Association Between Survival After Living Donor Liver Transplantation and Recipient Systemic Inflammation and Body Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sarcopenia Definition and Assessment
2.3. Systemic Inflammatory Marker
2.4. Data Collection
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Demographics
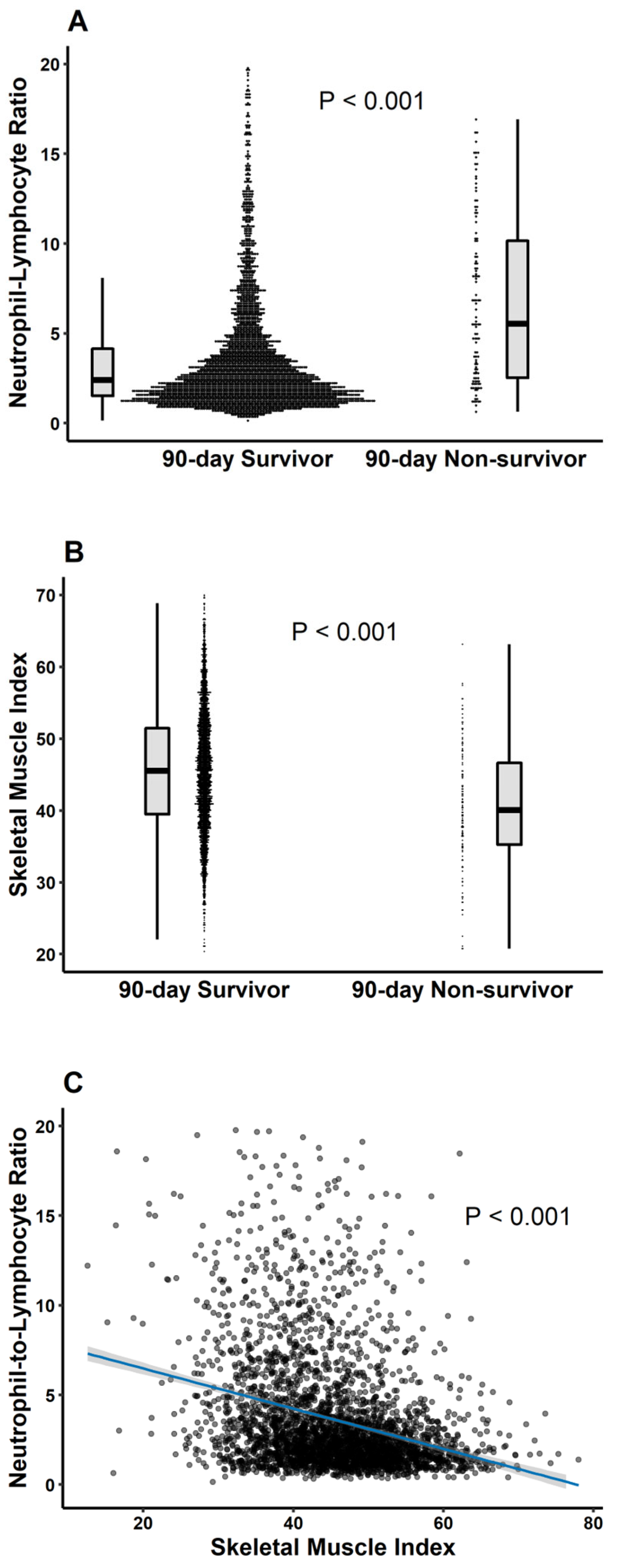
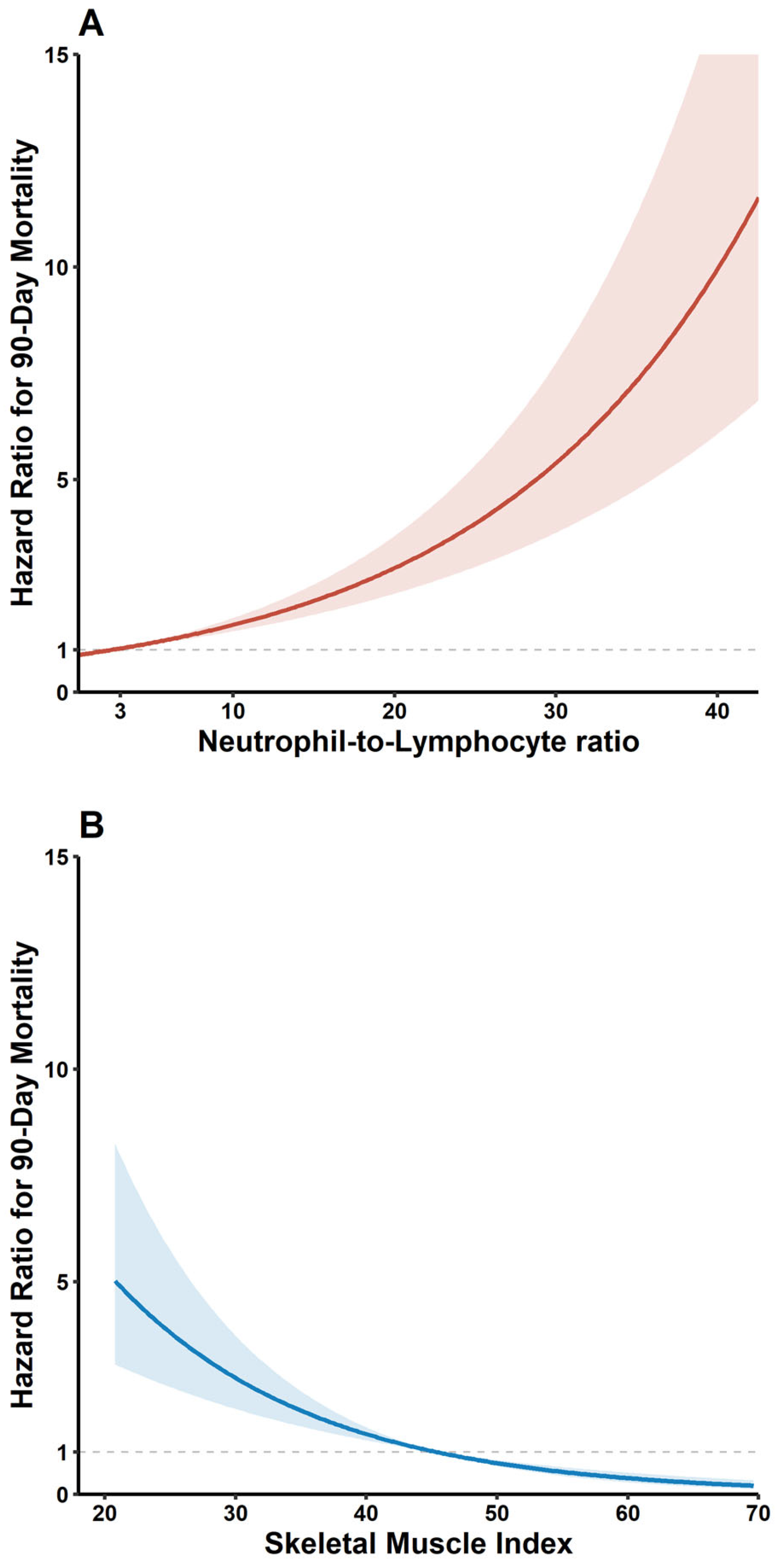
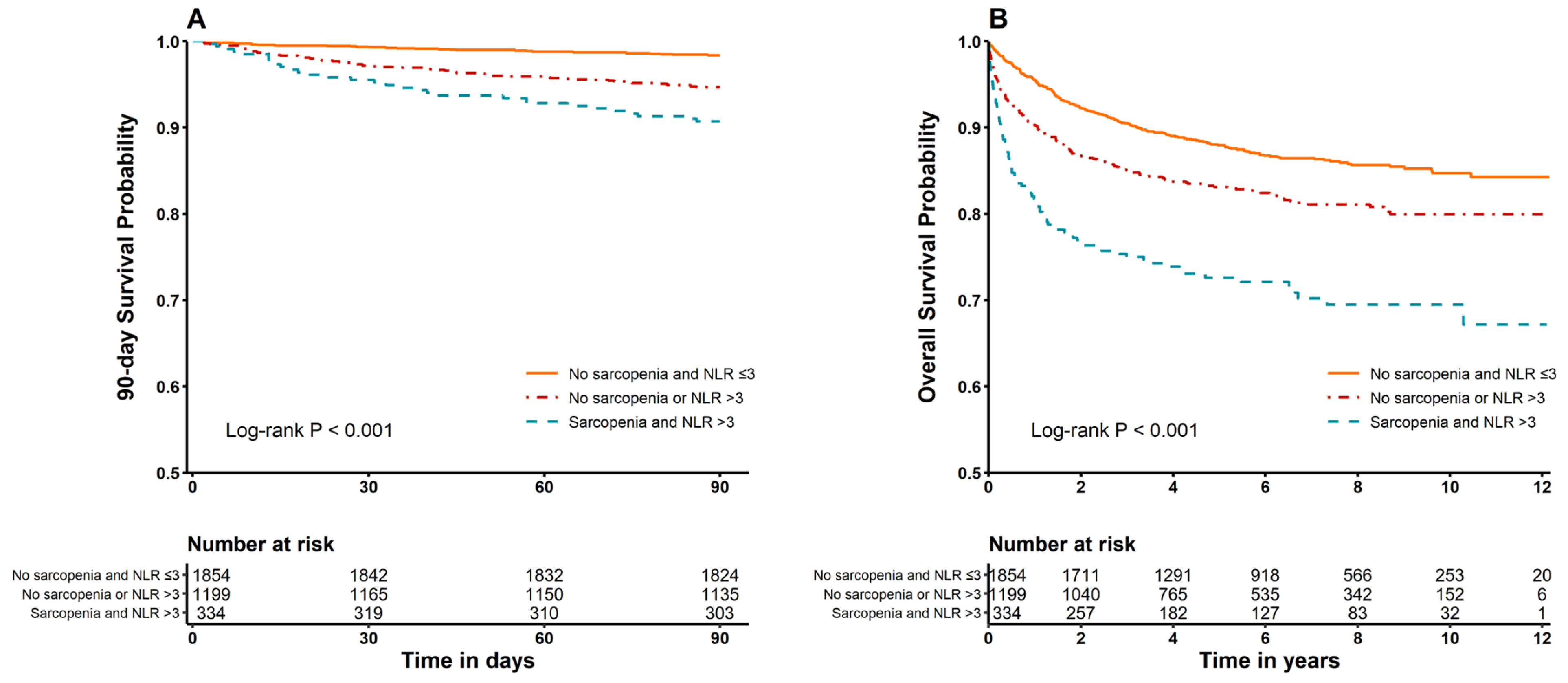
3.2. Mortality Compared According to Prevalence of Sarcopenia and NLR Categories (≤3, >3)
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| DM | diabetes mellitus |
| ESLD | end-stage liver disease |
| HR | hazard ratio |
| HTN | hypertension |
| IL-1β | interleukin-1β |
| IQR | interquartile range |
| LC | liver cirrhosis |
| LT | liver transplantation |
| MELD | Model for End-stage Liver Disease |
| MPB | muscle protein breakdown |
| MPS | muscle protein synthesis |
| NLR | neutrophil-to-lymphocyte ratio |
| SD | standard deviation |
| SMA | skeletal muscle area |
| SMI | skeletal muscle index |
| TNF-α | tumor necrosis factor-α |
References
- van Vugt, J.L.A.; Alferink, L.J.M.; Buettner, S.; Gaspersz, M.P.; Bot, D.; Darwish Murad, S.; Feshtali, S.; van Ooijen, P.M.A.; Polak, W.G.; Porte, R.J.; et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort. J. Hepatol. 2018, 68, 707–714. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeong, W.K.; Baik, S.K.; Cha, S.H.; Kim, M.Y. Impact of sarcopenia on prognostic value of cirrhosis: Going beyond the hepatic venous pressure gradient and MELD score. J. Cachexia Sarcopenia Muscle 2018, 9, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.H.; Lim, T.S.; Jeon, M.Y.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H.; Kim, S.U. Predictors of Discordance in the Assessment of Skeletal Muscle Mass between Computed Tomography and Bioimpedance Analysis. J. Clin. Med. 2019, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, J.L.; Levolger, S.; de Bruin, R.W.; van Rosmalen, J.; Metselaar, H.J.; IJzermans, J.N. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am. J. Transplant. 2016, 16, 2277–2292. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Jang, J.W. Sarcopenia in the prognosis of cirrhosis: Going beyond the MELD score. World J. Gastroenterol. 2015, 21, 7637–7647. [Google Scholar] [CrossRef]
- Kuo, S.Z.; Ahmad, M.; Dunn, M.A.; Montano-Loza, A.J.; Carey, E.J.; Lin, S.; Moghe, A.; Chen, H.W.; Ebadi, M.; Lai, J.C. Sarcopenia Predicts Post-transplant Mortality in Acutely Ill Men Undergoing Urgent Evaluation and Liver Transplantation. Transplantation 2019, 103, 2312–2317. [Google Scholar] [CrossRef]
- Cirera, I.; Bauer, T.M.; Navasa, M.; Vila, J.; Grande, L.; Taura, P.; Fuster, J.; Garcia-Valdecasas, J.C.; Lacy, A.; Suarez, M.J.; et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J. Hepatol. 2001, 34, 32–37. [Google Scholar] [CrossRef]
- Thabut, D.; Massard, J.; Gangloff, A.; Carbonell, N.; Francoz, C.; Nguyen-Khac, E.; Duhamel, C.; Lebrec, D.; Poynard, T.; Moreau, R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology 2007, 46, 1872–1882. [Google Scholar] [CrossRef]
- Malik, R.; Mookerjee, R.P.; Jalan, R. Infection and inflammation in liver failure: Two sides of the same coin. J. Hepatol. 2009, 51, 426–429. [Google Scholar] [CrossRef]
- Oweira, H.; Lahdou, I.; Daniel, V.; Opelz, G.; Schmidt, J.; Zidan, A.; Mehrabi, A.; Sadeghi, M. Early post-operative acute phase response in patients with early graft dysfunction is predictive of 6-month and 12-month mortality in liver transplant recipients. Hum. Immunol. 2016, 77, 952–960. [Google Scholar] [CrossRef]
- Rice, J.; Dodge, J.L.; Bambha, K.M.; Bajaj, J.S.; Reddy, K.R.; Gralla, J.; Ganapathy, D.; Mitrani, R.; Reuter, B.; Palecki, J.; et al. Neutrophil-to-Lymphocyte Ratio Associates Independently with Mortality in Hospitalized Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2018, 16, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Wedd, J.P.; Bambha, K.M.; Gralla, J.; Golden-Mason, L.; Collins, C.; Rosen, H.R.; Biggins, S.W. Neutrophil-to-lymphocyte ratio correlates with proinflammatory neutrophils and predicts death in low model for end-stage liver disease patients with cirrhosis. Liver Transpl. 2017, 23, 155–165. [Google Scholar] [CrossRef]
- Biyik, M.; Ucar, R.; Solak, Y.; Gungor, G.; Polat, I.; Gaipov, A.; Cakir, O.O.; Ataseven, H.; Demir, A.; Turk, S.; et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Leithead, J.A.; Rajoriya, N.; Gunson, B.K.; Ferguson, J.W. Neutrophil-to-lymphocyte ratio predicts mortality in patients listed for liver transplantation. Liver Int. 2015, 35, 502–509. [Google Scholar] [CrossRef]
- Halazun, K.J.; Hardy, M.A.; Rana, A.A.; Woodland, D.C.; Luyten, E.J.; Mahadev, S.; Witkowski, P.; Siegel, A.B.; Brown, R.S., Jr.; Emond, J.C. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann. Surg. 2009, 250, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Muller, M.J.; Baracos, V.; Bosy-Westphal, A.; Dulloo, A.G.; Eckel, J.; Fearon, K.C.; Hall, K.D.; Pietrobelli, A.; Sorensen, T.I.; Speakman, J.; et al. Functional body composition and related aspects in research on obesity and cachexia: Report on the 12th Stock Conference held on 6 and 7 September 2013 in Hamburg, Germany. Obes. Rev. 2014, 15, 640–656. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Sim, J.H.; Kwon, H.M.; Kim, K.W.; Ko, Y.S.; Jun, I.G.; Kim, S.H.; Kim, K.S.; Moon, Y.J.; Song, J.G.; Hwang, G.S. Associations of sarcopenia with graft failure and mortality in patients undergoing living donor liver transplantation. Liver Transpl. 2022, 28, 1345–1355. [Google Scholar] [CrossRef]
- Proctor, M.J.; McMillan, D.C.; Horgan, P.G.; Fletcher, C.D.; Talwar, D.; Morrison, D.S. Systemic inflammation predicts all-cause mortality: A glasgow inflammation outcome study. PLoS ONE 2015, 10, e0116206. [Google Scholar] [CrossRef]
- Montgomery, J.; Englesbe, M. Sarcopenia in Liver Transplantation. Curr. Transplant. Rep. 2019, 6, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.J.; Lai, J.C.; Sonnenday, C.; Tapper, E.B.; Tandon, P.; Duarte-Rojo, A.; Dunn, M.A.; Tsien, C.; Kallwitz, E.R.; Ng, V. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology 2019, 70, 1816–1829. [Google Scholar] [CrossRef]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of Systemic Inflammation and Sarcopenia with Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- Della Peruta, C.; Lozanoska-Ochser, B.; Renzini, A.; Moresi, V.; Sanchez Riera, C.; Bouché, M.; Coletti, D. Sex differences in inflammation and muscle wasting in aging and disease. Int. J. Mol. Sci. 2023, 24, 4651. [Google Scholar] [CrossRef]
- Seol, A.; Kim, S.I.; Song, Y.S. Sarcopenia: Clinical implications in ovarian cancer, diagnosis, etiology, and management. Sports Med. Health Sci. 2020, 2, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Dirchwolf, M.; Ruf, A.E. Role of systemic inflammation in cirrhosis: From pathogenesis to prognosis. World J. Hepatol. 2015, 7, 1974–1981. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Reid, M.B.; Li, Y.P. Tumor necrosis factor-alpha and muscle wasting: A cellular perspective. Respir. Res. 2001, 2, 269–272. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef]
| No Sarcopenia | Sarcopenia | |||||
|---|---|---|---|---|---|---|
| NLR ≤ 3 (n = 1854) | NLR > 3 (n = 1058) | p Value | NLR ≤ 3 (n = 141) | NLR > 3 (n = 334) | p Value | |
| Patients’ demographics | ||||||
| Age, years | 53 (48–58) | 53 (47–58) | 0.380 | 54 (48–59) | 54 (47–60) | 0.947 |
| Male sex, n (%) | 1390 (75.0) | 699 (66.1) | <0.001 | 126 (89.4) | 297 (88.9) | 1.000 |
| Body mass index, kg m−2 | 24.6 (22.7–26.7) | 24.1 (22.1–26.5) | <0.001 | 21.1 (19.1–23.4) | 20.8 (19.4–23.2) | 0.992 |
| MELD score | 12 (9–16) | 21 (13–33) | <0.001 | 17 (11–22) | 26 (16–36) | <0.001 |
| Diabetes, n (%) | 394 (21.3) | 271 (25.6) | 0.008 | 43 (30.5) | 84 (25.1) | 0.276 |
| Hypertension, n (%) | 330 (17.8) | 156 (14.7) | 0.038 | 25 (17.7) | 39 (11.7) | 0.106 |
| Beta blocker, n (%) | 545 (29.4) | 332 (a31.4) | 0.280 | 41 (29.1) | 109 (32.6) | 0.513 |
| Diuretics, n (%) | 675 (36.4) | 698 (66.0) | <0.001 | 81 (57.4) | 238 (71.3) | 0.005 |
| Preoperative laboratory data | ||||||
| Hemoglobin g dL−1 | 11.3 (9.7–13.0) | 9.5 (8.4–11.0) | <0.001 | 9.4 (8.2–11.1) | 8.9 (7.9–10.3) | 0.002 |
| Albumin, g dL−1 | 3.1 (2.7–3.6) | 3.1 (2.7–3.5) | 0.818 | 3.0 ± 0.6 | 3.1 ± 0.6 | 0.774 |
| Sodium, mmol L−1 | 140 (137–141) | 137 (133–140) | <0.001 | 137 (133–140) | 135 (130–139) | 0.003 |
| Etiology of cirrhosis | ||||||
| HBV, n (%) | 1255 (67.7) | 606 (57.3) | <0.001 | 73 (51.8) | 154 (46.1) | 0.304 |
| HCV, n (%) | 143 (7.7) | 52 (4.9) | 0.005 | 11 (7.8) | 20 (6.0) | 0.598 |
| Alcoholic, n (%) | 260 (14.0) | 218 (20.6) | <0.001 | 34 (24.1) | 113 (33.8) | 0.047 |
| Other disease, n (%) | 234 (12.6) | 197 (18.6) | < 0.001 | 26 (18.4) | 59 (17.7) | 0.944 |
| Combined HCC, n (%) | 1064 (57.4) | 369 (34.9) | <0.001 | 49 (34.8) | 87 (26.0) | 0.071 |
| Donor-related variables | ||||||
| Donor Age, year | 27 (22–33) | 29 (23–39) | <0.001 | 28 (21–36) | 32 (24–41) | 0.001 |
| Donor male sex, n (%) | 1276 (68.8) | 714 (67.5) | 0.481 | 98 (69.5) | 230 (68.9) | 0.976 |
| Donor body mass index, kg m−2 | 22.8 (20.9–24.7) | 23.0 (20.9–25.0) | 0.138 | 22.4 (20.2–24.8) | 22.7 (20.8–24.9) | 0.142 |
| Graft-to-recipient weight ratio | 1.05 (0.92–1.23) | 1.19 (0.99–1.48) | <0.001 | 1.21 (1.01–1.41) | 1.31 (1.06–1.91) | 0.001 |
| Deceased-donor graft | 65 (3.5) | 224 (21.2) | <0.001 | 17 (12.1) | 104 (31.1) | <0.001 |
| Intraoperative data | ||||||
| Operation time, min | 770 (691–865) | 770 (679–881) | 0.930 | 767 (682–856) | 756 (655–875) | 0.888 |
| Cold ischemic time, min | 82 (66–101) | 86 (70–127) | <0.001 | 85 (66–101) | 99 (78–193) | <0.001 |
| Warm ischemic time, min | 40 (33–50) | 42 (35–52) | <0.001 | 40 (33–48) | 43 (36–53) | 0.013 |
| Postreperfusion syndrome, n (%) | 933 (50.3) | 601 (56.8) | 0.001 | 91 (64.5) | 221 (66.2) | 0.814 |
| Massive transfusion, n (%) | 540 (29.1) | 625 (59.1) | <0.001 | 71 (50.4) | 244 (73.1) | <0.001 |
| Outcome | ||||||
| 90-day mortality | 30 (1.6) | 59 (5.6) | <0.001 | 5 (3.5) | 31 (9.3) | 0.049 |
| Overall mortality | 240 (12.9) | 186 (17.6) | 0.001 | 27 (19.1) | 95 (28.4) | 0.045 |
| Univariate | Multivariate * | |||
|---|---|---|---|---|
| HR [95% CI] | p Value | HR [95% CI] | p Value | |
| 90-day mortality | ||||
| Sarcopenia | ||||
| No | 1 [reference] | 1 [reference] | ||
| Yes | 2.54 [1.72–3.74] | <0.001 | 1.73 [1.14–2.61] | 0.009 |
| NLR | ||||
| ≤3 | 1 [reference] | 1 [reference] | ||
| >3 | 3.78 [2.56–5.59] | <0.001 | 1.58 [1.01–2.47] | 0.045 |
| Sarcopenia and NLR > 3 | ||||
| Neither | 1 [reference] | 1 [reference] | ||
| Both | 5.97 [3.62–9.87] | <0.001 | 2.48 [1.40–4.40] | 0.002 |
| Overall mortality | ||||
| Sarcopenia | ||||
| No | 1 [reference] | 1 [reference] | ||
| Yes | 1.93 [1.58–2.37] | <0.001 | 1.60 [1.30–1.98] | <0.001 |
| NLR | ||||
| ≤3 | 1 [reference] | 1 [reference] | ||
| >3 | 1.63 [1.38–1.92] | <0.001 | 1.17 [0.95–1.42] | 0.134 |
| Sarcopenia and NLR > 3 | ||||
| Neither | 1 [reference] | 1 [reference] | ||
| Both | 2.55 [2.02–3.24] | <0.001 | 1.81 [1.37–2.38] | <0.001 |
| Overall Mortality | ||
|---|---|---|
| HR [95% CI] a | p Value | |
| Age (years) | ||
| <55 | 2.03 [1.35–3.04] | <0.001 |
| ≥55 | 1.59 [1.11–2.67] | 0.011 |
| Sex | ||
| Male | 1.54 [1.12–2.12] | 0.008 |
| Female | 3.43 [1.83–6.43] | <0.001 |
| MELD score | ||
| <25 | 1.62 [1.09–2.40] | 0.016 |
| ≥25 | 1.68 [1.03–2.73] | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Kim, Y.J.; Kwon, H.-M.; Kim, K.-W.; YanZhen, J.; Kang, S.-J.; Jun, I.-G.; Song, J.-G.; Hwang, G.-S. Association Between Survival After Living Donor Liver Transplantation and Recipient Systemic Inflammation and Body Composition. J. Clin. Med. 2025, 14, 5889. https://doi.org/10.3390/jcm14165889
Kim JH, Kim YJ, Kwon H-M, Kim K-W, YanZhen J, Kang S-J, Jun I-G, Song J-G, Hwang G-S. Association Between Survival After Living Donor Liver Transplantation and Recipient Systemic Inflammation and Body Composition. Journal of Clinical Medicine. 2025; 14(16):5889. https://doi.org/10.3390/jcm14165889
Chicago/Turabian StyleKim, Jae Hwan, Yeon Ju Kim, Hye-Mee Kwon, Kyung-Won Kim, Jin YanZhen, Sa-Jin Kang, In-Gu Jun, Jun-Gol Song, and Gyu-Sam Hwang. 2025. "Association Between Survival After Living Donor Liver Transplantation and Recipient Systemic Inflammation and Body Composition" Journal of Clinical Medicine 14, no. 16: 5889. https://doi.org/10.3390/jcm14165889
APA StyleKim, J. H., Kim, Y. J., Kwon, H.-M., Kim, K.-W., YanZhen, J., Kang, S.-J., Jun, I.-G., Song, J.-G., & Hwang, G.-S. (2025). Association Between Survival After Living Donor Liver Transplantation and Recipient Systemic Inflammation and Body Composition. Journal of Clinical Medicine, 14(16), 5889. https://doi.org/10.3390/jcm14165889





