Long-Term Impact of Chronic Obstructive Pulmonary Disease and Atrial Fibrillation on Post-Acute Myocardial Infarction Long-Term All-Cause Mortality: Insights from the SAMI III Project
Abstract
1. Introduction
2. Methods
2.1. Study Population and Outcomes
2.2. Data Collection and Definitions
2.3. Statistical Analysis
3. Results
3.1. Study Population and Groups
3.2. Patient Characteristics
3.3. Follow-Up and Primary Outcome
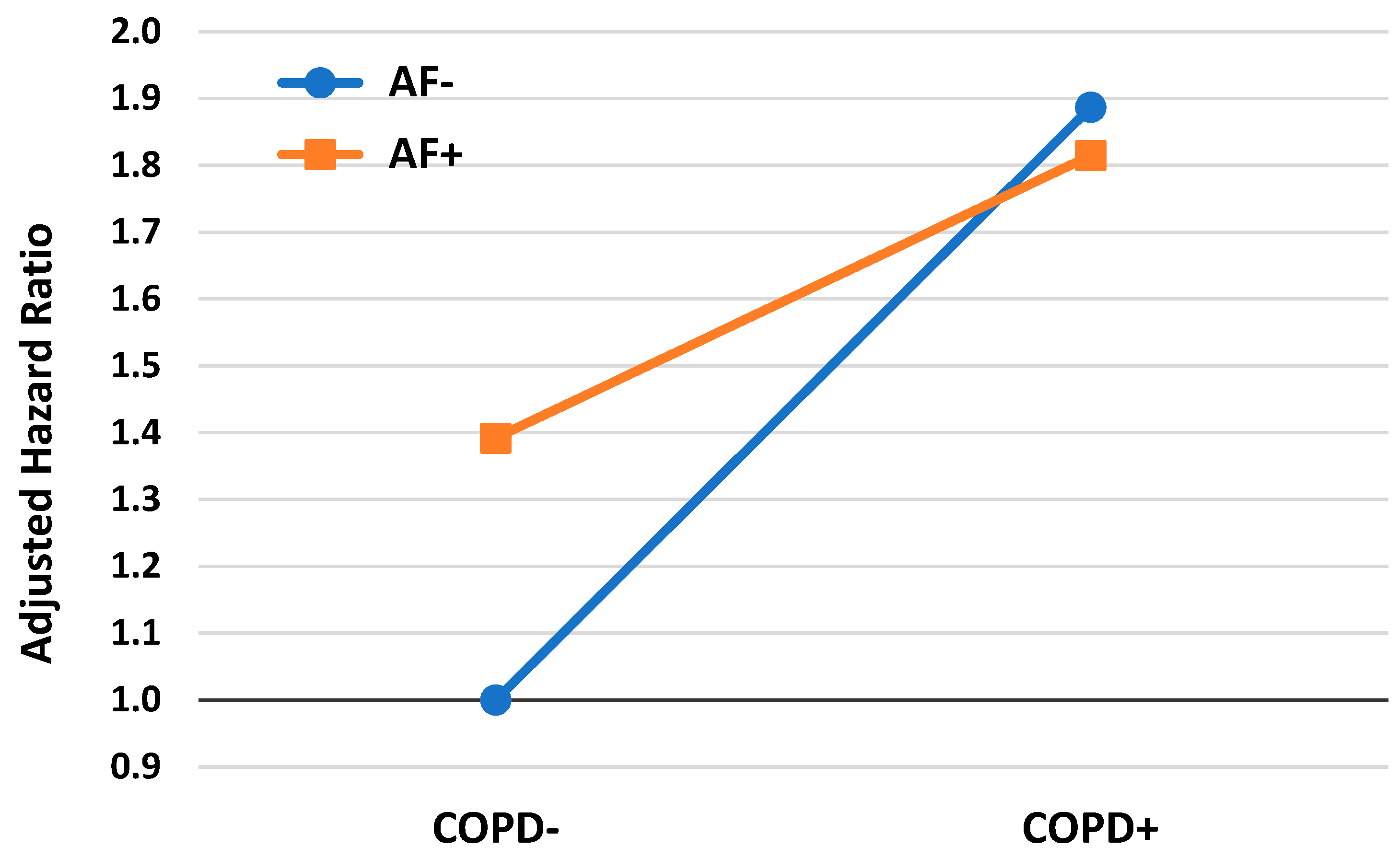
3.4. COPD, AF, and the Risk for Mortality
3.5. Multivariable Analysis
3.6. Sub-Group Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AMI | Acute myocardial infarction |
| CABG | Coronary artery bypass grafting |
| CAD | Coronary artery disease |
| COPD | Chronic obstructive pulmonary disease |
| ECG | Electrocardiogram |
| FEV1 | Forced expiratory volume in 1 s |
| HR | Hazard ratio |
| ICD-9-CM | International Classification of Diseases, Ninth Revision, Clinical Modification |
| IQR | Interquartile range |
| LV | Left ventricular |
| MMPs | Metalloproteinases |
| NSTEMI | Non-ST-elevation myocardial infarction |
| PCI | Percutaneous coronary intervention |
| STEMI | ST-elevation myocardial infarction |
References
- Plakht, Y.; Gilutz, H.; Shiyovich, A. Excess long-term mortality among hospital survivors of acute myocardial infarction. Soroka Acute Myocardial Infarction (SAMI) project. Public Health 2017, 143, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Plakht, Y.; Abu Tailakh, M.; Barabi, T.; Shiyovich, A. Ethnic disparities in emergency department utilization patterns in southern Israel: A population-based study. Intern. Emerg. Med. 2012, 7, 547–555. [Google Scholar] [CrossRef]
- Shiyovich, A.; Berman, A.N.; Besser, S.A.; Biery, D.W.; Cardoso, R.; Divakaran, S.; Singh, A.; Huck, D.M.; Weber, B.; Plutzky, J.; et al. Lipoprotein(a) as a cardiovascular risk factor among patients with and without diabetes Mellitus: The Mass General Brigham Lp(a) Registry. Cardiovasc. Diabetol. 2024, 23, 257. [Google Scholar] [CrossRef]
- Bucci, T.; Romiti, G.F.; Shantsila, A.; Teo, W.S.; Park, H.W.; Shimizu, W.; Corica, B.; Proietti, M.; Tse, H.F.; Chao, T.F.; et al. Risk of Death and Cardiovascular Events in Asian Patients with Atrial Fibrillation and Chronic Obstructive Pulmonary Disease: A Report from the Prospective APHRS Registry. J. Am. Heart Assoc. 2024, 13, e032785. [Google Scholar] [CrossRef]
- Shiyovich, A.; Axelrod, M.; Gilutz, H.; Plakht, Y. Early Versus Late New-Onset Atrial Fibrillation in Acute Myocardial Infarction: Differences in Clinical Characteristics and Predictors. Angiology 2019, 70, 921–928. [Google Scholar] [CrossRef]
- Shiyovich, A.; Chodick, G.; Azani, L.; Tirosh, M.; Shuvy, M.; Pereg, D.; Katz, A.; Minha, S. Sex-specific contemporary trends in incidence, prevalence and survival of patients with non-valvular atrial fibrillation: A long-term real-world data analysis. PLoS ONE 2021, 16, e0247097. [Google Scholar] [CrossRef]
- Wallström, O.; Stridsman, C.; Lindberg, A.; Nyberg, F.; Vanfleteren, L. Exacerbation History and Risk of Myocardial Infarction and Pulmonary Embolism in COPD. Chest 2024, 166, 1347–1359. [Google Scholar] [CrossRef]
- Romiti, G.F.; Corica, B.; Pipitone, E.; Vitolo, M.; Raparelli, V.; Basili, S.; Boriani, G.; Harari, S.; Lip, G.Y.H.; Proietti, M. Prevalence, management and impact of chronic obstructive pulmonary disease in atrial fibrillation: A systematic review and meta-analysis of 4,200,000 patients. Eur. Heart J. 2021, 42, 3541–3554. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Corica, B.; Mei, D.A.; Frost, F.; Bisson, A.; Boriani, G.; Bucci, T.; Olshansky, B.; Chao, T.F.; Huisman, M.V.; et al. Impact of chronic obstructive pulmonary disease in patients with atrial fibrillation: An analysis from the GLORIA-AF registry. EP Eur. 2023, 26, euae021. [Google Scholar] [CrossRef] [PubMed]
- Warming, P.E.; Garcia, R.; Hansen, C.J.; Simons, S.O.; Torp-Pedersen, C.; Linz, D.; Tfelt-Hansen, J. Atrial fibrillation and chronic obstructive pulmonary disease: Diagnostic sequence and mortality risk. Eur. Heart J.-Qual. Care Clin. Outcomes 2023, 9, 128–134. [Google Scholar] [CrossRef]
- Camm, A.J.; Lip, G.Y.; De Caterina, R.; Savelieva, I.; Atar, D.; Hohnloser, S.H.; Hindricks, G.; Kirchhof, P. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2012, 33, 2719–2747. [Google Scholar] [CrossRef]
- Ioannides, A.E.; Tayal, U.; Quint, J.K. Spirometry in atrial fibrillation: What’s the catch? Expert Rev. Respir. Med. 2023, 17, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S.; Lyubavin, A. Early Detection of Atrial Fibrillation in Chronic Obstructive Pulmonary Disease Patients. Med. 2024, 60, 352. [Google Scholar] [CrossRef]
- Sá-Sousa, A.; Rodrigues, C.; Jácome, C.; Cardoso, J.; Fortuna, I.; Guimarães, M.; Pinto, P.; Sarmento, P.M.; Baptista, R. Cardiovascular Risk in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review. J. Clin. Med. 2024, 13, 5173. [Google Scholar] [CrossRef] [PubMed]
- Maclagan, L.C.; Croxford, R.; Chu, A.; Sin, D.D.; Udell, J.A.; Lee, D.S.; Austin, P.C.; Gershon, A.S. Quantifying COPD as a risk factor for cardiac disease in a primary prevention cohort. Eur. Respir. J. 2023, 62, 2202364. [Google Scholar] [CrossRef]
- Qi, S.; Li, X.; Jiang, Y.; Zhu, T.; Ze, L.; Li, Z.; Wang, W. Analysis of risk factors and development of predictive model for acute myocardial injury in patients with acute exacerbation of chronic obstructive pulmonary disease. J. Thorac. Dis. 2025, 17, 1977–1990. [Google Scholar] [CrossRef]
- Goedemans, L.; Abou, R.; Montero-Cabezas, J.M.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Chronic Obstructive Pulmonary Disease and Risk of Atrial Arrhythmias After ST-Segment Elevation Myocardial Infarction. J. Atr. Fibrillation 2020, 13, 2360. [Google Scholar] [CrossRef]
- Gu, J.; Liu, X.; Tan, H.; Zhou, L.; Jiang, W.; Wang, Y.; Liu, Y. Impact of chronic obstructive pulmonary disease on procedural outcomes and quality of life in patients with atrial fibrillation undergoing catheter ablation. J. Cardiovasc. Electrophysiol. 2013, 24, 148–154. [Google Scholar] [CrossRef]
- Méndez-Bailón, M.; Lopez-de-Andrés, A.; de Miguel-Diez, J.; de Miguel-Yanes, J.M.; Hernández-Barrera, V.; Muñoz-Rivas, N.; Lorenzo-Villalba, N.; Jiménez-García, R. Chronic obstructive pulmonary disease predicts higher incidence and in hospital mortality for atrial fibrillation. An observational study using hospital discharge data in Spain (2004–2013). Int. J. Cardiol. 2017, 236, 209–215. [Google Scholar] [CrossRef]
- Plakht, Y.; Shiyovich, A.; Weitzman, S.; Fraser, D.; Zahger, D.; Gilutz, H. A new risk score predicting 1- and 5-year mortality following acute myocardial infarction Soroka Acute Myocardial Infarction (SAMI) Project. Int. J. Cardiol. 2012, 154, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Plakht, Y.; Shiyovich, A.; Gilutz, H. Predictors of long-term (10-year) mortality postmyocardial infarction: Age-related differences. Soroka Acute Myocardial Infarction (SAMI) Project. J. Cardiol. 2015, 65, 216–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Boriani, G.; Proietti, M.; Laroche, C.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.; Dan, G.A.; Kalarus, Z.; Diemberger, I.; et al. Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: A report from the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) Long-Term General Registry. EP Eur. 2018, 20, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Konecny, T.; Park, J.Y.; Somers, K.R.; Konecny, D.; Orban, M.; Soucek, F.; Parker, K.O.; Scanlon, P.D.; Asirvatham, S.J.; Brady, P.A.; et al. Relation of chronic obstructive pulmonary disease to atrial and ventricular arrhythmias. Am. J. Cardiol. 2014, 114, 272–277. [Google Scholar] [CrossRef]
- Chen, W.; Thomas, J.; Sadatsafavi, M.; FitzGerald, J.M. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Respir. Med. 2015, 3, 631–639. [Google Scholar] [CrossRef]
- Goedemans, L.; Bax, J.J.; Delgado, V. COPD and acute myocardial infarction. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2020, 29, 190139. [Google Scholar] [CrossRef]
- Li, J.; Agarwal, S.K.; Alonso, A.; Blecker, S.; Chamberlain, A.M.; London, S.J.; Loehr, L.R.; McNeill, A.M.; Poole, C.; Soliman, E.Z.; et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2014, 129, 971–980. [Google Scholar] [CrossRef]
- Saleh, M.; Coleman, K.; Fishbein, J.; Gandomi, A.; Yang, B.; Kossack, A.; Varrias, D.; Jauhar, R.; Lasic, Z.; Kim, M.; et al. In-hospital outcomes and postdischarge mortality in patients with acute coronary syndrome and atrial fibrillation. Heart Rhythm 2024, 21, 1658–1668. [Google Scholar] [CrossRef]
- Santos, H.; Santos, M.; Almeida, I.; Paula, S.B.; Almeida, S.; Chin, J.; Almeida, L. Early and late new-onset of atrial fibrillation in acute coronary syndromes: Their differences in mortality and cardiac event. J. Arrhythmia 2022, 38, 299–306. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Agbaedeng, T.A.; Nyaga, U.F.; Lau, D.H.; Worthley, M.I.; Nicholls, S.J.; Sanders, P. Atrial fibrillation incidence, prevalence, predictors, and adverse outcomes in acute coronary syndromes: A pooled analysis of data from 8 million patients. J. Cardiovasc. Electrophysiol. 2022, 33, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Buchta, P.; Kalarus, Z.; Mizia-Stec, K.; Myrda, K.; Skrzypek, M.; Ga Sior, M. De novo and pre-existing atrial fibrillation in acute coronary syndromes: Impact on prognosis and cardiovascular events in long-term follow-up. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 1129–1139. [Google Scholar] [CrossRef]
- Worme, M.D.; Tan, M.K.; Armstrong, D.W.J.; Yan, A.T.; Tan, N.S.; Brieger, D.; Budaj, A.; Gore, J.M.; López-Sendón, J.; Van de Werf, F.; et al. Previous and New Onset Atrial Fibrillation and Associated Outcomes in Acute Coronary Syndromes (from the Global Registry of Acute Coronary Events). Am. J. Cardiol. 2018, 122, 944–951. [Google Scholar] [CrossRef] [PubMed]
- LaFon, D.C.; Helgeson, E.S.; Lindberg, S.; Voelker, H.; Bhatt, S.P.; Casaburi, R.; Cassady, S.J.; Connett, J.; Criner, G.J.; Hatipoglu, U.; et al. β-Blocker Use and Clinical Outcomes in Patients With COPD Following Acute Myocardial Infarction. JAMA Netw. Open 2024, 7, e247535. [Google Scholar] [CrossRef]
- Kundu, A.; O’Day, K.; Shaikh, A.Y.; Lessard, D.M.; Saczynski, J.S.; Yarzebski, J.; Darling, C.E.; Thabet, R.; Akhter, M.W.; Floyd, K.C.; et al. Relation of Atrial Fibrillation in Acute Myocardial Infarction to In-Hospital Complications and Early Hospital Readmission. Am. J. Cardiol. 2016, 117, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.H.; Dabbous, O.H.; Granger, C.B.; Kuznetsova, P.; Kline-Rogers, E.M.; Anderson, F.A., Jr.; Fox, K.A.; Gore, J.M.; Goldberg, R.J.; Eagle, K.A. Comparison of outcomes of patients with acute coronary syndromes with and without atrial fibrillation. Am. J. Cardiol. 2003, 92, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Agrawal, S.; Garg, L.; Garg, A.; Bhatia, N.; Kadaria, D.; Reed, G. Effect of Chronic Obstructive Pulmonary Disease on In-Hospital Mortality and Clinical Outcomes After ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2017, 119, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.M.; Huang, Z.; Pieper, K.S.; Solomon, S.D.; Kober, L.; Velazquez, E.J.; Swedberg, K.; Pfeffer, M.A.; McMurray, J.J.; Maggioni, A.P. Chronic obstructive pulmonary disease is an independent predictor of death but not atherosclerotic events in patients with myocardial infarction: Analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT). Eur. J. Heart Fail. 2009, 11, 292–298. [Google Scholar] [CrossRef]
- Sin, D.D.; Man, S.F. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 2003, 107, 1514–1519. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Seemungal, T.A.; MacCallum, P.K.; Paul, E.A.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Meade, T.W. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb. Haemost. 2000, 84, 210–215. [Google Scholar] [CrossRef]
- Pelgrim, C.E.; Peterson, J.D.; Gosker, H.R.; Schols, A.; van Helvoort, A.; Garssen, J.; Folkerts, G.; Kraneveld, A.D. Psychological co-morbidities in COPD: Targeting systemic inflammation, a benefit for both? Eur. J. Pharmacol. 2019, 842, 99–110. [Google Scholar] [CrossRef]
- Schamroth Pravda, N.; Golovchiner, G.; Goldenberg, G.; Plakht, Y.; Wiessman, M.; Tal, S.; Barsheshet, A.; Kadmon, E.; Erez, A.; Skalsky, K.; et al. Albumin as a Prognostic Marker for Atrial Fibrillation Recurrence following Cryoballoon Ablation of Pulmonary Venous. J. Clin. Med. 2022, 12, 264. [Google Scholar] [CrossRef]
- Corban, M.T.; Godo, S.; Burczak, D.R.; Noseworthy, P.A.; Toya, T.; Lewis, B.R.; Lerman, L.O.; Gulati, R.; Lerman, A. Coronary Endothelial Dysfunction Is Associated With Increased Risk of Incident Atrial Fibrillation. J. Am. Heart Assoc. 2020, 9, e014850. [Google Scholar] [CrossRef] [PubMed]
- Corban, M.T.; Toya, T.; Ahmad, A.; Lerman, L.O.; Lee, H.C.; Lerman, A. Atrial Fibrillation and Endothelial Dysfunction: A Potential Link? Mayo Clin. Proc. 2021, 96, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Caram, L.M.; Ferrari, R.; Naves, C.R.; Tanni, S.E.; Coelho, L.S.; Zanati, S.G.; Minicucci, M.F.; Godoy, I. Association between left ventricular diastolic dysfunction and severity of chronic obstructive pulmonary disease. Clinics 2013, 68, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Søyseth, V.; Kononova, N.; Neukamm, A.; Holmedahl, N.H.; Hagve, T.A.; Omland, T.; Einvik, G. Systemic inflammation induced by exacerbation of COPD or pneumonia in patients with COPD induces cardiac troponin elevation. BMJ Open Respir. Res. 2021, 8, e000997. [Google Scholar] [CrossRef]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Durheim, M.T.; Cyr, D.D.; Lopes, R.D.; Thomas, L.E.; Tsuang, W.M.; Gersh, B.J.; Held, C.; Wallentin, L.; Granger, C.B.; Palmer, S.M.; et al. Chronic obstructive pulmonary disease in patients with atrial fibrillation: Insights from the ARISTOTLE trial. Int. J. Cardiol. 2016, 202, 589–594. [Google Scholar] [CrossRef]
- Liao, K.M.; Chen, P.J.; Chen, C.Y. Prescribing patterns in patients with chronic obstructive pulmonary disease and atrial fibrillation. Open Med. 2023, 18, 20230864. [Google Scholar] [CrossRef]
- Metzner, A.; Suling, A.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.; Eckardt, L.; Elvan, A.; Goette, A.; Haegeli, L.M.; et al. Anticoagulation, therapy of concomitant conditions, and early rhythm control therapy: A detailed analysis of treatment patterns in the EAST—AFNET 4 trial. EP Eur. 2022, 24, 552–564. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Pisters, R.; Nieuwlaat, R.; Prins, M.H.; Le Heuzey, J.Y.; Maggioni, A.P.; Camm, A.J.; Crijns, H.J. Clinical correlates of immediate success and outcome at 1-year follow-up of real-world cardioversion of atrial fibrillation: The Euro Heart Survey. EP Eur. 2012, 14, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, B.; Skinner, D.; Devereux, G.; Thomas, V.; Ling Zhi Jie, J.; Martin, J.; Carter, V.; Price, D.B. Underuse of β-blockers in heart failure and chronic obstructive pulmonary disease. Heart (Br. Card. Soc.) 2016, 102, 1909–1914. [Google Scholar] [CrossRef]
- Maraey, A.M.; Maqsood, M.H.; Khalil, M.; Hashim, A.; Elzanaty, A.M.; Elsharnoby, H.R.; Elsheikh, E.; Elbatanony, L.; Ong, K.; Chacko, P. Impact of Chronic Obstructive Pulmonary Disease on Atrial Fibrillation Ablation Outcomes According to the National Readmission Database. J. Innov. Card. Rhythm Manag. 2022, 13, 5112–5119. [Google Scholar] [CrossRef]
- Steer, J.; Gibson, J.; Bourke, S.C. The DECAF Score: Predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax 2012, 67, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Popovic, D.S.; Pamporis, K.; Theofilis, P.; Nasoufidou, A.; Stachteas, P.; Samaras, A.; Tzikas, A.; Giannakoulas, G.; et al. Effects of mineralocorticoid receptor antagonists on new-onset or recurrent atrial fibrillation: A Bayesian and frequentist network meta-analysis of randomized trials. Curr. Probl. Cardiol. 2024, 49, 102742. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value | Study Group | Total | p | |||
|---|---|---|---|---|---|---|---|
| 1 (COPD−, AF−) | 2 (COPD+, AF−) | 3 (COPD−, AF+) | 4 (COPD+, AF+) | ||||
| n | 11,892 | 1010 | 2171 | 376 | 15,449 | ||
| Demographics | |||||||
| Age, years | Mean (SD) | 63.38 (13.72) † | 70.50 (11.11) #† | 75.28 (11.38) # | 75.42 (9.65) # | 65.81 (13.94) | <0.001 |
| <65 | 6833 (57.5) † | 312 (30.9) #† | 402 (18.5) # | 56 (14.9) # | 7603 (49.2) | <0.001 | |
| 65–75 | 2478 (20.8) † | 346 (34.3) # | 544 (25.1) #† | 123 (32.7) # | 3491 (22.6) | ||
| ≥75 | 2581 (21.7) † | 352 (34.9) #† | 1225 (56.4) # | 197 (52.4) # | 4355 (28.2) | ||
| Sex | Male | 8691 (73.1) | 746 (73.9) | 1188 (54.7) #† | 266 (70.7) | 10,891 (70.5) | <0.001 |
| Ethnicity | Arab/other | 2051 (17.2) | 265 (26.2) # | 215 (9.9) #† | 75 (19.9) | 2606 (16.9) | <0.001 |
| Cardiac diseases | |||||||
| Cardiomegaly | 937 (7.9) † | 163 (16.1) #† | 368 (17.0) #† | 100 (26.6) # | 1568 (10.1) | <0.001 | |
| CHF | 1816 (15.3) † | 291 (28.8) #† | 728 (33.5) #† | 162 (43.1) # | 2997 (19.4) | <0.001 | |
| Pulmonary heart disease | 752 (6.3) † | 184 (18.2) #† | 460 (21.2) #† | 137 (36.4) # | 1533 (9.9) | <0.001 | |
| CIHD | 10,023 (84.3) | 836 (82.8) | 1637 (75.4) # | 305 (81.1) | 12,801 (82.9) | <0.001 | |
| s/p MI | 2118 (17.8) † | 302 (29.9) # | 542 (25.0) #† | 131 (34.8) # | 3093 (20.0) | <0.001 | |
| s/p PCI | 2328 (19.6) | 283 (28) | 479 (22.1) | 113 (30.1) | 3203 (20.7) | <0.001 | |
| s/p CABG | 997 (8.4) † | 126 (12.5) #† | 324 (14.9) #† | 79 (21.0) # | 1526 (9.9) | <0.001 | |
| AV block | 410 (3.4) † | 30 (3.0) † | 96 (4.4) | 27 (7.2) # | 563 (3.6) | <0.001 | |
| Cardiovascular risk factors | |||||||
| Chronic kidney disease | 931 (7.8) † | 126 (12.5) #† | 355 (16.4) # | 70 (18.6) # | 1482 (9.6) | <0.001 | |
| Diabetes mellitus | 4715 (39.6) † | 517 (51.2) # | 1004 (46.2) # | 189 (50.3) # | 6425 (41.6) | <0.001 | |
| Dyslipidemia | 9924 (83.5) † | 798 (79.0) # | 1677 (77.2) # | 292 (77.7) # | 12,691 (82.1) | <0.001 | |
| Hypertension | 6176 (51.9) † | 564 (55.8) | 1463 (67.4) # | 239 (63.6) # | 8442 (54.6) | <0.001 | |
| Obesity | 2667 (22.4) | 226 (22.4) | 414 (19.1) # | 83 (22.1) | 3390 (21.9) | 0.007 | |
| Smoking | 5540 (46.6) | 656 (65.0) #† | 426 (19.6) #† | 198 (52.7) | 6820 (44.1) | <0.001 | |
| PVD | 1195 (10.0) † | 182 (18.0) # | 356 (16.4) # | 73 (19.4) # | 1806 (11.7) | <0.001 | |
| Family history of IHD | 1434 (12.1) † | 51 (5.0) # | 74 (3.4) # | 7 (1.9) # | 1566 (10.1) | <0.001 | |
| Other disorders | |||||||
| Neurological disorders | 1748 (14.7) † | 183 (18.1) # | 565 (26.0) # | 84 (22.3) # | 2580 (16.7) | <0.001 | |
| Malignancy | 447 (3.8) | 50 (5.0) | 117 (5.4) # | 24 (6.4) | 638 (4.1) | <0.001 | |
| Anemia | 4846 (40.8) † | 534 (52.9) #† | 1229 (56.6) # | 236 (62.8) # | 6845 (44.3) | <0.001 | |
| GI bleeding | 206 (1.7) † | 24 (2.4) † | 83 (3.8) # | 21 (5.6) # | 334 (2.2) | <0.001 | |
| Schizophrenia/Psychosis | 171 (1.4) | 24 (2.4) | 46 (2.1) | 6 (1.6) | 247 (1.6) | 0.023 | |
| Alcohol/drug addiction | 245 (2.1) | 44 (4.4) # | 22 (1.0) #† | 10 (2.7) | 321 (2.1) | <0.001 | |
| History of malignancy | 575 (4.8) † | 75 (7.4) # | 180 (8.3) # | 37 (9.8) # | 867 (5.6) | <0.001 | |
| Characteristics of AMI | |||||||
| Type of AMI | STEMI | 5679 (47.8) † | 311 (30.8) # | 593 (27.3) # | 90 (23.9) # | 6673 (43.2) | <0.001 |
| Admitted/transposed to ICCU | 8238 (69.3) † | 527 (52.2) #† | 984 (45.3) # | 150 (39.9) # | 9899 (64.1) | <0.001 | |
| Length of hospital stay, days | Mean (SD) | 9.30 (8.81) † | 10.88 (9.81) #† | 11.73 (11.18) #† | 13.53 (15.82) # | 9.85 (9.53) | <0.001 |
| ≥7 | 5057 (42.5) † | 519 (51.4) # | 1187 (54.7) # | 211 (56.1) # | 6974 (45.1) | <0.001 | |
| Type of treatment | Noninvasive | 2798 (23.5) † | 405 (40.1) #† | 1043 (48.0) #† | 220 (58.5) # | 4466 (28.9) | <0.001 |
| PCI | 7378 (62.0) † | 502 (49.7) #† | 913 (42.1) # | 134 (35.6) # | 8927 (57.8) | ||
| CABG | 1716 (14.4) † | 103 (10.2) # | 215 (9.9) # | 22 (5.9) # | 2056 (13.3) | ||
| Acute in-hospital events | |||||||
| Cardiac arrest | 36 (0.3) | 4 (0.4) | 12 (0.6) | 2 (0.5) | 54 (0.3) | 0.291 | |
| Cardiogenic shock | 180 (1.5) | 23 (2.3) | 70 (3.2) # | 12 (3.2) | 285 (1.8) | <0.001 | |
| Intra-aortic balloon pulsation | 257 (2.2) | 13 (1.3) | 61 (2.8) | 6 (1.6) | 337 (2.2) | 0.038 | |
| Any form of pacing | 201 (1.7) † | 16 (1.6) † | 74 (3.4) # | 19 (5.1) # | 310 (2.0) | <0.001 | |
| Mechanical ventilation | 368 (3.1) † | 69 (6.8) # | 122 (5.6) #† | 39 (10.4) # | 598 (3.9) | <0.001 | |
| Blood transfusion | 1413 (11.9) † | 136 (13.5) | 353 (16.3) # | 71 (18.9) # | 1973 (12.8) | <0.001 | |
| Sepsis | 92 (0.8) | 15 (1.5) | 37 (1.7) # | 6 (1.6) | 150 (1) | <0.001 | |
| Results of echocardiography | |||||||
| Echocardiography performance | n | 8780 | 617 | 1305 | 206 | 10,908 | |
| Severe LV dysfunction | 908 (10.3) † | 105 (17.0) # | 224 (17.2) # | 43 (20.9) # | 1280 (11.7) | <0.001 | |
| LV hypertrophy | 398 (4.5) † | 40 (6.5) | 112 (8.6) # | 21 (10.2) # | 571 (5.2) | <0.001 | |
| Mitral regurgitation | 339 (3.9) † | 41 (6.6) #† | 159 (12.2) # | 28 (13.6) # | 567 (5.2) | <0.001 | |
| Tricuspid regurgitation | 177 (2.0) † | 26 (4.2) #† | 150 (11.5) # | 27 (13.1) # | 380 (3.5) | <0.001 | |
| Pulmonary hypertension | 420 (4.8) † | 80 (13.0) #† | 247 (18.9) #† | 56 (27.2) # | 803 (7.4) | <0.001 | |
| Results of angiography | |||||||
| Angiography performance | n | 8074 | 538 | 993 | 148 | 9753 | |
| Measure of CAD | No/non-significant | 354 (4.4) | 40 (7.4) # | 105 (10.6) # | 10 (6.8) | 509 (5.2) | <0.001 |
| One vessel | 2358 (29.2) | 122 (22.7) # | 196 (19.7) # | 33 (22.3) | 2709 (27.8) | <0.001 | |
| Two vessels | 2304 (28.5) | 150 (27.9) | 229 (23.1) # | 44 (29.7) | 2727 (28.0) | <0.001 | |
| Three vessels/LM | 3058 (37.9) | 226 (42.0) | 463 (46.6) # | 61 (41.2) | 3808 (39.0) | <0.001 | |
| Parameter | Values | B (SE) | AdjHR | (95% CI) | p |
|---|---|---|---|---|---|
| Study group | 1 (COPD−, AF−) | 1 (ref.) | |||
| 2 (COPD+, AF−) | 0.635 (0.041) | 1.887 | (1.742; 2.045) | <0.001 | |
| 3 (COPD−, AF+) | 0.331 (0.031) | 1.392 | (1.311; 1.479) | <0.001 | |
| 4 (COPD+, AF+) | 0.596 (0.060) | 1.815 | (1.614; 2.040) | <0.001 | |
| Age, years | <65 | 1 (ref.) | |||
| 65–75 | 0.814 (0.037) | 2.258 | (2.100; 2.428) | <0.001 | |
| ≥75 | 1.303 (0.037) | 3.681 | (3.425; 3.956) | <0.001 | |
| Sex | Male vs. Female | −0.121 (0.027) | 0.886 | (0.841; 0.934) | <0.001 |
| CHF | 0.325 (0.028) | 1.385 | (1.310; 1.463) | <0.001 | |
| s/p MI | 0.160 (0.028) | 1.173 | (1.110; 1.240) | <0.001 | |
| Chronic kidney disease | 0.453 (0.033) | 1.573 | (1.474; 1.680) | <0.001 | |
| Diabetes mellitus | 0.276 (0.025) | 1.318 | (1.254; 1.386) | <0.001 | |
| Dyslipidemia | −0.181 (0.030) | 0.834 | (0.787; 0.884) | <0.001 | |
| Obesity | −0.135 (0.033) | 0.874 | (0.819; 0.932) | <0.001 | |
| PVD | 0.338 (0.032) | 1.403 | (1.317; 1.494) | <0.001 | |
| Neurological disorders | 0.421 (0.028) | 1.524 | (1.443; 1.609) | <0.001 | |
| Malignancy | 0.573 (0.047) | 1.774 | (1.617; 1.946) | <0.001 | |
| Anemia | 0.327 (0.027) | 1.387 | (1.315; 1.463) | <0.001 | |
| Alcohol/drug addiction | 0.755 (0.082) | 2.128 | (1.811; 2.500) | <0.001 | |
| Type of AMI | NSTEMI vs. STEMI | 0.174 (0.028) | 1.191 | (1.126; 1.258) | <0.001 |
| Type of treatment | Noninvasive | 1 (ref.) | |||
| PCI | −0.682 (0.052) | 0.506 | (0.456; 0.560) | <0.001 | |
| CABG | −1.110 (0.061) | 0.329 | (0.292; 0.371) | <0.001 | |
| Mechanical ventilation | 0.109 (0.053) | 1.115 | (1.005; 1.238) | 0.041 | |
| Blood transfusion | 0.140 (0.036) | 1.150 | (1.071; 1.235) | <0.001 | |
| Severe LV dysfunction | 0.386 (0.042) | 1.471 | (1.357; 1.596) | <0.001 | |
| LV hypertrophy | 0.246 (0.059) | 1.279 | (1.140; 1.436) | <0.001 | |
| Pulmonary hypertension | 0.275 (0.045) | 1.317 | (1.206; 1.439) | <0.001 | |
| Measure of CAD | No/non-significant/One vessel | 1 (ref.) | |||
| Two vessels | 0.118 (0.052) | 1.125 | (1.016; 1.246) | 0.024 | |
| Three vessels/LM | 0.322 (0.047) | 1.380 | (1.259; 1.512) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiyovich, A.; Gilutz, H.; Skalsky, K.; Plakht, Y. Long-Term Impact of Chronic Obstructive Pulmonary Disease and Atrial Fibrillation on Post-Acute Myocardial Infarction Long-Term All-Cause Mortality: Insights from the SAMI III Project. J. Clin. Med. 2025, 14, 5907. https://doi.org/10.3390/jcm14165907
Shiyovich A, Gilutz H, Skalsky K, Plakht Y. Long-Term Impact of Chronic Obstructive Pulmonary Disease and Atrial Fibrillation on Post-Acute Myocardial Infarction Long-Term All-Cause Mortality: Insights from the SAMI III Project. Journal of Clinical Medicine. 2025; 14(16):5907. https://doi.org/10.3390/jcm14165907
Chicago/Turabian StyleShiyovich, Arthur, Harel Gilutz, Keren Skalsky, and Ygal Plakht. 2025. "Long-Term Impact of Chronic Obstructive Pulmonary Disease and Atrial Fibrillation on Post-Acute Myocardial Infarction Long-Term All-Cause Mortality: Insights from the SAMI III Project" Journal of Clinical Medicine 14, no. 16: 5907. https://doi.org/10.3390/jcm14165907
APA StyleShiyovich, A., Gilutz, H., Skalsky, K., & Plakht, Y. (2025). Long-Term Impact of Chronic Obstructive Pulmonary Disease and Atrial Fibrillation on Post-Acute Myocardial Infarction Long-Term All-Cause Mortality: Insights from the SAMI III Project. Journal of Clinical Medicine, 14(16), 5907. https://doi.org/10.3390/jcm14165907






