A.; Lee, J.; Valimahomed, A.; Gulati, A. Ultrasound-Guided Peripheral Nerve Stimulation of Cervical, Thoracic, and Lumbar Spinal Nerves for Dermatomal Pain: A Case Series. Neuromodulation 2021, 24, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Kollenburg, L.; Kurt, E.; Mulleners, W.; Abd-Elsayed, A.; Yazdi, C.; Schatman, M.E.; Yong, R.J.; Cerda, I.H.; Pappy, A.; Ashina, S.; et al. Four Decades of Occipital Nerve Stimulation for Headache Disorders: A Systematic Review. Curr. Pain Headache Rep. 2024, 28, 1015–1034. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Silberstein, S.D.; Reed, K.L.; Deer, T.R.; Slavin, K.V.; Huh, B.; Sharan, A.D.; Narouze, S.; Mogilner, A.Y.; Trentman, T.L.; et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: Long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 2015, 35, 344–358. [Google Scholar] [CrossRef]
- Mekhail, N.A.; Estemalik, E.; Azer, G.; Davis, K.; Tepper, S.J. Safety and Efficacy of Occipital Nerves Stimulation for the Treatment of Chronic Migraines: Randomized, Double-blind, Controlled Single-center Experience. Pain Pract. 2017, 17, 669–677. [Google Scholar] [CrossRef]
- Saper, J.R.; Dodick, D.W.; Silberstein, S.D.; McCarville, S.; Sun, M.; Goadsby, P.J. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia 2011, 31, 271–285. [Google Scholar] [CrossRef]
- Serra, G.; Marchioretto, F. Occipital nerve stimulation for chronic migraine: A randomized trial. Pain Physician 2012, 15, 245–253. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Dodick, D.W.; Saper, J.; Huh, B.; Slavin, K.V.; Sharan, A.; Reed, K.; Narouze, S.; Mogilner, A.; Goldstein, J.; et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: Results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia 2012, 32, 1165–1179, https://doi.org/10.1177/0333102412462642. Reprinted in Cephalalgia 2014, 34, 944. [Google Scholar] [CrossRef]
- Antony, A.B.; Mazzola, A.J.; Dhaliwal, G.S.; Hunter, C.W. Neurostimulation for the Treatment of Chronic Head and Facial Pain: A Literature Review. Pain Physician 2019, 22, 447–477. [Google Scholar] [CrossRef]
- Salmasi, V.; Olatoye, O.O.; Terkawi, A.S.; Hah, J.M.; Ottestad, E.; Pingree, M. Peripheral Nerve Stimulation for Occipital Neuralgia. Pain Med. 2020, 21 (Suppl. 1), S13–S17. [Google Scholar] [CrossRef]
- Moman, R.N.; Olatoye, O.O.; Pingree, M.J. Temporary, Percutaneous Peripheral Nerve Stimulation for Refractory Occipital Neuralgia. Pain Med. 2022, 23, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Valenti, K.; Robinson, C.L.; Orhurhu, V.; Mahmood, S.; Hasoon, J. Case Report: Percutaneous Peripheral Nerve Stimulation for the Treatment of Occipital Neuralgia. Orthop. Rev. 2025, 17, 128100. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Gilmore, C.; Kapural, L.; Hanling, S.; Plunkett, A.; McGee, M.; Boggs, J. Percutaneous Peripheral Nerve Stimulation for Pain Reduction and Improvements in Functional Outcomes in Chronic Low Back Pain. Mil. Med. 2019, 184 (Suppl. S1), 537–541, https://doi.org/10.1093/milmed/usy310. Reprinted in Mil. Med. 2019, 184, e954. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, C.A.; Kapural, L.; McGee, M.J.; Boggs, J.W. Percutaneous Peripheral Nerve Stimulation for Chronic Low Back Pain: Prospective Case Series With 1 Year of Sustained Relief Following Short-Term Implant. Pain Pract. 2020, 20, 310–320. [Google Scholar] [CrossRef]
- Deer, T.R.; Gilmore, C.A.; Desai, M.J.; Li, S.; DePalma, M.J.; Hopkins, T.J.; Burgher, A.H.; Spinner, D.A.; Cohen, S.P.; McGee, M.J.; et al. Percutaneous Peripheral Nerve Stimulation of the Medial Branch Nerves for the Treatment of Chronic Axial Back Pain in Patients After Radiofrequency Ablation. Pain Med. 2021, 22, 548–560, https://doi.org/10.1093/pm/pnaa432; Reprinted in Pain Med. 2021, 22, 1890. [Google Scholar] [CrossRef]
- Gilmore, C.A.; Desai, M.J.; Hopkins, T.J.; Li, S.; DePalma, M.J.; Deer, T.R.; Grace, W.; Burgher, A.H.; Sayal, P.K.; Amirdelfan, K.; et al. Treatment of chronic axial back pain with 60-day percutaneous medial branch PNS: Primary end point results from a prospective, multicenter study. Pain Pract. 2021, 21, 877–889. [Google Scholar] [CrossRef]
- Strand, N.H.; D’sOuza, R.; Wie, C.; Covington, S.; Maita, M.; Freeman, J.; Maloney, J. Mechanism of Action of Peripheral Nerve Stimulation. Curr. Pain Headache Rep. 2021, 25, 47. [Google Scholar] [CrossRef]
- Malinowski, M.N.; Chopra, P.R.; Tieppo Francio, V.; Budwany, R.; Deer, T.R. A narrative review and future considerations of spinal cord stimulation, dorsal root ganglion stimulation and peripheral nerve stimulation. Curr. Opin. Anaesthesiol. 2021, 34, 774–780. [Google Scholar] [CrossRef]
- Lam, C.M.; Keim, S.A.; Latif, U. Novel implantation technique for pudendal nerve peripheral nerve stimulation for treatment of chronic pelvic pain. Reg. Anesth. Pain Med. 2023, 48, 567–571. [Google Scholar] [CrossRef]
- Lam, C.M.; Keim, S.; Sayed, D.; Abd-Elsayed, A.; Gulati, A.; Schatman, M.; Deer, T.; Latif, U. Novel Implantation Technique for Thoracoabdominal Peripheral Nerve Stimulation via a Transversus Abdominal Plane Approach for Treatment of Chronic Abdominal Pain. J Pain Res. 2024, 17, 981–987. [Google Scholar] [CrossRef]
- Goree, J.H.; Grant, S.A.; Dickerson, D.M.; Ilfeld, B.M.; Eshraghi, Y.; Vaid, S.; Valimahomed, A.K.; Shah, J.R.; Smith, G.L.; Finneran, J.J.; et al. Randomized Placebo-Controlled Trial of 60-Day Percutaneous Peripheral Nerve Stimulation Treatment Indicates Relief of Persistent Postoperative Pain, and Improved Function After Knee Replacement. Neuromodulation 2024, 27, 847–861. [Google Scholar] [CrossRef]
- Hatheway, J.; Hersel, A.; Song, J.; Engle, M.; Gutierrez, G.; Khemlani, V.; Kapural, L.; Moore, G.; Ajakwe, R.; Trainor, D.; et al. Clinical study of a micro-implantable pulse generator for the treatment of peripheral neuropathic pain: 3-month and 6-month results from the COMFORT-randomised controlled trial. Reg. Anesth. Pain Med. 2024, 50, 561–567. [Google Scholar] [CrossRef]
- Pritzlaff, S.G.; Latif, U.; Rosenow, J.M.; Chae, J.; Wilson, R.D.; Huffman, W.J.; Crosby, N.D.; Boggs, J.W. A review of prospective studies regarding percutaneous peripheral nerve stimulation treatment in the management of chronic pain. Pain Manag. 2024, 14, 209–222. [Google Scholar] [CrossRef] [PubMed]
- West, T.; Hussain, N.; Bhatia, A.; ElSaban, M.; E Kilgore, A.; Palettas, M.; Abdel-Rasoul, M.; Javed, S.; D’SOuza, R.S. Pain intensity and opioid consumption after temporary and permanent peripheral nerve stimulation: A 2-year multicenter analysis. Reg. Anesth. Pain Med. 2024; ahead of print. [Google Scholar] [CrossRef]
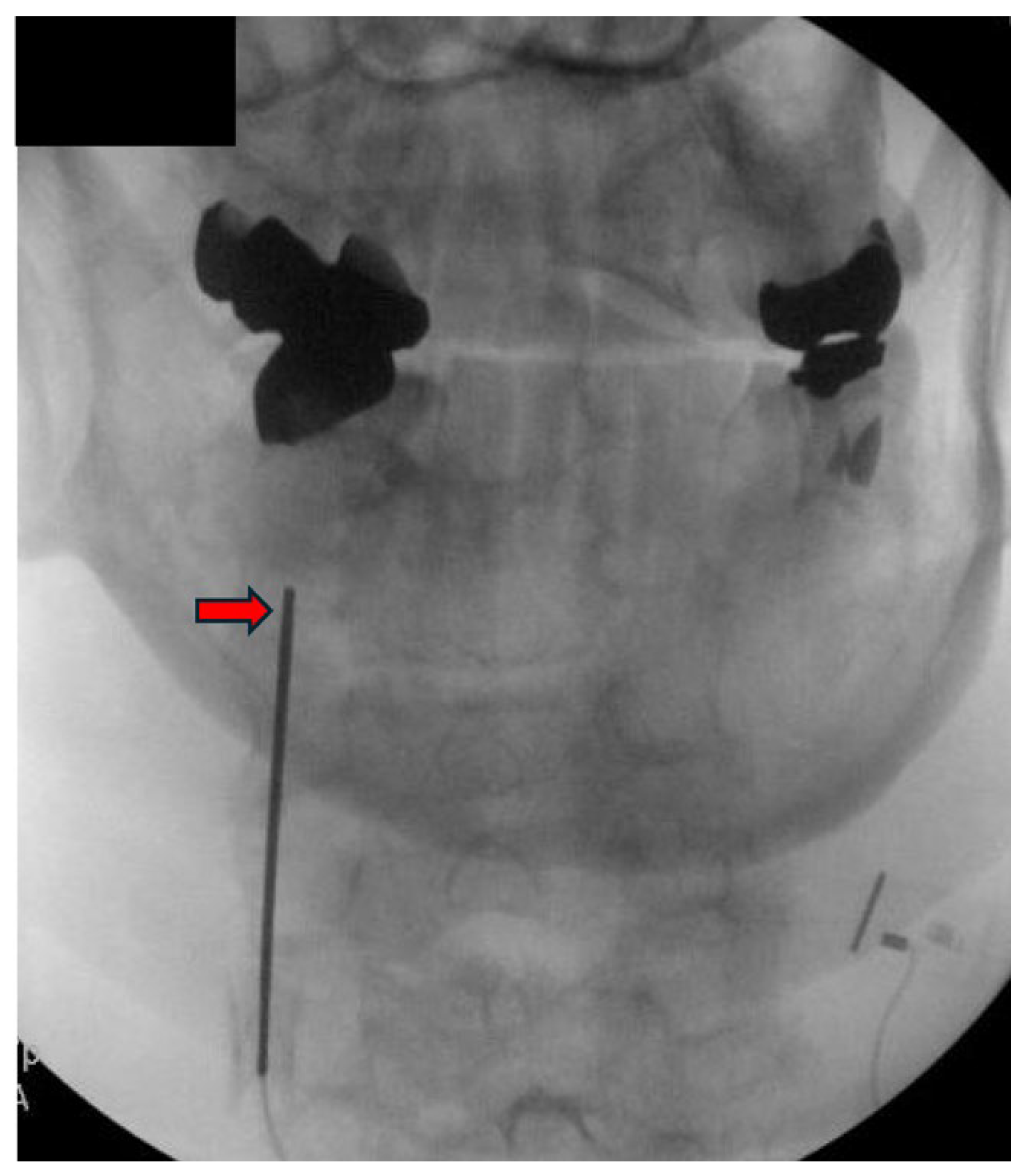

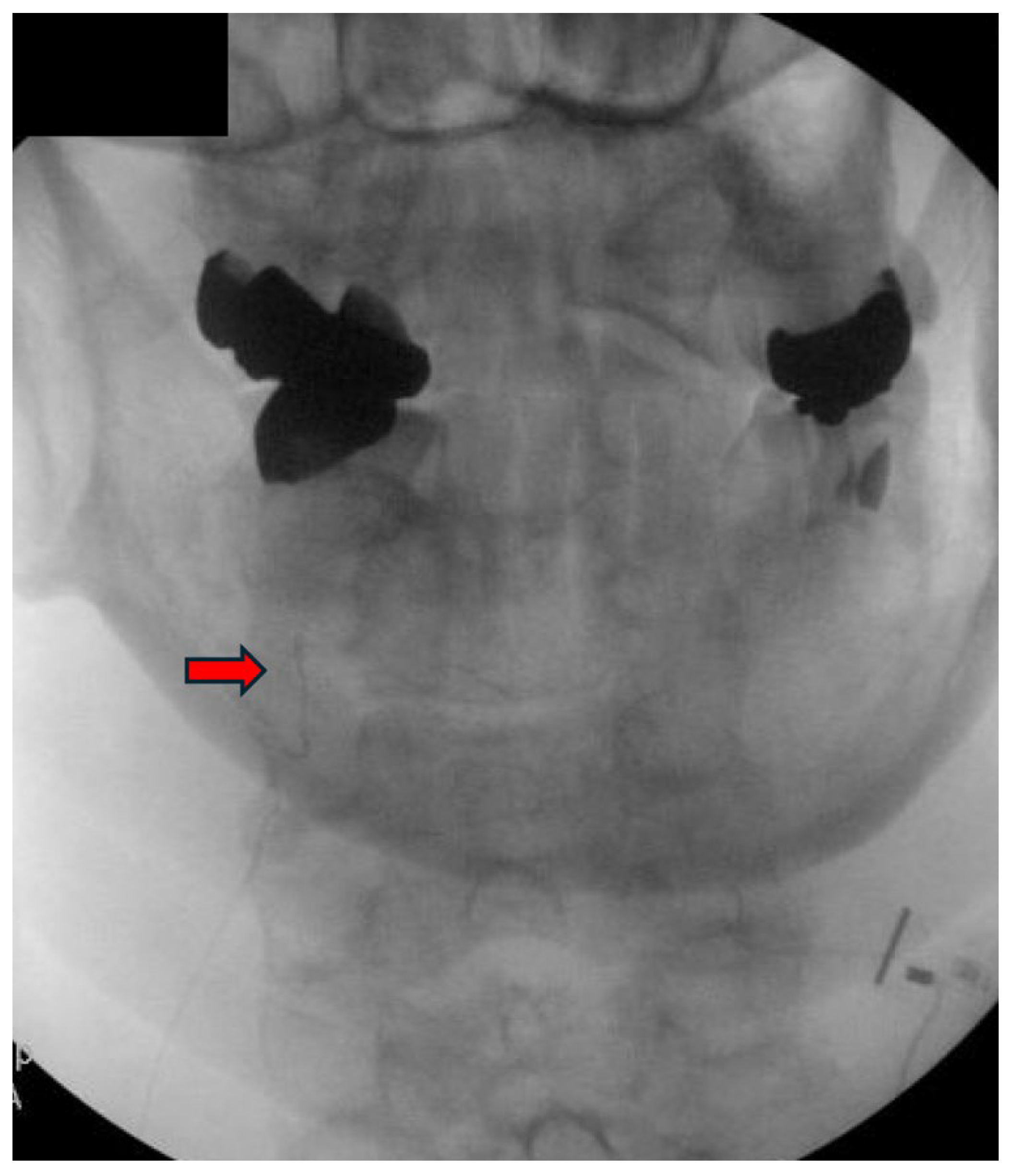
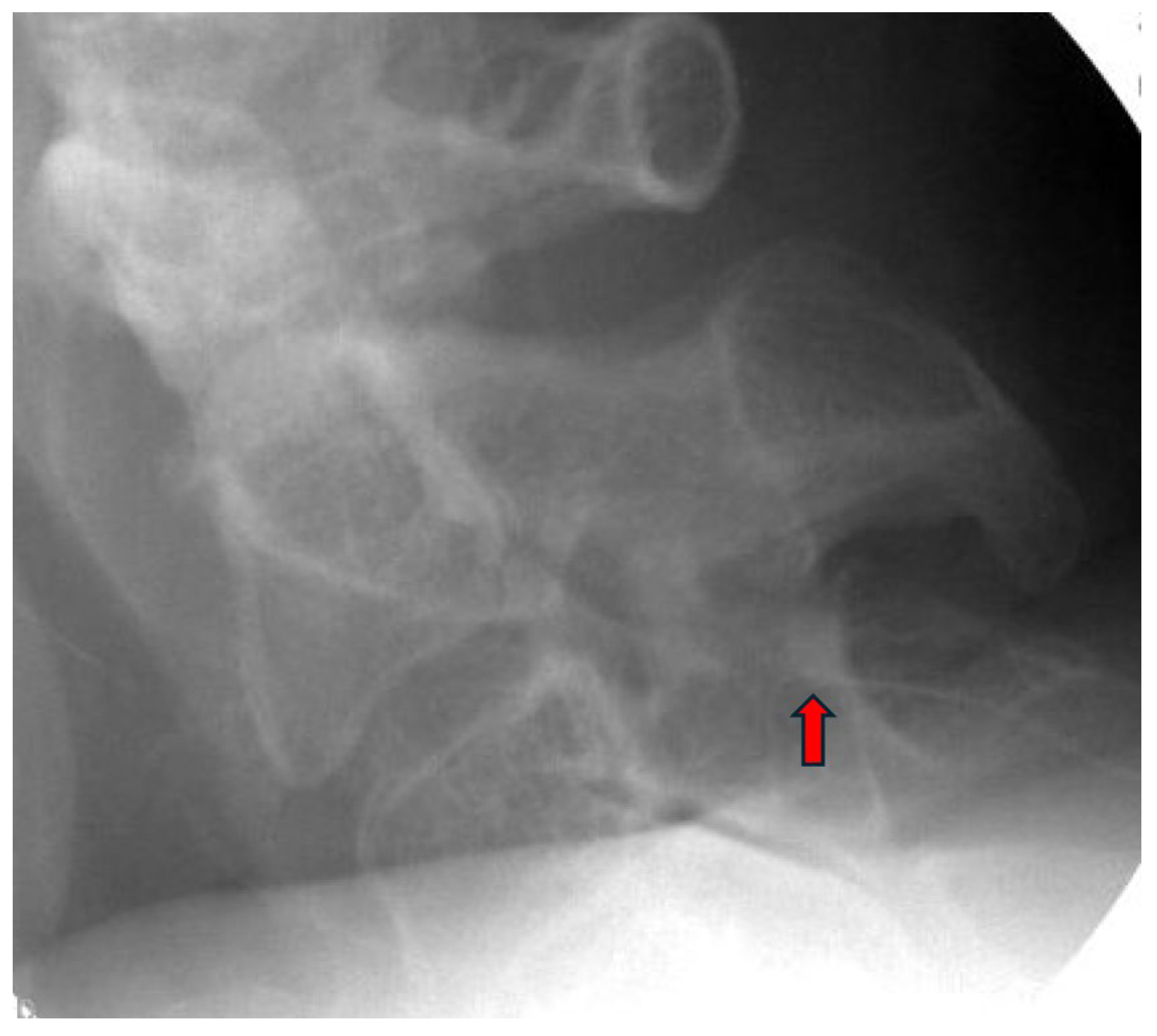
| Author, Year | Study Design | Type of Stimulation | Duration of Stimulation | Results | Comments |
|---|---|---|---|---|---|
| Huntoon et al., 2023 [23] | Retrospective Study | PNS | Temporary 60-day | -Out of a total of 6160 patients who underwent PNS in the study, 76 received PNS targeting the CMBN -Nearly 65% of those patients experienced greater than 50% pain relief and/or clinically significant improvement in the Patient Global Impression of Change (PGIC) | -This study was not limited to the CMBN PNS but encompassed a diverse range of chronic pain etiologies -No subgroup analyses of the CMBN PNS group were conducted -The study did not specify the underlying diagnoses or etiologies of neck pain that were treated |
| Haider et al., 2023 [20] | Case Report | PNS | Temporary 60-day | -A 50% reduction in pain intensity on the NRS was observed, along with subjective improvement in cervical range of motion | -This is the first case report of PNS of the CMBN for the treatment of axial neck pain |
| Owada et al., 2021 [19] | Case Report | PFNS | Permanent | -The patient reported a 70% decrease in both occipital and axial neck pain; however, this improvement was not quantified using a standardized outcome measure | -This is the first case report of PNFS being used to treat both axial neck and occipital neuralgia |
| Mainkar et al., 2021 [24] | Case Series | PNS | Temporary 60-day | -One out of the eleven patients (1/11) underwent targeting of the left C8 nerve root for the treatment of post-mastectomy pain and intercostobrachial neuritis -The patient experienced good paresthesia coverage at implant and two days following; however, they developed a rash shortly after and the device was removed | -This study evaluated PNS of the C8 nerve root and not the CMBN |
| Burgher et al., 2012 [18] | Case Series | PFNS | Permanent | -Ten patients underwent a trial of subcutaneous PFNS, with ten proceeding to permanent implantation -The primary outcome measure was patient-reported pain relief at the last follow-up, with an average improvement of 45%; however, this was not assessed using a standardized outcome measure | -Electrodes were placed in the deep subcutaneous tissue, positioned at the interface between the hypodermis and the first major fascial plane, corresponding to the identified area of pain -The majority of patients in the study had a diagnosis of failed back surgery syndrome, which may limit the generalizability of the findings |
| Lipov et al., 2009 [17] | Case Report | PFNS | Permanent | -The patient reported 100% pain relief sustained through the nine-month follow-up period; however, this outcome was not evaluated using a standardized or validated assessment tool | -This is the first case report of PNFS being used for axial neck pain |
| Case # | Age | Gender | Smoker | Diabetic | Prior CRFA | Prior Neck Surgery | Level of Intervention | NRS at Baseline | NRS at 60-Day Follow-Up | % Pain Relief Reported | ODI at Baseline | ODI at 60-Day Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77 | Female | No | No | Yes | No | Bilateral C4 | 4 | 1 | 80% | 44 | 8 |
| 2 | 79 | Male | Yes | Yes | No | No | Bilateral C6 | 4 | 2 | 70% | 22 | 12 |
| 3 | 81 | Male | No | No | No | No | Bilateral C6 | 6 | 5 | 60% | 32 | 4 |
| 4 | 66 | Female | No | No | Yes | Yes | Bilateral C6 | 4 | 0 | 50% | 40 | 32 |
| 5 | 92 | Female | No | No | No | No | Right C3 and Occipital | 5 | 0 | 30% | 44 | 21 |
| 6 | 83 | Female | Former | No | No | Yes | Bilateral C5 | 7 | 5 | 0% | 40 | 26 |
| 7 | 68 | Male | No | No | Yes | Yes | Bilateral C6 | 3 | 2 | 20% | 24 | 24 |
| 8 | 46 | Male | Yes | No | Yes | Yes | Bilateral C6 | 8 | 8 | 30% | 42 | 32 |
| 9 | 60 | Female | Former | No | Yes | No | Bilateral C5 | 7 | 3 | 60% | 72 | 23 |
| Baseline (Standard Deviation) | 60-Day Follow-Up (Standard Deviation) | Minimal Clinical Important Difference Achieved? | 95% Confidence Interval (CI) | p-Value | |
|---|---|---|---|---|---|
| Pain | 5.3 (SD 1.7) | 2.8 (SD 2.6) | Yes, 60% of subjects | −4.6951 to −0.3049 | 0.02 * |
| Disability | 40.0 (SD 14.5) | 20.2 (SD 10.8) | Yes, 70% of subjects | −33.0729 to −6.527 | 0.006 * |
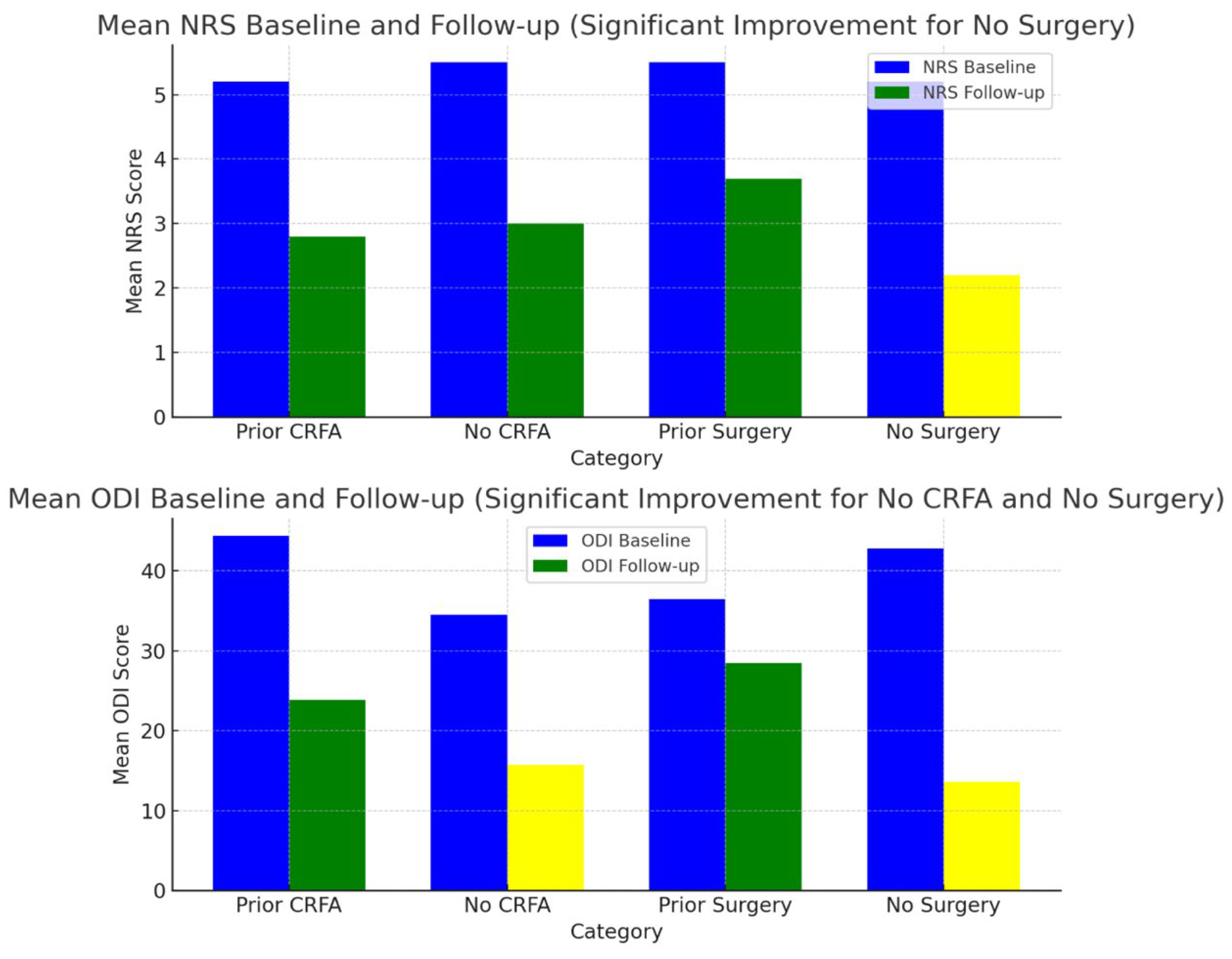
| Total (N) | Mean NRS Baseline (SD) | Mean NRS Follow-Up (SD) | 95% Confidence Interval Related to NRS | p-Value Related to NRS | Mean ODI Baseline (SD) | Mean ODI Follow-Up (SD) | 95% Confidence Interval Related to ODI | p-Value Related to ODI | |
|---|---|---|---|---|---|---|---|---|---|
| Prior CRFA | 5 | 5.2 (2.1) | 2.8 (3.1) | −6.2614 to 1.4614 | 0.18 | 44.4 (17.3) | 23.9 (9.8) | −41.0048 to 0.0048 | 0.05 |
| No CRFA | 4 | 5.5 (1.2) | 3 (2.4) | −5.7829 to 0.7829 | 0.11 | 34.5 (9.7) | 15.7 (9.7) | −35.5832 to −2.0168 | 0.03 * |
| Prior Surgery | 4 | 5.5 (2.3) | 3.7 (3.5) | −6.9239 to 3.3239 | 0.4 | 36.5 (8.3) | 28.5 (4.1) | −19.3261 to 3.3261 | 0.13 |
| No Surgery | 5 | 5.2 (1.3) | 2.2 (1.9) | −5.3742 to −0.6258 | 0.01 * | 42.8 (18.7) | 13.6 (8.2) | −50.2575 to −8.1425 | 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tieppo Francio, V.; Gustafson, K.; Leavitt, L.; Zwick, R.; Lam, C.M.; Sack, A.; Sayed, D.; Latif, U. Temporary Peripheral Nerve Stimulation (PNS) of the Cervical Medial Branch Nerve (CMBN) for Chronic Axial Neck Pain—A Literature Review and Case Series. J. Clin. Med. 2025, 14, 5910. https://doi.org/10.3390/jcm14165910
Tieppo Francio V, Gustafson K, Leavitt L, Zwick R, Lam CM, Sack A, Sayed D, Latif U. Temporary Peripheral Nerve Stimulation (PNS) of the Cervical Medial Branch Nerve (CMBN) for Chronic Axial Neck Pain—A Literature Review and Case Series. Journal of Clinical Medicine. 2025; 14(16):5910. https://doi.org/10.3390/jcm14165910
Chicago/Turabian StyleTieppo Francio, Vinicius, Kelsey Gustafson, Logan Leavitt, Ryan Zwick, Christopher M. Lam, Andrew Sack, Dawood Sayed, and Usman Latif. 2025. "Temporary Peripheral Nerve Stimulation (PNS) of the Cervical Medial Branch Nerve (CMBN) for Chronic Axial Neck Pain—A Literature Review and Case Series" Journal of Clinical Medicine 14, no. 16: 5910. https://doi.org/10.3390/jcm14165910
APA StyleTieppo Francio, V., Gustafson, K., Leavitt, L., Zwick, R., Lam, C. M., Sack, A., Sayed, D., & Latif, U. (2025). Temporary Peripheral Nerve Stimulation (PNS) of the Cervical Medial Branch Nerve (CMBN) for Chronic Axial Neck Pain—A Literature Review and Case Series. Journal of Clinical Medicine, 14(16), 5910. https://doi.org/10.3390/jcm14165910






