Uncommon and Accessory Electrocardiographic Findings in Brugada Syndrome: A Review
Abstract
1. Introduction
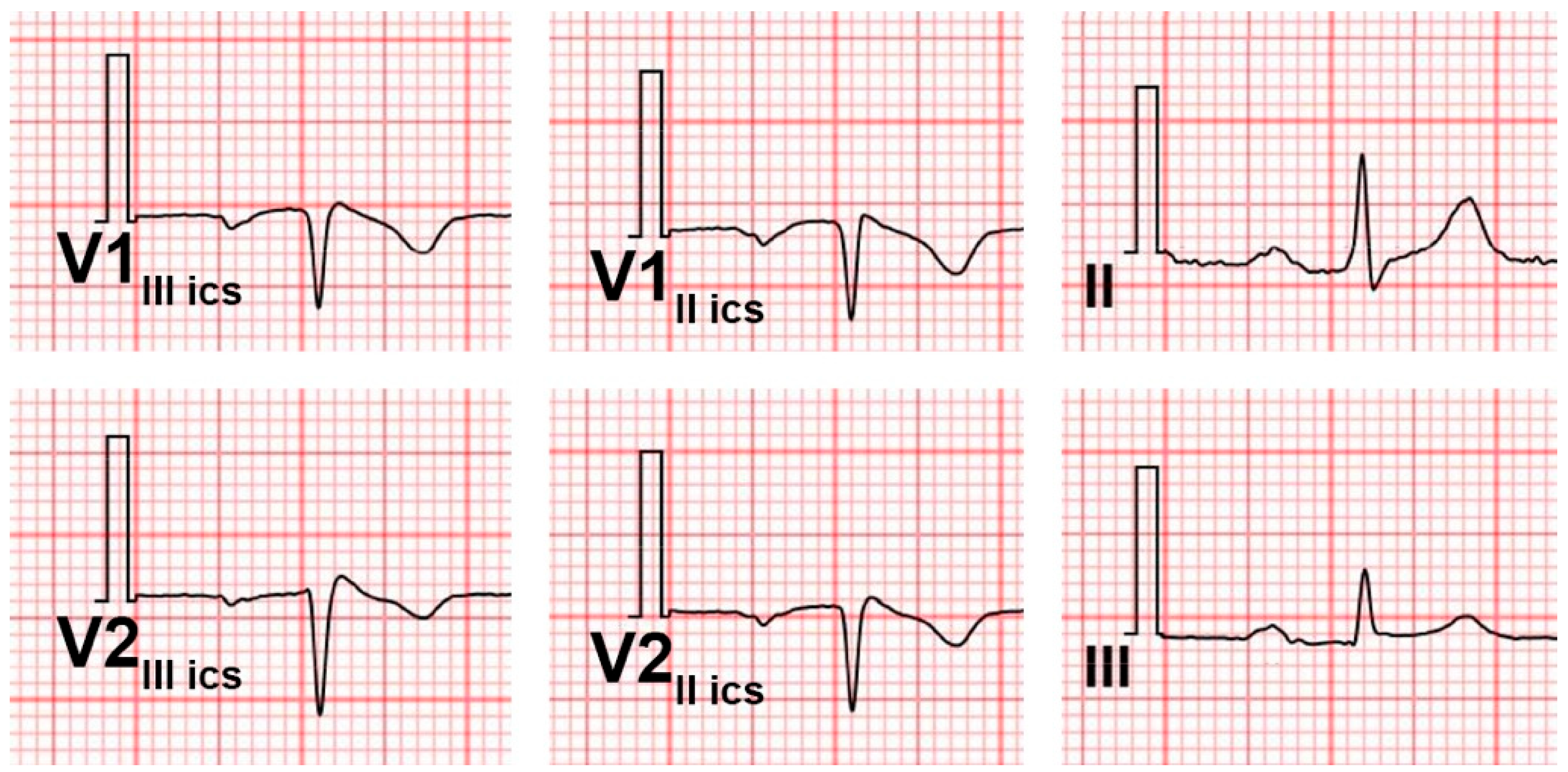
2. Coexistence of Right Bundle Branch Block and Brugada Pattern
3. Brugada Pattern and Left Bundle Branch Block
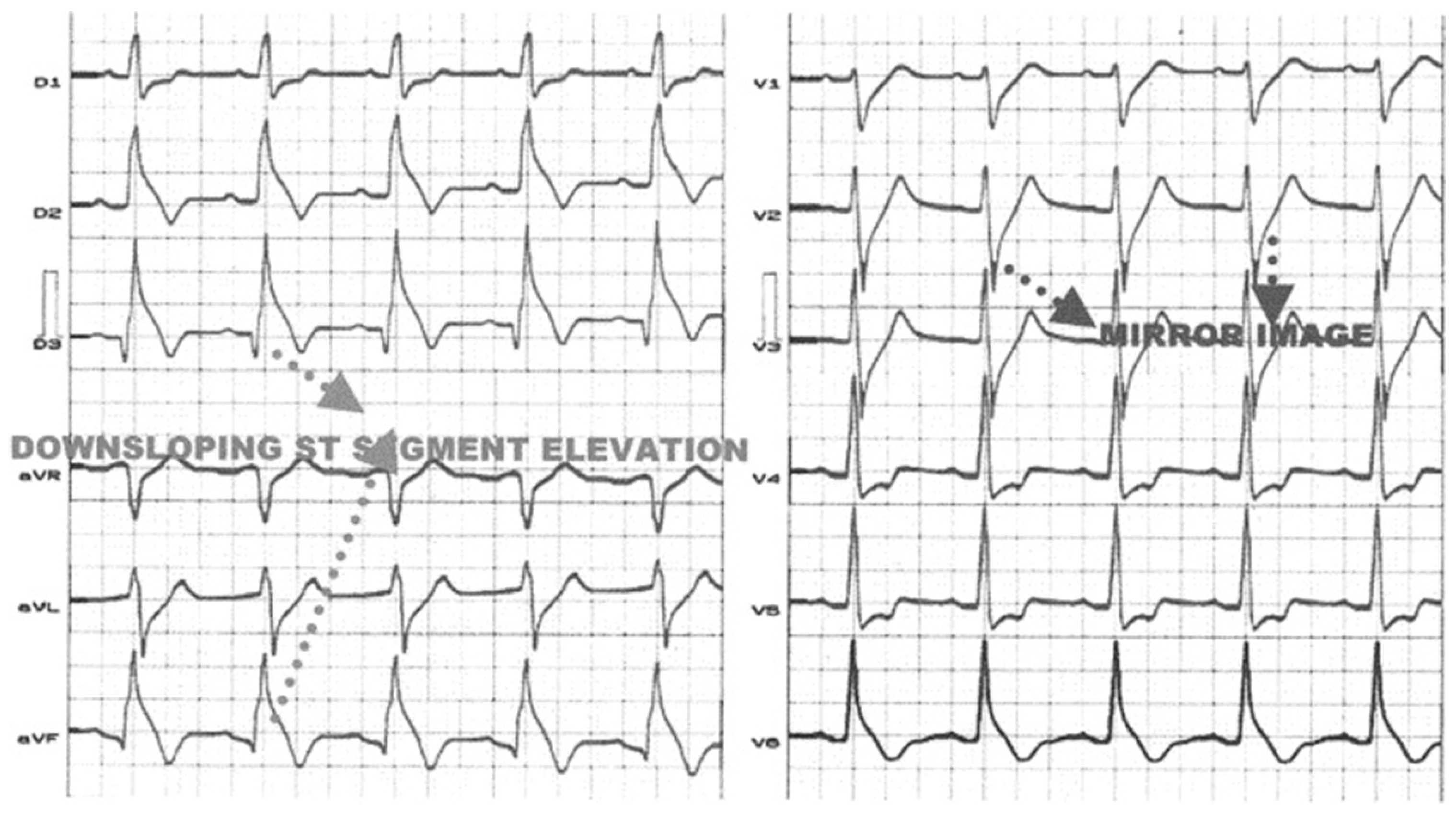
4. ST-Segment Depression in Inferior Leads
5. Other Anomalies of the Peripheral Leads
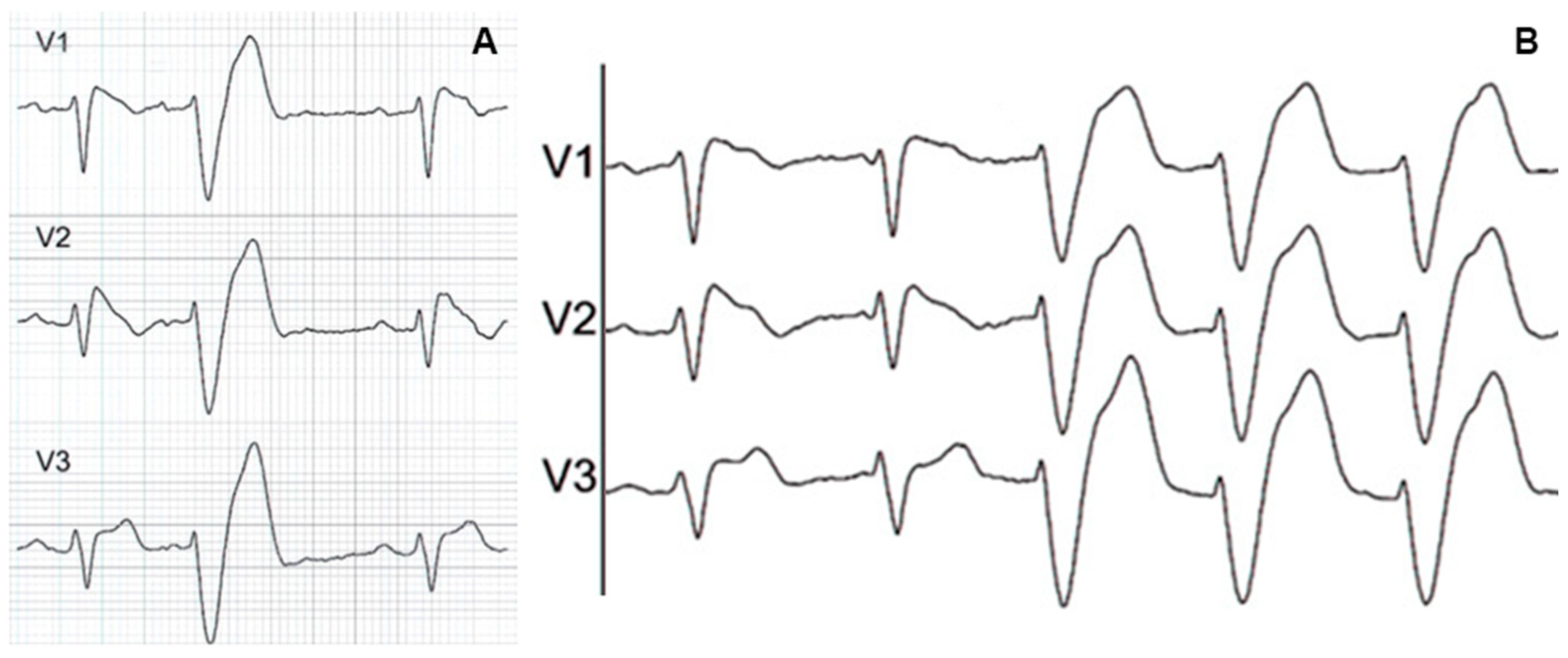
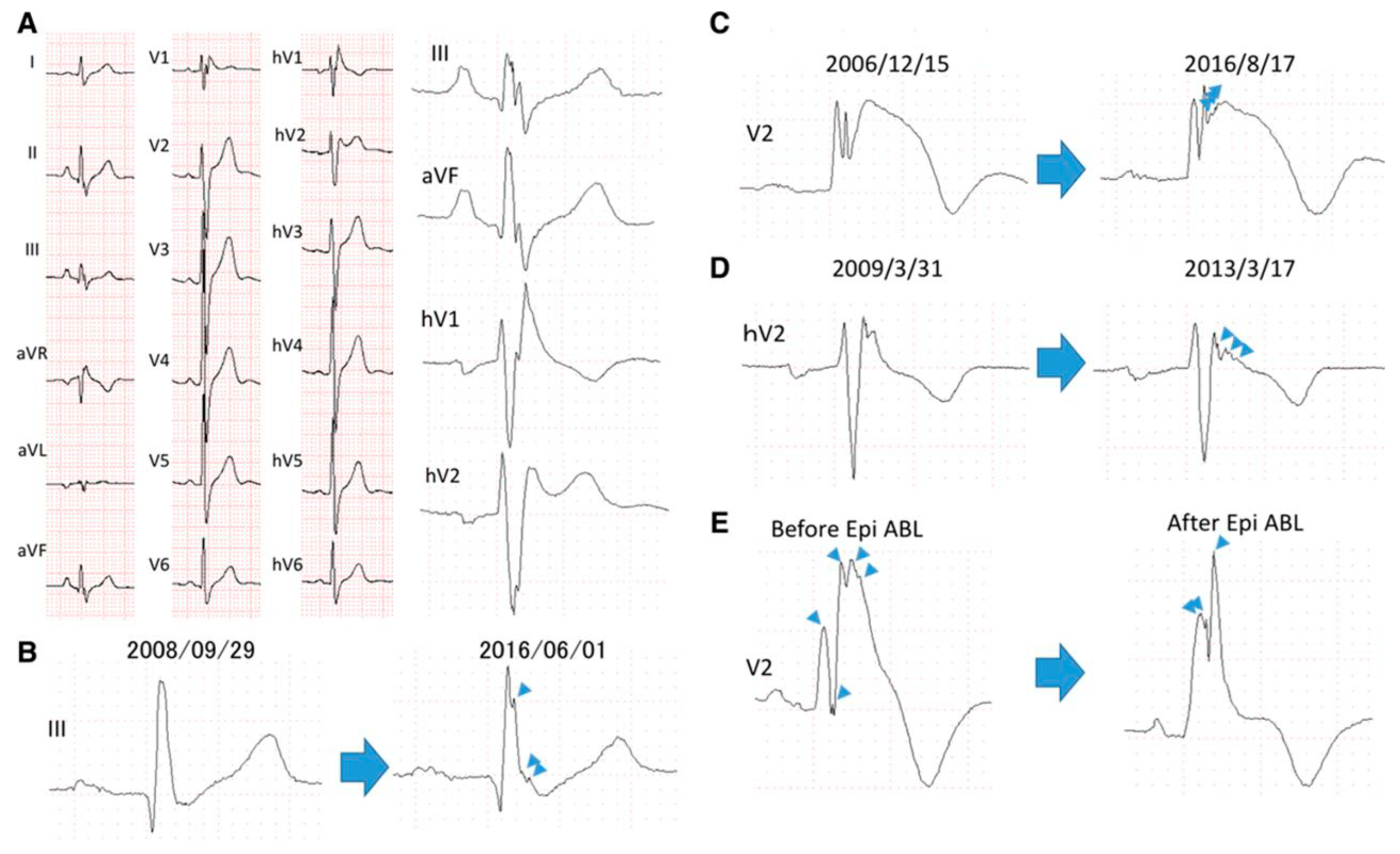
6. QRS Fragmentation in Patients with Brugada Syndrome
7. Alterations of Atrioventricular Conduction
8. Conclusions
9. Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BrS | (Brugada Syndrome) |
| ECG | (Electrocardiogram) |
| RBBB | (Right Bundle Branch Block) |
| LBBB | (Left Bundle Branch Block) |
| RVOT | (Right Ventricular Outflow Tract) |
| AVB | (Atrioventricular Block) |
References
- Hiss, R.G.; Lamb, L.E. Electrocardiographic findings in 122,043 individuals. Circulation 1962, 25, 947–961. [Google Scholar] [CrossRef]
- Fleg, J.L.; Das, D.N.; Lakatta, E.G. Right bundle branch block: Long-term prognosis in apparently healthy men. J. Am. Coll. Cardiol. 1983, 1, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, Y.; Takatsuki, S.; Kimura, T.; Nishiyama, N.; Fukumoto, K.; Tanimoto, Y.; Tanimoto, K.; Miyoshi, S.; Suzuki, M.; Yokoyama, Y.; et al. Ventricular fibrillation associated with complete right bundle branch block. Heart Rhythm 2013, 10, 1028–1035. [Google Scholar] [CrossRef][Green Version]
- Bussink, B.E.; Holst, A.G.; Jespersen, L.; Deckers, J.W.; Jensen, G.B.; Prescott, E. Right bundle branch block: Prevalence, risk factors, and outcome in the general population: Results from the Copenhagen City Heart Study. Eur. Heart J. 2013, 34, 138–146. [Google Scholar] [CrossRef]
- Gaba, P.; Pedrotty, D.; DeSimone, C.V.; Bonikowske, A.R.; Allison, T.G.; Kapa, S. Mortality in Patients With Right Bundle-Branch Block in the Absence of Cardiovascular Disease. J. Am. Heart Assoc. 2020, 9, e017430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maury, P.; Rollin, A.; Sacher, F.; Gourraud, J.B.; Raczka, F.; Pasquié, J.L.; Duparc, A.; Mondoly, P.; Cardin, C.; Delay, M.; et al. Prevalence and prognostic role of various conduction disturbances in patients with the Brugada syndrome. Am. J. Cardiol. 2013, 112, 1384–1389. [Google Scholar] [CrossRef]
- Tomita, M.; Kitazawa, H.; Sato, M.; Okabe, M.; Antzelevitch, C.; Aizawa, Y. A complete right bundle-branch block masking Brugada syndrome. J. Electrocardiol. 2012, 45, 780–782. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Márquez, M.F.; Bisteni, A.; Medrano, G.; De Micheli, A.; Guevara, M.; Iturralde, P.; Colín, L.; Hermosillo, A.G.; Cárdenas, M. Dynamic electrocardiographic changes after aborted sudden death in a patient with Brugada syndrome and rate-dependent right bundle branch block. J. Electrocardiol. 2005, 38, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, Y.; Takatsuki, S.; Sano, M.; Kimura, T.; Nishiyama, N.; Fukumoto, K.; Tanimoto, Y.; Tanimoto, K.; Murata, M.; Komatsu, T.; et al. Brugada syndrome behind complete right bundle-branch block. Circulation 2013, 128, 1048–1054, Erratum in Circulation 2014, 129, e465. [Google Scholar] [CrossRef] [PubMed]
- Chiale, P.A.; Garro, H.A.; Fernández, P.A.; Elizari, M.V. High-degree right bundle branch block obscuring the diagnosis of Brugada electrocardiographic pattern. Heart Rhythm 2012, 9, 974–976. [Google Scholar] [CrossRef]
- Shinohara, T.; Takahashi, N.; Saikawa, T.; Yoshimatsu, H. Brugada syndrome with complete right bundle branch block disclosed by a febrile illness. Intern. Med. 2008, 47, 843–846. [Google Scholar] [CrossRef]
- Veltmann, C.; Wolpert, C.; Sacher, F.; Mabo, P.; Schimpf, R.; Streitner, F.; Brade, J.; Kyndt, F.; Kuschyk, J.; Le Marec, H.; et al. Response to intravenous ajmaline: A retrospective analysis of 677 ajmaline challenges. Europace 2009, 11, 1345–1352. [Google Scholar] [CrossRef]
- Conte, G.; Levinstein, M.; Sarkozy, A.; Sieira, J.; de Asmundis, C.; Chierchia, G.B.; Di Giovanni, G.; Baltogiannis, G.; Ciconte, G.; Wauters, K.; et al. The clinical impact of ajmaline challenge in elderly patients with suspected atrioventricular conduction disease. Int. J Cardiol. 2014, 172, 423–427. [Google Scholar] [CrossRef]
- Laporte, P.; Porretta, A.P.; Charles, M.; Algalarrondo, V.; Extramiana, F. Wide QRS monomorphic tachycardia induced by ajmaline infusion. Hear. Case Rep. 2025, 11, 442–445. [Google Scholar] [CrossRef]
- Pérez-Riera, A.R.; Barbosa-Barros, R.; de Rezende Barbosa, M.P.C.; Daminello-Raimundo, R.; de Abreu, L.C.; Nikus, K. Left bundle branch block: Epidemiology, etiology, anatomic features, electrovectorcardiography, and classification proposal. Ann. Noninvasive Electrocardiol. 2019, 24, e12572. [Google Scholar] [CrossRef]
- Kumar, V.; Venkataraman, R.; Aljaroudi, W.; Osorio, J.; Heo, J.; Iskandrian, A.E.; Hage, F.G. Implications of left bundle branch block in patient treatment. Am. J. Cardiol. 2013, 111, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Guandalini, G.S.; Marchlinski, F.E. Parahisian pacing to unmask Brugada pattern with concomitant left bundle branch block and to document epicardial ablation endpoint in Brugada syndrome. Hear. Case Rep. 2021, 7, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Narula, O.S. Longitudinal dissociation in the His bundle. Bundle branch block due to asynchronous conduction within the His bundle in man. Circulation 1977, 56, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Ozin, B.; Atar, I.; Altunkeser, B.; Ertan, C.; Yildirir, A.; Müderrisoğlu, H. Typical ECG changes unmasked by ajmaline in a patient with Brugada syndrome and left bundle branch block. Ann. Noninvasive Electrocardiol. 2005, 10, 378–381. [Google Scholar] [CrossRef]
- Arana-Rueda, E.; Pezzotti, M.R.; Pedrote, A.; Acosta, J.; Frutos-López, M.; Varela, L.M.; García-Fernández, N.; Castellano, A. Brugada syndrome masked by complete left bundle branch block: A clinical and functional study of its association with the p.1449Y>H SCN5A variant. J. Cardiovasc. Electrophysiol. 2021, 32, 2785–2790. [Google Scholar] [CrossRef]
- Crea, P.; Picciolo, G.; Luzza, F.; Oreto, G. ST Segment Depression in the Inferior Leads in Brugada Pattern: A New Sign. Ann. Noninvasive Electrocardiol. 2015, 20, 561–565. [Google Scholar] [CrossRef]
- Luzza, F.; Crea, P.; Nicotera, A.; Picciolo, G.; Pugliatti, P.; Oreto, G. A Case of Brugada Pattern Migrant from Right Precordial Leads to Peripheral Leads. Ann. Noninvasive Electrocardiol. 2016, 21, 316–318. [Google Scholar] [CrossRef]
- Crea, P.; Oreto, G. Usefulness of inferior leads analysis in Brugada pattern. Pacing Clin. Electrophysiol. 2019, 42, 769–770. [Google Scholar] [CrossRef]
- Crea, P.; Picciolo, G.; Luzza, F.; Oreto, G. A three-dimensional computed model of ST segment abnormality in type 1 Brugada Pattern: A key role of right ventricular outflow tract orientation? J. Electrocardiol. 2019, 53, 31–35. [Google Scholar] [CrossRef]
- Chinushi, M.; Tagawa, M.; Izumi, D.; Furushima, H.; Aizawa, Y. Pilsicainide-induced ST segment elevation and ST segment depression in two patients with variant forms of Brugada-type electrocardiographic abnormalities. Pacing Clin. Electrophysiol. 2009, 32, 811–815. [Google Scholar] [CrossRef]
- Rollin, A.; Sacher, F.; Gourraud, J.B.; Pasquié, J.L.; Raczka, F.; Duparc, A.; Mondoly, P.; Cardin, C.; Delay, M.; Chatel, S.; et al. Prevalence, characteristics, and prognosis role of type 1 ST elevation in the peripheral ECG leads in patients with Brugada syndrome. Heart Rhythm 2013, 10, 1012–1018. [Google Scholar] [CrossRef]
- Takagi, M.; Aihara, N.; Takaki, H.; Taguchi, A.; Shimizu, W.; Kurita, T.; Suyama, K.; Kamakura, S. Clinical characteristics of patients with spontaneous or inducible ventricular fibrillation without apparent heart disease presenting with J wave and ST segment elevation in inferior leads. J. Cardiovasc. Electrophysiol. 2000, 11, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Kalla, H.; Yan, G.X.; Marinchak, R. Ventricular fibrillation in a patient with prominent J (Osborn) waves and ST segment elevation in the inferior electrocardiographic leads: A Brugada syndrome variant? J. Cardiovasc. Electrophysiol. 2000, 11, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Sahara, M.; Sagara, K.; Yamashita, T.; Abe, T.; Kirigaya, H.; Nakada, M.; Iinuma, H.; Fu, L.T.; Watanabe, H. J wave and ST segment elevation in the inferior leads: A latent type of variant Brugada syndrome? Jpn. Heart J. 2002, 43, 55–60. [Google Scholar] [CrossRef]
- Riera, A.R.; Ferreira, C.; Schapachnik, E.; Sanches, P.C.; Moffa, P.J. Brugada syndrome with atypical ECG: Downsloping ST-segment elevation in inferior leads. J. Electrocardiol. 2004, 37, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, W.; Segawa, K.; Ito, H.; Tanaka, S.; Yoshimoto, N. Class IC antiarrhythmic drugs, flecainide and pilsicainide, produce ST segment elevation simulating inferior myocardial ischemia. J. Cardiovasc. Electrophysiol. 1998, 9, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Potet, F.; Mabo, P.; Le Coq, G.; Probst, V.; Schott, J.J.; Airaud, F.; Guihard, G.; Daubert, J.C.; Escande, D.; Le Marec, H. Novel brugada SCN5A mutation leading to ST segment elevation in the inferior or the right precordial leads. J. Cardiovasc. Electrophysiol. 2003, 14, 200–203. [Google Scholar] [CrossRef]
- Macfarlane, P.W.; Antzelevitch, C.; Haissaguerre, M.; Huikuri, H.V.; Potse, M.; Rosso, R.; Sacher, F.; Tikkanen, J.T.; Wellens, H.; Yan, G.X. The Early Repolarization Pattern: A Consensus Paper. J. Am. Coll. Cardiol. 2015, 66, 470–477. [Google Scholar] [CrossRef]
- Conte, G.; Caputo, M.L.; Regoli, F.; Moccetti, T.; Brugada, P.; Auricchio, A. Brugada Syndrome and Early Repolarisation: Distinct Clinical Entities or Different Phenotypes of the Same Genetic Disease? Arrhythm. Electrophysiol. Rev. 2016, 5, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Rosso, R.; Glikson, E.; Belhassen, B.; Katz, A.; Halkin, A.; Steinvil, A.; Viskin, S. Distinguishing “benign” from “malignant early repolarization”: The value of the ST-segment morphology. Heart Rhythm 2012, 9, 225–229. [Google Scholar] [CrossRef]
- Tikkanen, J.T.; Huikuri, H.V. Characteristics of “malignant” vs. “benign” electrocardiographic patterns of early repolarization. J. Electrocardiol. 2015, 48, 390–394. [Google Scholar] [CrossRef]
- Sarkozy, A.; Chierchia, G.B.; Paparella, G.; Boussy, T.; De Asmundis, C.; Roos, M.; Henkens, S.; Kaufman, L.; Buyl, R.; Brugada, R.; et al. Inferior and lateral electrocardiographic repolarization abnormalities in Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2009, 2, 154–161. [Google Scholar] [CrossRef]
- Letsas, K.P.; Sacher, F.; Probst, V.; Weber, R.; Knecht, S.; Kalusche, D.; Haïssaguerre, M.; Arentz, T. Prevalence of early repolarization pattern in inferolateral leads in patients with Brugada syndrome. Heart Rhythm 2008, 5, 1685–1689. [Google Scholar] [CrossRef] [PubMed]
- Kawata, H.; Morita, H.; Yamada, Y.; Noda, T.; Satomi, K.; Aiba, T.; Isobe, M.; Nagase, S.; Nakamura, K.; Fukushima Kusano, K.; et al. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: A novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm 2013, 10, 1161–1168. [Google Scholar] [CrossRef]
- Adler, A.; Topaz, G.; Heller, K.; Zeltser, D.; Ohayon, T.; Rozovski, U.; Halkin, A.; Rosso, R.; Ben-Shachar, S.; Antzelevitch, C.; et al. Fever-induced Brugada pattern: How common is it and what does it mean? Heart Rhythm 2013, 10, 1375–1382. [Google Scholar] [CrossRef]
- Benito, B.; Guasch, E.; Rivard, L.; Nattel, S. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J. Am. Coll. Cardiol. 2010, 56, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Sarkozy, A.; Veltmann, C.; Chierchia, G.B.; Boussy, T.; Wolpert, C.; Schimpf, R.; Brugada, J.; Brugada, R.; Borggrefe, M.; et al. Variability of the diagnostic ECG pattern in an ICD patient population with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2009, 20, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Zipes, D.P. Fragmented QRS: A predictor of mortality and sudden cardiac death. Heart Rhythm 2009, 6 (Suppl. S3), S8–S14. [Google Scholar] [CrossRef]
- Das, M.K.; Khan, B.; Jacob, S.; Kumar, A.; Mahenthiran, J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006, 113, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Maskoun, W.; Shen, C.; Michael, M.A.; Suradi, H.; Desai, M.; Subbarao, R.; Bhakta, D. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm 2010, 7, 74–80. [Google Scholar] [CrossRef]
- Morita, H.; Kusano, K.F.; Miura, D.; Nagase, S.; Nakamura, K.; Morita, S.T.; Ohe, T.; Zipes, D.P.; Wu, J. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation 2008, 118, 1697–1704. [Google Scholar] [CrossRef]
- Nademanee, K.; Raju, H.; de Noronha, S.V.; Papadakis, M.; Robinson, L.; Rothery, S.; Makita, N.; Kowase, S.; Boonmee, N.; Vitayakritsirikul, V.; et al. Fibrosis, Connexin-43, and Conduction Abnormalities in the Brugada Syndrome. J. Am. Coll. Cardiol. 2015, 66, 1976–1986. [Google Scholar] [CrossRef]
- Morita, H.; Watanabe, A.; Morimoto, Y.; Kawada, S.; Tachibana, M.; Nakagawa, K.; Nishii, N.; Ito, H. Distribution and Prognostic Significance of Fragmented QRS in Patients With Brugada Syndrome. Circ. Arrhythm. Electrophysiol. 2017, 10, e004765. [Google Scholar] [CrossRef]
- Notarstefano, P.; Pieroni, M.; Guida, R.; Rio, T.; Oliva, A.; Grotti, S.; Fraticelli, A.; Bolognese, L. Progression of electroanatomic substrate and electric storm recurrence in a patient with Brugada syndrome. Circulation 2015, 131, 838–841. [Google Scholar] [CrossRef][Green Version]
- Rivaud, M.R.; Marchal, G.A.; Wolswinkel, R.; Jansen, J.A.; van der Made, I.; Beekman, L.; Ruiz-Villalba, A.; Baartscheer, A.; Rajamani, S.; Belardinelli, L.; et al. Functional modulation of atrio-ventricular conduction by enhanced late sodium current and calcium-dependent mechanisms in Scn5a1798insD/+ mice. Europace 2020, 22, 1579–1589. [Google Scholar] [CrossRef]
- Wang, D.W.; Viswanathan, P.C.; Balser, J.R.; George, A.L., Jr.; Benson, D.W. Clinical, genetic, and biophysical characterization of SCN5A mutations associated with atrioventricular conduction block. Circulation 2002, 105, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Sumiyoshi, M.; Nakazato, Y.; Daida, H. Brugada syndrome and sinus node dysfunction. J. Arrhythm. 2018, 34, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Arabia, G.; Cerini, M.; Cersosimo, A.; Vinciguerra, P.; Calvi, E.; Mitacchione, G.; Aboelhassan, M.; Giacopelli, D.; Curnis, A. Implantable loop recorder in Brugada syndrome: Insights from a single-center experience. Int. J. Cardiol. Heart Vasc. 2024, 51, 101371. [Google Scholar] [CrossRef]
- Migliore, F.; Ottaviano, L.; Arestia, A.; Nigro, G.; Dello Russo, A.; Viani, S.; Bianchi, V.; Bisignani, A.; Pieragnoli, P.; Vitulano, G.; et al. S-ICD Rhythm Detect Investigators. Subcutaneous Implantable Defibrillator Therapy in Patients With Brugada Syndrome: Data From a Large Multicenter Registry. JACC Clin. Electrophysiol. 2025, 11, 1572–1582. [Google Scholar] [CrossRef]
- Knops, R.E.; Lloyd, M.S.; Roberts, P.R.; Wright, D.J.; Boersma, L.V.A.; Doshi, R.; Friedman, P.A.; Neuzil, P.; Blomström-Lundqvist, C.; Bongiorni, M.G.; et al. MODULAR ATP Investigators. A Modular Communicative Leadless Pacing-Defibrillator System. N. Engl. J. Med. 2024, 391, 1402–1412. [Google Scholar] [CrossRef]
- Kamakura, T.; Sacher, F.; Katayama, K.; Ueda, N.; Nakajima, K.; Wada, M.; Yamagata, K.; Ishibashi, K.; Inoue, Y.; Miyamoto, K.; et al. High-risk atrioventricular block in Brugada syndrome patients with a history of syncope. J. Cardiovasc. Electrophysiol. 2021, 32, 772–781. [Google Scholar] [CrossRef]
- Migliore, F.; Testolina, M.; Zorzi, A.; Bertaglia, E.; Silvano, M.; Leoni, L.; Bellin, A.; Basso, C.; Thiene, G.; Allocca, G.; et al. First-degree atrioventricular block on basal electrocardiogram predicts future arrhythmic events in patients with Brugada syndrome: A long-term follow-up study from the Veneto region of Northeastern Italy. Europace 2019, 21, 322–331. [Google Scholar] [CrossRef]
- Amin, A.S.; Boink, G.J.; Atrafi, F.; Spanjaart, A.M.; Asghari-Roodsari, A.; Molenaar, R.J.; Ruijter, J.M.; Wilde, A.A.; Tan, H.L. Facilitatory and inhibitory effects of SCN5A mutations on atrial fibrillation in Brugada syndrome. Europace 2011, 13, 968–975. [Google Scholar] [CrossRef]
- Morita, H.; Kusano-Fukushima, K.; Nagase, S.; Fujimoto, Y.; Hisamatsu, K.; Fujio, H.; Haraoka, K.; Kobayashi, M.; Morita, S.T.; Nakamura, K.; et al. Atrial fibrillation and atrial vulnerability in patients with Brugada syndrome. J. Am. Coll. Cardiol. 2002, 40, 1437–1444. [Google Scholar] [CrossRef] [PubMed]


| Associate Finding | Arrhythmic Risk | References |
|---|---|---|
| ST elevation in peripheral leads | Increased | [27,28,29,30] |
| Early repolarization | Increased (HR intermittent 2.5; persistent 4.88) | [39] |
| QRS fragmentation | Shorter time to arrhythmic events (2.77%/year vs. 0.46%/year) | [48] |
| AV Block | 1st degree: predictor of arrhythmic events (HR 3.84) | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micari, A.; Bellocchi, P.; Cautela, A.; Moncada, A.; Pluchino, M.; Cusmà-Piccione, M.; Oreto, L.; Vizzari, G.; Dattilo, G.; Crea, P. Uncommon and Accessory Electrocardiographic Findings in Brugada Syndrome: A Review. J. Clin. Med. 2025, 14, 5895. https://doi.org/10.3390/jcm14165895
Micari A, Bellocchi P, Cautela A, Moncada A, Pluchino M, Cusmà-Piccione M, Oreto L, Vizzari G, Dattilo G, Crea P. Uncommon and Accessory Electrocardiographic Findings in Brugada Syndrome: A Review. Journal of Clinical Medicine. 2025; 14(16):5895. https://doi.org/10.3390/jcm14165895
Chicago/Turabian StyleMicari, Antonino, Paolo Bellocchi, Asya Cautela, Alice Moncada, Matteo Pluchino, Maurizio Cusmà-Piccione, Lilia Oreto, Giampiero Vizzari, Giuseppe Dattilo, and Pasquale Crea. 2025. "Uncommon and Accessory Electrocardiographic Findings in Brugada Syndrome: A Review" Journal of Clinical Medicine 14, no. 16: 5895. https://doi.org/10.3390/jcm14165895
APA StyleMicari, A., Bellocchi, P., Cautela, A., Moncada, A., Pluchino, M., Cusmà-Piccione, M., Oreto, L., Vizzari, G., Dattilo, G., & Crea, P. (2025). Uncommon and Accessory Electrocardiographic Findings in Brugada Syndrome: A Review. Journal of Clinical Medicine, 14(16), 5895. https://doi.org/10.3390/jcm14165895






