The Endometrial Receptivity Test: The Impact of Combined Treatment with Pentoxifylline and Alpha-Tocopherol in Patients with Recurrent Implantation Failure or Recurrent Pregnancy Loss
Abstract
1. Introduction
2. Materials and Methods
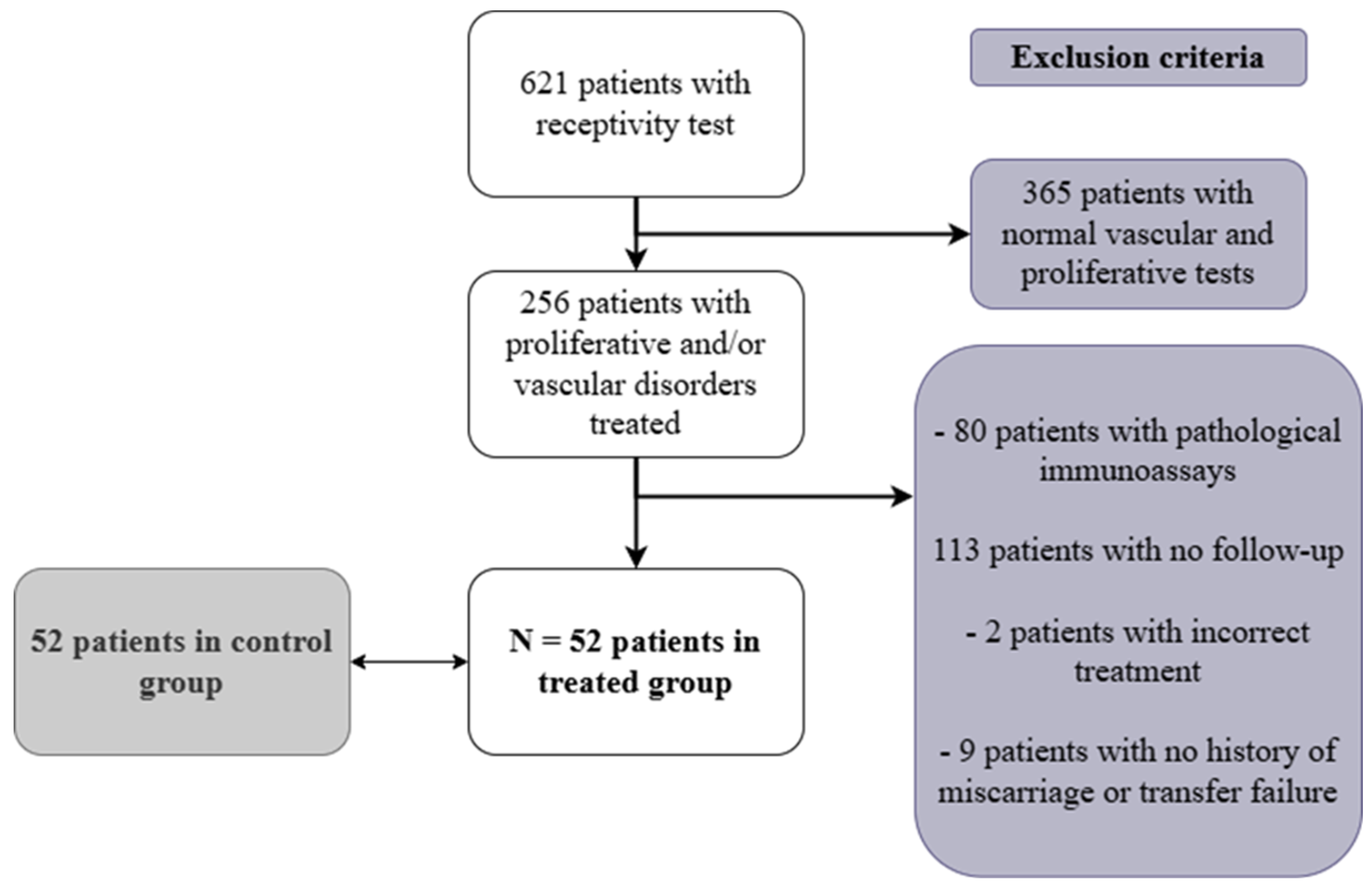
2.1. Patient Selection
2.2. Endometrial Receptivity Testing and Ultrasound Measurements
2.3. Treatment Protocol
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Effect of Pentoxifylline and Alpha-Tocopherol on Endometrial Vascularization and Proliferation
3.3. Outcome of the First Embryo Transfer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muter, J.; Lynch, V.J.; McCoy, R.C.; Brosens, J.J. Human embryo implantation. Development 2023, 150, dev201507. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.; Alecsandru, D.; Forman, E.; Gemmell, L.; Goldberg, J.; Llarena, N.; Seli, E. A review of the pathophysiology of recurrent implantation failure. Fertil. Steril. 2021, 116, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Kaluanga Bwanga, P.; Tremblay-Lemoine, P.-L.; Timmermans, M.; Ravet, S.; Munaut, C.; Nisolle, M.; Henry, L. The Endometrial Microbiota: Challenges and Prospects. Medicina 2023, 59, 1540. [Google Scholar] [CrossRef] [PubMed]
- Craciunas, L.; Gallos, I.; Chu, J.; Bourne, T.; Quenby, S.; Brosens, J.J.; Coomarasamy, A. Conventional and modern markers of endometrial receptivity: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 202–223. [Google Scholar] [CrossRef] [PubMed]
- Zollner, U.; Zollner, K.-P.; Specketer, M.; Blissing, S.; Müller, T.; Steck, T.; Dietl, J. Endometrial volume as assessed by three-dimensional ultrasound is a predictor of pregnancy outcome after in vitro fertilization and embryo transfer. Fertil. Steril. 2003, 80, 1515–1517. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Shaikh, A.; Ratnani, R. Ultrasonography and Doppler Study to Predict Uterine Receptivity in Infertile Patients Undergoing Embryo Transfer. J. Obstet. Gynecol. India 2016, 66, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chiang, C.; Huang, H.; Chao, A.; Wang, H.; Soong, Y. Detection of the subendometrial vascularization flow index by three-dimensional ultrasound may be useful for predicting the pregnancy rate for patients undergoing in vitro fertilization–embryo transfer. Fertil. Steril. 2003, 79, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.; Kamel, A.; Abu-Hamila, F.; Elkomy, R.; Ohida, O.; Hassan, S.; Ramadan, W. The measurement of endometrial volume and sub-endometrial vascularity to replace the traditional endometrial thickness as predictors of in-vitro fertilization success. Gynecol. Endocrinol. 2019, 35, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Cakiroglu, Y.; Tiras, B.; Franasiak, J.; Seli, E. Treatment options for endometrial hypoproliferation. Curr. Opin. Obstet. Gynecol. 2023, 35, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Letur-Könirsch, H.; Guis, F.; Delanian, S. Uterine restoration by radiation sequelae regression with combined pentoxifylline– tocopherol: A phase II study. Fertil. Steril. 2002, 77, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Lédée-Bataille, N.; Olivennes, F.; Lefaix, J.-L.; Chaouat, G.; Frydman, R.; Delanian, S. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Hum. Reprod. 2002, 17, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Lédée, N.; Chaouat, G.; Serazin, V.; Lombroso, R.; Dubanchet, S.; Oger, P.; Louafi, N.; Ville, Y. Endometrial vascularity by three-dimensional power Doppler ultrasound and cytokines: A complementary approach to assess uterine receptivity. J. Reprod. Immunol. 2008, 77, 57–62. [Google Scholar] [CrossRef] [PubMed]
- ESHRE Working Group on Recurrent Implantation Failure; Cimadomo, D.; de Los Santos, M.J.; Griesinger, G.; Lainas, G.; Le Clef, N.; McLernon, D.J.; Montjean, D.; Toth, B.; Vermeulen, N.; et al. ESHRE good practice recommendations on recurrent implantation failure. Hum. Reprod. Open 2023, 2023, hoad023. [Google Scholar] [CrossRef] [PubMed]
- The ESHREGuideline Group on RPL; Atik, R.B.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenb, S.; et al. ESHRE guideline: Recurrent pregnancy los. Hum. Reprod. Open 2018, 2018, hoy004. [Google Scholar]
- Acharya, S.; Yasmin, E.; Balen, A. The use of a combination of pentoxifylline and tocopherol in women with a thin endometrium undergoing assisted conception therapies—A report of 20 cases. Hum. Fertil. 2009, 12, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Raga, F.; Bonilla-Musoles, F.; Casan, E.M.; Klein, O.; Bonilla, F. Assessment of endometrial volume bu three-dimensional ultrasound priori to embryo transfert: Clues to endometrial receptivity. Hum. Reprod. 1999, 14, 2851–2854. [Google Scholar] [CrossRef] [PubMed]
- Zollner, U.; Specketer, M.; Dietl, J.; Zollner, K. 3D-Endometrial volume and outcome of cryopreserved embryo replacement cycles. Gynecol. Obstet. 2012, 286, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Krief, F.; Simon, C.; Goldstein, R.; Ellenberg, L.P.; Ledee, N. Efficacy of tocopherol and pentoxifylline combined therapy for women undergoing assisted reproductive treatment with poor endometrial development: A retrospective cohort study on 143 patients. Hum. Fertil. 2021, 24, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Collège de Médecins Médecine de la Reproduction. Medicine IVF Report 2021; Belrap: Liege, Belgique, 2023. [Google Scholar]
- Poseidon Group Patient-Oriented Strategies Encomp. A new more detailed stratification of low responders to ovarian stimulation: From a poor ovarian response to a low prognosis concept. Fertil. Steril. 2016, 105, 1452–1453. [Google Scholar] [CrossRef] [PubMed]

| Control Group | Treated Group | ||||
|---|---|---|---|---|---|
| Variable | N | Mean ± SD /Median [Q1–Q3] /Number (%) | Mean ± SD /Median [Q1–Q3] /Number (%) | p-Value | 95% CI |
| Age (years) | 52 | 35.06 ± 4.56 | 34.46 ± 4.70 | 0.22 | [−1.92; 3.12] |
| Age (years) (categories) * | 52 | 1.00 | |||
| <36 | 32 (61.5) | 32 (61.5) | |||
| 36–39 | 8 (15.4) | 8 (15.4) | |||
| 40–42 | 12 (23.1) | 12 (23.1) | |||
| >42 | 0 (0.0) | 0 (0.0) | |||
| Tobacco | 48 | 0.13 | [−0.029; 0.279] | ||
| No | 42 (87.5) | 36 (75.0) | |||
| Yes | 6 (12.5) | 12 (25.0) | |||
| BMI (kg/m2) | 46 | 25.60 ± 4.93 | 24.48 ± 4.33 | 0.22 | [−1.56; 3.80] |
| AMH (ng/mL) | 45 | 1.31 [0.81–1.80] | 1.77 [1.00–3.63] | 0.0017 | [−0.41; 1.33] |
| 1st or 2nd infertility | 52 | 0.36 | [−0.109; 0.263] | ||
| Primary | 20 (38.5) | 24 (46.2) | |||
| Secondary | 28 (53.9) | 27 (51.9) | |||
| Couple of women | 4 (7.7) | 1 (1.9) | |||
| Spermogram | 46 | 0.99 | |||
| Teratozoospermia | 12 (26.1) | 12 (26.1) | |||
| Cryptozoospermia | 1 (2.2) | 2 (4.4) | |||
| Azoospermia | 3 (6.5) | 1 (2.2) | |||
| Oligozoospermia | 1 (2.2) | 3 (6.5) | |||
| Oligoasthenozoospermia | 0 (0.0) | 4 (8.7) | |||
| Oligoteratozoospermia | 7 (15.2) | 5 (10.9) | |||
| OAT | 5 (10.9) | 6 (13.0) | |||
| Donor | 9 (19.6) | 6 (13.0) | |||
| Normal | 8 (17.4) | 7 (15.2) | |||
| Number of previous IVF/ICSI | 51 | 2.41 ± 1.00 | 2.14 ± 1.64 | 0.22 | [−1.01; 0.47] |
| Number of embryos previously transferred | 52 | 3.94 ± 3.15 | 4.46 ± 3.48 | 0.18 | [−2.32; 1.28] |
| Number of embryos previously transferred (categories) * | 52 | 1.00 | |||
| 0 | 9 (17.3) | 9 (17.3) | |||
| 1–4 | 19 (36.5) | 19 (36.5) | |||
| 5 and over | 24 (46.2) | 24 (46.2) | |||
| Number of previous miscarriages | 52 | 0.71 ± 1.00 | 1.15 ± 1.60 | 0.0008 | [−1.17; 0.29] |
| Number of previous miscarriages (categories) * | 52 | 1.00 | |||
| 0–1 | 36 (69.2) | 36 (69.2) | |||
| 2 and more | 16 (30.8) | 16 (30.8) | |||
| Egg donation | 52 | 0.16 | [−0.0135; 0.0915] | ||
| Yes | 0 (0.0) | 2 (3.9) |
| Variable | N | Test 1 Mean ± SD /Number (%) | Test 2 Mean ± SD /Number (%) | p-Value | 95% CI/95% CI of the Difference |
|---|---|---|---|---|---|
| Exam performed under: | 52 | 0.052 | [0.005; 0.263] | ||
| Artificial cycle | 49 (94.2) | 42 (80.8) | |||
| Natural cycle | 3 (5.8) | 10 (19.2) | |||
| Endometrial thickness (mm) | 52 | 6.63 ± 2.15 | 7.41 ± 2.38 | 0.019 | [0.13; 1.43] |
| Endometrial volume (cm3) | 51 | 1.57 ± 0.95 | 1.89 ± 1.12 | 0.0054 | [0.10; 0.54] |
| Endometrial volume > 2 cm3 | 51 | 10 (19.6) | 17 (33.3) | 0.035 | [−0.006; 0.280] |
| Endometrial volume > 2.5 cm3 | 51 | 9 (17.6) | 11 (21.6) | 0.48 | [−0.114; 0.194] |
| Endometrial volume > 3.2 cm3 | 51 | 3 (5.9) | 7 (13.7) | 0.10 | [−0.036; 0.192] |
| Sub-endometrial VFI | 49 | 1.00 ± 1.52 | 1.50 ± 1.74 | 0.041 | [0.02; 0.96] |
| Sub-endometrial VFI > 0.25 | 49 | 33 (67.3) | 44 (89.8) | 0.0076 | [0.086; 0.364] |
| IP right uterine artery | 51 | 2.32 ± 0.70 | 2.07 ± 0.54 | 0.029 | [−0.48; −0.02] |
| IP left uterine artery | 52 | 2.53 ± 0.99 | 2.27 ± 0.79 | 0.083 | [−0.57; 0.03] |
| IP of the uterine arteries | 51 | 0.083 | [−0.005; 0.241] | ||
| 0 PI > 1.8 | 3 (5.9) | 9 (17.7) | |||
| 1–2 IP > 1.8 | 48 (94.1) | 42 (82.3) | |||
| Proliferation disorder present | 49 | 40 (81.6) | 31 (63.3) | 0.020 | [−0.326; −0.040] |
| Vascularization disorder present | 49 | 28 (57.1) | 11 (22.4) | 0.0004 | [−0.495; −0.199] |
| Variable | N | Control Group Number (%) | Treated Group Number (%) | p-Value | 95% CI of the Difference |
|---|---|---|---|---|---|
| hCG | 34 | ||||
| Positive | 9 (26.5) | 13 (38.2) | 0.28 | [−0.104; 0.338] | |
| CPR | 34 | ||||
| Yes | 8 (23.5) | 13 (38.2) | 0.17 | [−0.069; 0.363] | |
| LBR | 34 | ||||
| Yes | 5 (14.7) | 6 (17.7) | 0.72 | [−0.145; 0.205] |
| Variable | N | Control Group Number (%) | Treated Group Number (%) | p-Value | 95% CI of the Difference |
|---|---|---|---|---|---|
| Number of transfers carried out over 6 months | 43 | ||||
| 0 | 0 (0.0) | 9 (20.9) | |||
| 1 | 21 (40.4) | 25 (58.1) | |||
| 2 | 21 (40.4) | 6 (14.0) | |||
| 3 | 7 (13.5) | 3 (7.0) | |||
| 4 | 2 (3.8) | ||||
| 5 | 1 (1.9) | ||||
| hCG | 34 | ||||
| Positive | 12 (35.3) | 18 (52.9) | 0.12 | [−0.056; 0.408] | |
| Clinical pregnancy | 34 | ||||
| Yes | 12 (35.3) | 17 (50.0) | 0.18 | [−0.085; 0.379] | |
| Live birth | 34 | ||||
| Yes | 10 (29.4) | 11 (32.3) | 0.77 | [−0.190; 0.248] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prudhomme, L.; Habran, C.; Labied, S.; Wenders, F.; Rousseau, L.; Munaut, C.; Henry, L. The Endometrial Receptivity Test: The Impact of Combined Treatment with Pentoxifylline and Alpha-Tocopherol in Patients with Recurrent Implantation Failure or Recurrent Pregnancy Loss. J. Clin. Med. 2025, 14, 5903. https://doi.org/10.3390/jcm14165903
Prudhomme L, Habran C, Labied S, Wenders F, Rousseau L, Munaut C, Henry L. The Endometrial Receptivity Test: The Impact of Combined Treatment with Pentoxifylline and Alpha-Tocopherol in Patients with Recurrent Implantation Failure or Recurrent Pregnancy Loss. Journal of Clinical Medicine. 2025; 14(16):5903. https://doi.org/10.3390/jcm14165903
Chicago/Turabian StylePrudhomme, Laurine, Cécile Habran, Soraya Labied, Frédéric Wenders, Laetitia Rousseau, Carine Munaut, and Laurie Henry. 2025. "The Endometrial Receptivity Test: The Impact of Combined Treatment with Pentoxifylline and Alpha-Tocopherol in Patients with Recurrent Implantation Failure or Recurrent Pregnancy Loss" Journal of Clinical Medicine 14, no. 16: 5903. https://doi.org/10.3390/jcm14165903
APA StylePrudhomme, L., Habran, C., Labied, S., Wenders, F., Rousseau, L., Munaut, C., & Henry, L. (2025). The Endometrial Receptivity Test: The Impact of Combined Treatment with Pentoxifylline and Alpha-Tocopherol in Patients with Recurrent Implantation Failure or Recurrent Pregnancy Loss. Journal of Clinical Medicine, 14(16), 5903. https://doi.org/10.3390/jcm14165903







