Artificial Intelligence Models for Predicting Outcomes in Spinal Metastasis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
- Survival: At the longest or most precise follow-up available, it is reported as either overall survival (OS) or progression-free survival (PFS). PFS is the period of time until disease progression or death, whereas OS is the period of time from diagnosis or the start of therapy to death from any cause.
- Ambulatory status: Usually classified as either ambulatory or non-ambulatory, this refers to the patient’s capacity to walk on their own or with the use of assistive technology.
- Complications: Contains any unfavorable events that occur during or after surgery, such as bleeding, infection, thrombosis, or neurological decline. Standard classification systems (e.g., Clavien–Dindo) were used to stratify these by severity whenever possible.
2. Materials and Methods
2.1. Ethical Review
2.2. Search Strategy
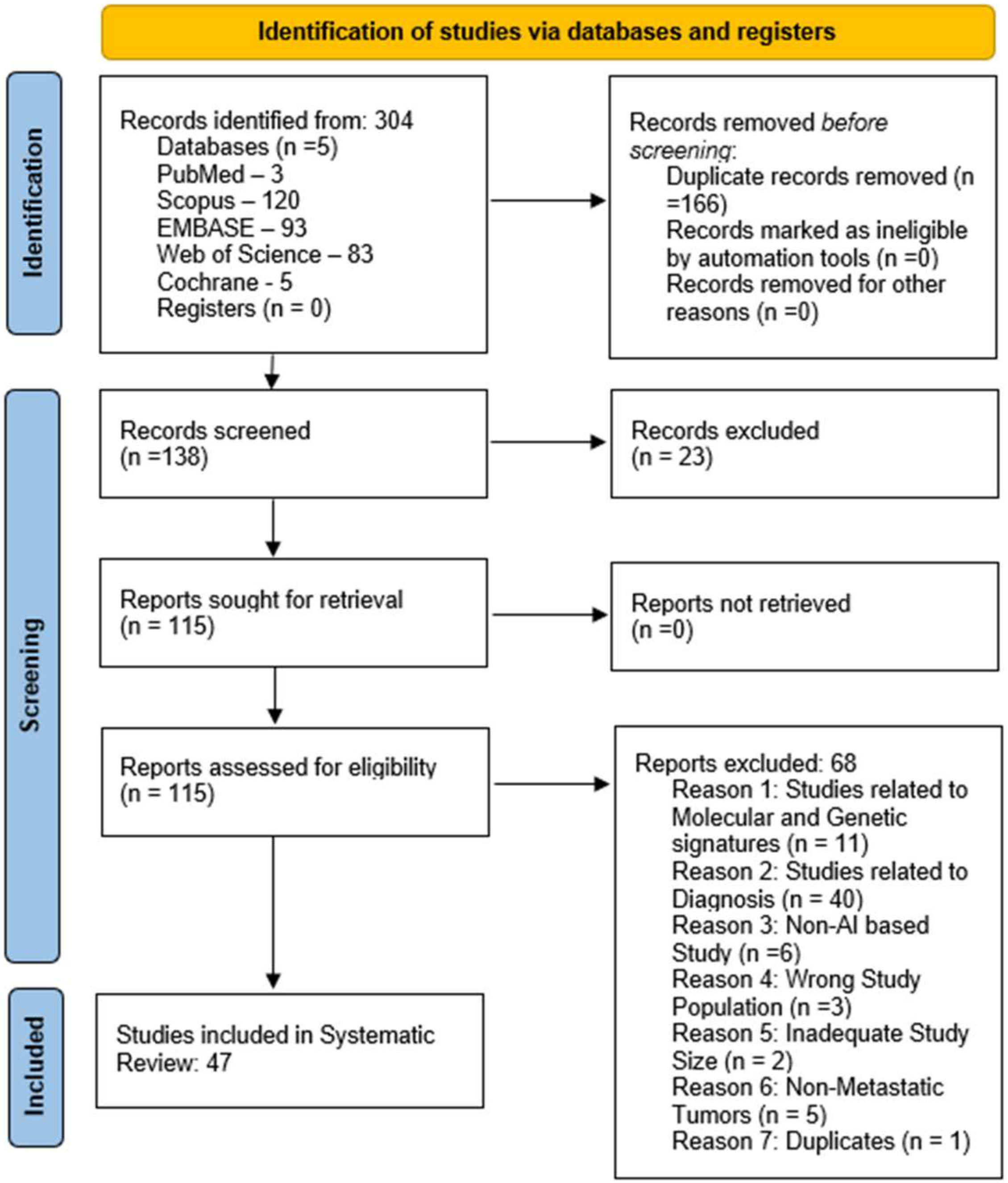
2.3. Screening of Studies
2.4. Data Extraction
2.5. Data Analysis
2.6. Quality Assessment
3. Results
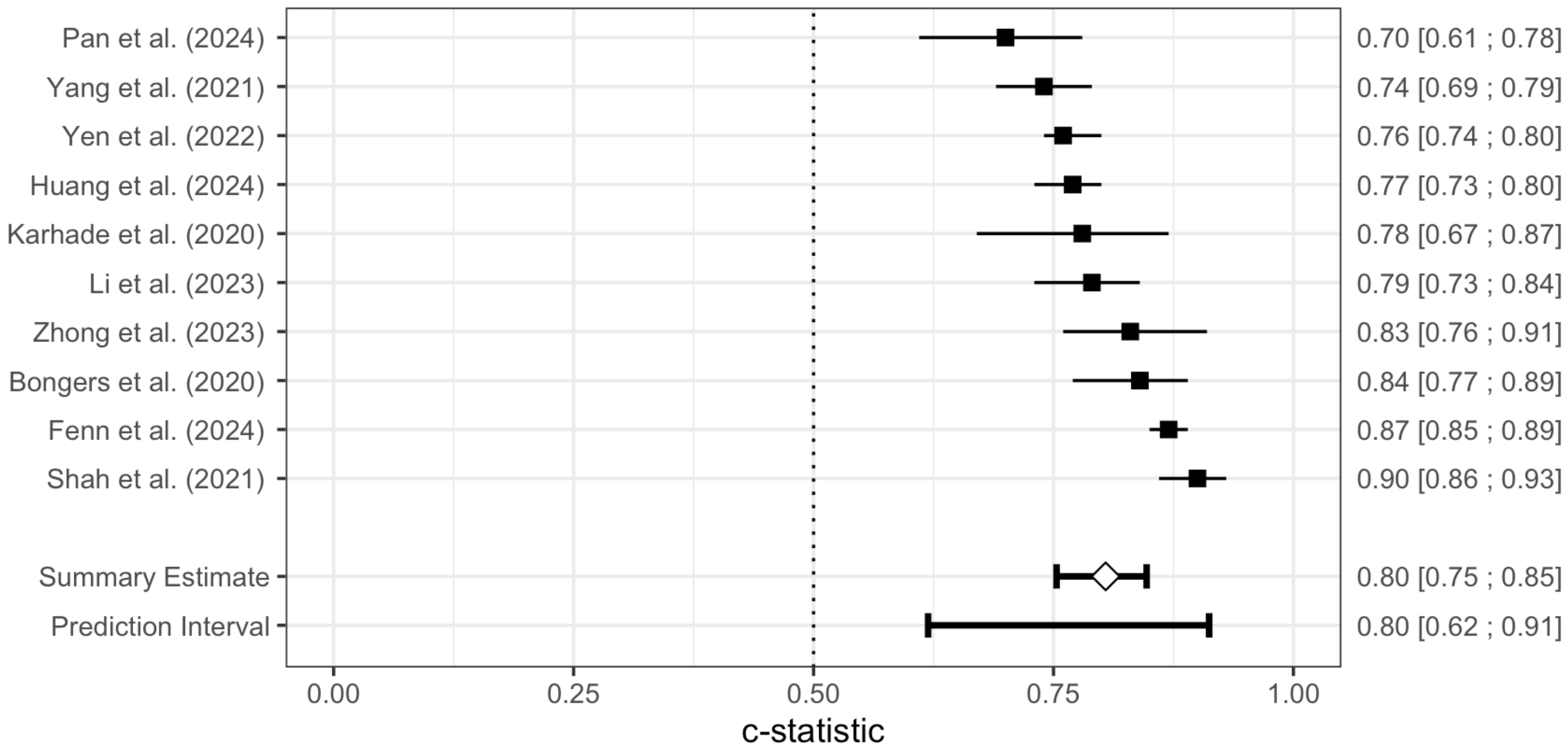
Meta-Analysis of the SORG-MLA Model
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wewel, J.T.; O’Toole, J.E. Epidemiology of spinal cord and column tumors. Neuro-Oncol. Pract. 2020, 7 (Suppl. S1), i5–i9. [Google Scholar] [CrossRef]
- Conti, A.; Acker, G.; Kluge, A.; Loebel, F.; Kreimeier, A.; Budach, V.; Vajkoczy, P.; Ghetti, I.; Germano’, A.F.; Senger, C.; et al. Decision Making in Patients with Metastatic Spine. The Role of Minimally Invasive Treatment Modalities. Front. Oncol. 2019, 9, 915. [Google Scholar] [CrossRef]
- Dixon, D.; Sattar, H.; Moros, N.; Kesireddy, S.R.; Ahsan, H.; Lakkimsetti, M.; Fatima, M.; Doshi, D.; Sadhu, K.; Junaid Hassan, M. Unveiling the Influence of AI Predictive Analytics on Patient Outcomes: A Comprehensive Narrative Review. Cureus 2024, 16, e59954. [Google Scholar] [CrossRef]
- Barton, L.B.; Arant, K.R.; Blucher, J.A.; Sarno, D.L.; Redmond, K.J.; Balboni, T.A.; Colman, M.; Goodwin, C.R.; Laufer, I.; Placide, R.; et al. Clinician Experiences in Treatment Decision-Making for Patients with Spinal Metastases. J. Bone Jt. Surg. 2021, 103, e1. [Google Scholar] [CrossRef] [PubMed]
- Sanker, V.; Sanikommu, S.; Thaller, A.; Li, Z.; Heesen, P.; Hariharan, S.; Nordin, E.O.R.; Cavagnaro, M.J.; Ratliff, J.; Desai, A. Artificial Intelligence for Non-Invasive Prediction of Molecular Signatures in Spinal Metastases: A Systematic Review. Bioengineering 2025, 12, 791. [Google Scholar] [CrossRef]
- Zhao, W.; Qin, S.; Wang, Q.; Chen, Y.; Liu, K.; Xin, P.; Lang, N. Assessment of Hidden Blood Loss in Spinal Metastasis Surgery: A Comprehensive Approach with MRI-Based Radiomics Models. J. Magn. Reson. Imaging 2024, 59, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshian, J.; Shahrestani, S.; Buser, Z.; Hah, R.; Hsieh, P.C.; Liu, J.C.; Wang, J.C. The performance of frailty in predictive modeling of short-term outcomes in the surgical management of metastatic tumors to the spine. Spine J. 2022, 22, 605–615. [Google Scholar] [CrossRef]
- Massaad, E.; Bridge, C.P.; Kiapour, A.; Fourman, M.S.; Duvall, J.B.; Connolly, I.D.; Hadzipasic, M.; Shankar, G.M.; Andriole, K.P.; Rosenthal, M.; et al. Evaluating frailty, mortality, and complications associated with metastatic spine tumor surgery using machine learning–derived body composition analysis. J. Neurosurg. Spine 2022, 37, 263–273. [Google Scholar] [CrossRef]
- Shi, Y.J.; Zhu, H.T.; Li, X.T.; Zhang, X.Y.; Wei, Y.Y.; Yan, S.; Sun, Y.S. Radiomics analysis based on multiple parameters MR imaging in the spine: Predicting treatment response of osteolytic bone metastases to chemotherapy in breast cancer patients. Magn. Reson. Imaging 2022, 92, 10–18. [Google Scholar] [CrossRef]
- Massaad, E.; Williams, N.; Hadzipasic, M.; Patel, S.S.; Fourman, M.S.; Kiapour, A.; Schoenfeld, A.J.; Shankar, G.M.; Shin, J.H. Performance assessment of the metastatic spinal tumor frailty index using machine learning algorithms: Limitations and future directions. Neurosurg. Focus 2021, 50, E5. [Google Scholar] [CrossRef]
- Santipas, B.; Suvithayasiri, S.; Trathitephun, W.; Wilartratsami, S.; Luksanapruksa, P. Developmental and Validation of Machine Learning Model for Prediction Complication After Cervical Spine Metastases Surgery. Clin. Spine Surgery: A Spine Publ. 2024, 38, E81–E88. [Google Scholar] [CrossRef]
- Cui, Y.; Shi, X.; Qin, Y.; Wang, Q.; Cao, X.; Che, X.; Pan, Y.; Wang, B.; Lei, M.; Liu, Y. Establishment and validation of an interactive artificial intelligence platform to predict postoperative ambulatory status for patients with metastatic spinal disease: A multicenter analysis. Int. J. Surg. 2024, 110, 2738–2756. [Google Scholar] [CrossRef]
- Santipas, B.; Chanajit, A.; Wilartratsami, S.; Ittichaiwong, P.; Veerakanjana, K.; Luksanapruksa, P. Development of Machine Learning Algorithms for Predicting Preoperative and Postoperative venous Thromboembolism in Patients Undergoing Surgery for Spinal Metastasis. Siriraj Med. J. 2024, 76, 381–388. [Google Scholar] [CrossRef]
- Santipas, B.; Veerakanjana, K.; Ittichaiwong, P.; Chavalparit, P.; Wilartratsami, S.; Luksanapruksa, P. Development and internal validation of machine-learning models for predicting survival in patients who underwent surgery for spinal metastases. Asian Spine J. 2024, 18, 325–335. [Google Scholar] [CrossRef]
- Shi, X.; Cui, Y.; Wang, S.; Pan, Y.; Wang, B.; Lei, M. Development and validation of a web-based artificial intelligence prediction model to assess massive intraoperative blood loss for metastatic spinal disease using machine learning techniques. Spine J. 2024, 24, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Chavalparit, P.; Wilartratsami, S.; Santipas, B.; Ittichaiwong, P.; Veerakanjana, K.; Luksanapruksa, P. Development of Machine-Learning Models to Predict Ambulation Outcomes Following Spinal Metastasis Surgery. Asian Spine J. 2023, 17, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qin, S.; Zhao, W.; Wang, Q.; Liu, K.; Xin, P.; Yuan, H.; Zhuang, H.; Lang, N. MRI feature-based radiomics models to predict treatment outcome after stereotactic body radiotherapy for spinal metastases. Insights Into Imaging 2023, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cao, Y.; Cao, X.; Shi, X.; Lei, M.; Su, X.; Liu, Y. Machine learning-based algorithms to predict severe psychological distress among cancer patients with spinal metastatic disease. Spine J. 2023, 23, 1255–1269. [Google Scholar] [CrossRef]
- Hallinan, J.T.P.D.; Zhu, L.; Zhang, W.; Kuah, T.; Lim, D.S.W.; Low, X.Z.; Cheng, A.J.L.; Eide, S.E.; Ong, H.Y.; Muhamat Nor, F.E.; et al. Deep Learning Model for Grading Metastatic Epidural Spinal Cord Compression on Staging CT. Cancers 2022, 14, 3219. [Google Scholar] [CrossRef]
- Jabehdar Maralani, P.; Chen, H.; Moazen, B.; Mojtahed Zadeh, M.; Salehi, F.; Chan, A.; Zeng, L.K.; Abugharib, A.; Tseng, C.L.; Husain, Z.; et al. Proposing a quantitative MRI-based linear measurement framework for response assessment following stereotactic body radiation therapy in patients with spinal metastasis. J. Neurooncol. 2022, 160, 265–272. [Google Scholar] [CrossRef]
- Karhade, A.V.; Fenn, B.; Groot, O.Q.; Shah, A.A.; Yen, H.K.; Bilsky, M.H.; Hu, M.H.; Laufer, I.; Park, D.Y.; Sciubba, D.M.; et al. Development and external validation of predictive algorithms for six-week mortality in spinal metastasis using 4304 patients from five institutions. Spine J. 2022, 22, 2033–2041. [Google Scholar] [CrossRef]
- Gui, C.; Chen, X.; Sheikh, K.; Mathews, L.; Lo, S.L.; Lee, J.; Khan, M.A.; Sciubba, D.M.; Redmond, K.J. Radiomic modeling to predict risk of vertebral compression fracture after stereotactic body radiation therapy for spinal metastases. J. Neurosurg. Spine 2022, 36, 294–302. [Google Scholar] [CrossRef]
- Karhade, A.V.; Thio, Q.C.B.S.; Ogink, P.T.; Shah, A.A.; Bono, C.M.; Oh, K.S.; Saylor, P.J.; Schoenfeld, A.J.; Shin, J.H.; Harris, M.B.; et al. Development of Machine Learning Algorithms for Prediction of 30-Day Mortality After Surgery for Spinal Metastasis. Neurosurgery 2019, 85, E83–E91. [Google Scholar] [CrossRef]
- Karhade, A.V.; Thio, Q.C.B.S.; Ogink, P.T.; Bono, C.M.; Ferrone, M.L.; Oh, K.S.; Saylor, P.J.; Schoenfeld, A.J.; Shin, J.H.; Harris, M.B.; et al. Predicting 90-Day and 1-Year Mortality in Spinal Metastatic Disease: Development and Internal Validation. Neurosurgery 2019, 85, E671–E681. [Google Scholar] [CrossRef]
- Paulino Pereira, N.R.; Janssen, S.J.; van Dijk, E.; Harris, M.B.; Hornicek, F.J.; Ferrone, M.L.; Schwab, J.H. Development of a Prognostic Survival Algorithm for Patients with Metastatic Spine Disease. J. Bone Jt. Surg. 2016, 98, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Fenn, B.P.; Karhade, A.V.; Groot, O.Q.; Collins, A.K.; Balboni, T.A.; Oh, K.S.; Ferrone, M.L.; Schwab, J.H. Survival in Patients with Spinal Metastatic Disease Treated Nonoperatively with Radiotherapy. Clin. Spine Surgery A Spine Publ. 2024, 37, E290–E296. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Peng, K.P.; Hsieh, H.C.; Groot, O.Q.; Yen, H.K.; Tsai, C.C.; Karhade, A.V.; Lin, Y.P.; Kao, Y.T.; Yang, J.J.; et al. Does the Presence of Missing Data Affect the Performance of the SORG Machine-learning Algorithm for Patients with Spinal Metastasis? Development of an Internet Application Algorithm. Clin. Orthop. Relat. Res. 2024, 482, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.T.; Lin, Y.P.; Yen, H.K.; Yen, H.H.; Huang, C.C.; Hsieh, H.C.; Janssen, S.; Hu, M.H.; Lin, W.H.; Groot, O.Q. Are Current Survival Prediction Tools Useful When Treating Subsequent Skeletal-related Events From Bone Metastases? Clin. Orthop. Relat. Res. 2024, 482, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, L.; Guo, B.; Zhang, P.; Wang, J.; Wang, X.; Yao, W. Evaluation of different scoring systems for spinal metastases based on a Chinese cohort. Cancer Med. 2023, 12, 4125–4136. [Google Scholar] [CrossRef]
- Su, C.C.; Lin, Y.P.; Yen, H.K.; Pan, Y.T.; Zijlstra, H.; Verlaan, J.J.; Schwab, J.H.; Lai, C.Y.; Hu, M.H.; Yang, S.H.; et al. A Machine Learning Algorithm for Predicting 6-Week Survival in Spinal Metastasis: An External Validation Study Using 2768 Taiwanese Patients. J. Am. Acad. Orthop. Surg. 2023, 31, e645–e656. [Google Scholar] [CrossRef]
- Zhong, G.; Cheng, S.; Zhou, M.; Xie, J.; Xu, Z.; Lai, H.; Yan, Y.; Xie, Z.; Zhou, J.; Xie, X.; et al. External validation of the SORG machine learning algorithms for predicting 90-day and 1-year survival of patients with lung cancer-derived spine metastases: A recent bi-center cohort from China. Spine J. 2023, 23, 731–738. [Google Scholar] [CrossRef]
- Yen, H.K.; Hu, M.H.; Zijlstra, H.; Groot, O.Q.; Hsieh, H.C.; Yang, J.J.; Karhade, A.V.; Chen, P.C.; Chen, Y.H.; Huang, P.H.; et al. Prognostic significance of lab data and performance comparison by validating survival prediction models for patients with spinal metastases after radiotherapy. Radiother. Oncol. 2022, 175, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Karhade, A.V.; Park, H.Y.; Sheppard, W.L.; Macyszyn, L.J.; Everson, R.G.; Shamie, A.N.; Park, D.Y.; Schwab, J.H.; Hornicek, F.J. Updated external validation of the SORG machine learning algorithms for prediction of ninety-day and one-year mortality after surgery for spinal metastasis. Spine J. 2021, 21, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Chen, C.W.; Fourman, M.S.; Bongers, M.E.R.; Karhade, A.V.; Groot, O.Q.; Lin, W.H.; Yen, H.K.; Huang, P.H.; Yang, S.H.; et al. International external validation of the SORG machine learning algorithms for predicting 90-day and one-year survival of patients with spine metastases using a Taiwanese cohort. Spine J. 2021, 21, 1670–1678. [Google Scholar] [CrossRef]
- Bongers, M.E.R.; Karhade, A.V.; Villavieja, J.; Groot, O.Q.; Bilsky, M.H.; Laufer, I.; Schwab, J.H. Does the SORG algorithm generalize to a contemporary cohort of patients with spinal metastases on external validation? Spine J. 2020, 20, 1646–1652. [Google Scholar] [CrossRef]
- Karhade, A.V.; Ahmed, A.K.; Pennington, Z.; Chara, A.; Schilling, A.; Thio, Q.C.B.S.; Ogink, P.T.; Sciubba, D.M.; Schwab, J.H. External validation of the SORG 90-day and 1-year machine learning algorithms for survival in spinal metastatic disease. Spine J. 2020, 20, 14–21. [Google Scholar] [CrossRef]
- Lee, S.B.; Hong, Y.; Cho, Y.J.; Jeong, D.; Lee, J.; Choi, J.W.; Hwang, J.Y.; Lee, S.; Choi, Y.H.; Cheon, J.E. Enhancing Radiomics Reproducibility: Deep Learning-Based Harmonization of Abdominal Computed Tomography (CT) Images. Bioengineering 2024, 11, 1212. [Google Scholar] [CrossRef]
- He, X.; Jiao, Y.Q.; Yang, X.G.; Hu, Y.C. A Novel Prediction Tool for Overall Survival of Patients Living with Spinal Metastatic Disease. World Neurosurg. 2020, 144, e824–e836. [Google Scholar] [CrossRef]
- Kehayias, C.E.; Bontempi, D.; Quirk, S.; Friesen, S.; Bredfeldt, J.; Kosak, T.; Kearney, M.; Tishler, R.; Pashtan, I.; Huynh, M.A.; et al. A prospectively deployed deep learning-enabled automated quality assurance tool for oncological palliative spine radiation therapy. Lancet Digit. Health 2024, 7, e13–e22. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, X.; Lun, D.; Li, R.; Ma, R.; Hu, Y. Quantile regression-based prediction of intraoperative blood loss in patients with spinal metastases: Model development and validation. Eur. Spine J. 2023, 32, 2479–2492. [Google Scholar] [CrossRef]
- Mezei, T.; Horváth, A.; Nagy, Z.; Czigléczki, G.; Banczerowski, P.; Báskay, J.; Pollner, P. A Novel Prognostication System for Spinal Metastasis Patients Based on Network Science and Correlation Analysis. Clin. Oncol. 2022, 35, e20–e29. [Google Scholar] [CrossRef]
- Fourman, M.S.; Siraj, L.; Duvall, J.; Ramsey, D.C.; De La Garza Ramos, R.; Hadzipasic, M.; Connolly, I.; Williamson, T.; Shankar, G.M.; Schoenfeld, A.; et al. Can We Use Artificial Intelligence Cluster Analysis to Identify Patients with Metastatic Breast Cancer to the Spine at Highest Risk of Postoperative Adverse Events? World Neurosurg. 2023, 174, e26–e34. [Google Scholar] [CrossRef]
- Li, Z.; Huang, L.; Guo, B.; Zhang, P.; Wang, J.; Wang, X.; Yao, W. The predictive ability of routinely collected laboratory markers for surgically treated spinal metastases: A retrospective single institution study. BMC Cancer. MC Cancer 2022, 22, 1231. [Google Scholar] [CrossRef]
- Hu, M.H.; Yen, H.K.; Chen, I.H.; Wu, C.H.; Chen, C.W.; Yang, J.J.; Wang, Z.Y.; Yen, M.H.; Yang, S.H.; Lin, W.H. Decreased psoas muscle area is a prognosticator for 90-day and 1-year survival in patients undergoing surgical treatment for spinal metastasis. Clin. Nutr. 2022, 41, 620–629. [Google Scholar] [CrossRef]
- Walker, A.; Bassale, S.; Shukla, R.; Dai Kubicky, C. A Prognostic Index for Predicting Survival of Patients Undergoing Radiation Therapy for Spine Metastasis Using Recursive Partitioning Analysis. J. Palliat. Med. 2022, 25, 21–27. [Google Scholar] [CrossRef]
- Khalid, S.I.; Massaad, E.; Kiapour, A.; Bridge, C.P.; Rigney, G.; Burrows, A.; Shim, J.; De la Garza Ramos, R.; Tobert, D.G.; Schoenfeld, A.J.; et al. Machine learning–based detection of sarcopenic obesity and association with adverse outcomes in patients undergoing surgical treatment for spinal metastases. J. Neurosurg. Spine 2024, 40, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Rigney, G.H.; Massaad, E.; Kiapour, A.; Razak, S.S.; Duvall, J.B.; Burrows, A.; Khalid, S.I.; De La Garza Ramos, R.; Tobert, D.G.; Williamson, T.; et al. Implication of nutritional status for adverse outcomes after surgery for metastatic spine tumors. J. Neurosurg. Spine 2023, 39, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, J.T.P.D.; Zhu, L.; Zhang, W.; Lim, D.S.W.; Baskar, S.; Low, X.Z.; Yeong, K.Y.; Teo, E.C.; Kumarakulasinghe, N.B.; Yap, Q.V.; et al. Deep Learning Model for Classifying Metastatic Epidural Spinal Cord Compression on MRI. Front. Oncol. 2022, 12, 849447. [Google Scholar] [CrossRef] [PubMed]
- Arends, S.R.S.; Savenije, M.H.F.; Eppinga, W.S.C.; van der Velden, J.M.; van den Berg, C.A.T.; Verhoeff, J.J.C. Clinical utility of convolutional neural networks for treatment planning in radiotherapy for spinal metastases. Phys. Imaging Radiat. Oncol. 2022, 21, 42–47. [Google Scholar] [CrossRef]
- Rogé, M.; Henni, A.H.; Neggaz, Y.A.; Mallet, R.; Hanzen, C.; Dubray, B.; Colard, E.; Gensanne, D.; Thureau, S. Evaluation of a Dedicated Software “ElementsTM Spine SRS, Brainlab®” for Target Volume Definition in the Treatment of Spinal Bone Metastases with Stereotactic Body Radiotherapy. Front Oncol. 2022, 12, 827195. [Google Scholar] [CrossRef]
- Kowalchuk, R.O.; Waters, M.R.; Richardson, K.M.; Spencer, K.; Larner, J.M.; McAllister, W.H.; Sheehan, J.P.; Kersh, C.R. Stereotactic body radiation therapy for spinal metastases: A novel local control stratification by spinal region. J. Neurosurg. Spine 2021, 34, 267–276. [Google Scholar] [CrossRef]
- Korpics, M.C.; Polley, M.Y.; Bhave, S.R.; Redler, G.; Pitroda, S.P.; Luke, J.J.; Chmura, S.J. A Validated T Cell Radiomics Score Is Associated with Clinical Outcomes Following Multisite SBRT and Pembrolizumab. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 189–195. [Google Scholar] [CrossRef]
- Cui, Y.; Pan, Y.; Lei, M.; Mi, C.; Wang, B.; Shi, X. The First Algorithm Calculating Cement Injection Volumes in Patients with Spine Metastases Treated with Percutaneous Vertebroplasty. Ther. Clin. Risk Manag. 2020, 16, 417–4288. [Google Scholar] [CrossRef]

| Year of publishing (range) | 2016–2025 |
| Total number of patients | 25,790 |
| Median number of patients | 268 |
| Number of patients per study (range) | 30–2786 |
| Primary Tumor Type | Number of Studies (Percentage) |
|---|---|
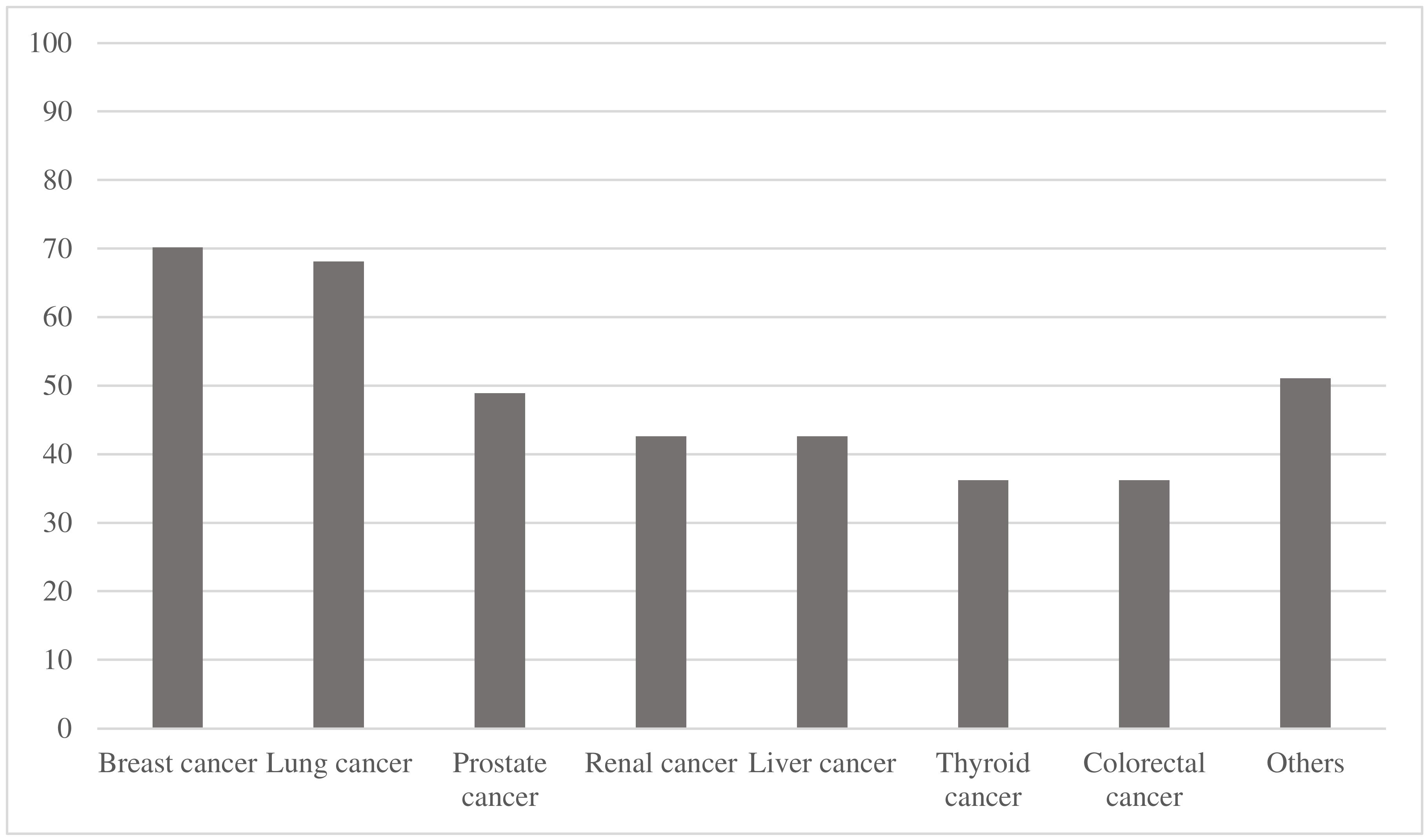
| Breast cancer | 33/47 (70.2%) |
| Lung cancer | 32/47 (68.1%) |
| Prostate cancer | 23/47 (48.9%) |
| Esophageal cancer | 4/47 (8.5%) |
| Bladder cancer | 3/47 (6.4%) |
| Neuroendocrine tumors | 3/47 (6.4%) |
| Study | Output/Prediction | Best-Performing Model | AUC | 95% CI |
|---|---|---|---|---|
| Zhao et al. (2024) [6] | Hidden blood loss in spinal metastasis surgery | MRI-Based Radiomics | 0.784 | - |
| Bakhsheshian et al. (2022) [7] | Mortality | Machine Learning Model using ECI 1 and Frailty | 0.788 | - |
| Medical complications | 0.723 | - | ||
| Massaad et al. (2022) [8] | 1-year mortality | Machine Learning Model using Body Composition and NESMS 2 | 0.73 | 0.67–0.78 |
| Shi et al. (2022) [9] | Response of osteolytic metastases to chemotherapy | Radiomics (T2WI + ADCall) | 0.908 | 0.86–0.96 |
| Massaad et al. (2021) [10] | Postoperative complications | Random Forest to develop MSTFI 3 | 0.62 | 0.56–0.68 |
| Study | Output/Prediction | Best-Performing Model | AUC | 95% CI | |||
|---|---|---|---|---|---|---|---|
| Santipas et al. (2024) [11] | Complications after cervical spine metastases surgery | Gradient Boosting | 0.939 1 | 0.873 2 | - | - | |
| Cui et al. (2024) [12] | Postoperative ambulatory status | Ensemble Machine Learning combining LR 6, eXGBM 7, SVM 8, RF 9, NN 10 and DT 11 | 0.911 1 | 0.854–0.968 1 | |||
| Santipas et al. (2024) [13] | 30-day preoperative VTE 5 | Gradient Boosted Trees | 0.77 1 | - | |||
| 90-day preoperative VTE 5 | Support Vector Machine | 0.72 1 | - | ||||
| 30-day postoperative VTE 5 | Gradient Boosted Trees | 0.71 1 | - | ||||
| 90-day postoperative VTE 5 | Support Vector Machine | 0.68 1 | - | ||||
| Santipas et al. (2024) [14] | 90-day survival | CatBoost | 0.750 1 | 0.758 2 | - | - | |
| 180-day survival | XGBoost | 0.726 1 | 0.744 2 | - | - | ||
| 365-day survival | XGBoost | 0.731 1 | 0.693 2 | - | |||
| Shi et al. (2024) [15] | Massive intraoperative blood loss | XGBoosting machine (XGBM; Machine Learning) | 0.857 2 | 0.827–0.877 2 | |||
| Zhao et al. (2024) [6] | Hidden blood loss | MRI-Based Radiomics | 0.744 2 | 0.576–0.914 2 | |||
| Chavalparit et al. (2023) [16] | 90-day postoperative ambulatory status | Decision Tree | 0.941 1 | - | |||
| 180-day postoperative ambulatory status | Extreme Gradient Boosting | 0.852 1 | - | ||||
| Chen et al. (2023) [17] | Treatment outcome after stereotactic body RT 12 | Gaussian Processes | 0.828 1 | - | |||
| Gao et al. (2023) [18] | Severe psychological distress | Gradient Boosting Machine (Machine Learning) | 0.865 2 | 0.788–0.941 2 | |||
| Hallinan et al. (2022) [19] | Grading metastatic epidural spinal cord compression (Bilsky grading (normal/low versus high) | Separated Window Learning (Max fusion model) | 0.971 2 | 0.961–0.981 2 | |||
| Grading metastatic epidural spinal cord compression (Bilsky grading (normal versus low/high) | Separated Window Learning (Spine-window) | 0.924 2 | 0.910–0.938 2 | ||||
| Jabehdar Maralani et al. (2022) [20] | Response following stereotactic body RT | Decision Tree | 0.923 1 | 0.959 2 | - | - | |
| Karhade et al. (2022) [21] | 6-week mortality | Elastic-net penalized logistic regression | 0.85 1 | 0.84 2 | 0.84–0.86 1 | 0.80–0.88 2 | |
| Shi et al. (2022) [9] | Response of osteolytic metastases to chemotherapy | Radiomics (FST2WI + ADCall) | 0.873 2 | 0.78–0.96 2 | |||
| Gui et al. (2022) [22] | Risk of vertebral compression fracture after stereotactic body RT 12 | Random Forest | 0.878 1 | 0.832–0.924 1 | |||
| Massaad et al. (2021) [10] | Postoperative complications | Random Forest to develop MSTFI 3 | 0.69 4 | 0.66–0.73 4 | |||
| Karhade et al. (2019) [23] | 30-day mortality | Bayes Point Machine (Machine Learning) | 0.786 1 | 0.782 2 | - | - | |
| Karhade et al. (2019) [24] | 90-day mortality | Stochastic gradient boosting | 0.83 1 | 0.83 2 | 0.81–0.85 1 | - | |
| 1-year mortality | 0.85 1 | 0.89 2 | 0.83–0.87 1 | - | |||
| Paulino Pereira et al. (2016) [25] | 30-day survival | Boosting Algorithm | Nomogram | 0.91 1 | 0.75 2 | 0.86–0.95 1 | 0.60–0.89 2 |
| 90-day survival | 0.86 1 | 0.73 2 | 0.83–0.90 1 | 0.63–0.83 2 | |||
| 1-year survival | 0.84 1 | 0.75 2 | 0.80–0.87 1 | 0.67–0.84 2 | |||
| Study | Output/Prediction | Best Performing Model | AUC | 95% CI |
|---|---|---|---|---|
| Cui et al. (2024) [12] | Postoperative ambulatory status | Ensemble Machine Learning combining LR 2, eXGBM 3, SVM 4, RF 5, NN 6 and DT 7 | 0.873 | 0.809–0.936 |
| 0.924 | 0.890–0.959 | |||
| Fenn et al. (2024) [26] | 90-day mortality | Machine Learning Algorithm (SORG-MLA) | 0.85 | 0.83–0.87 |
| 1-year mortality | 0.87 | 0.85–0.89 | ||
| Huang et al. (2024) [27] | 6-week survival | Machine Learning Algorithm (SORG-MLA) | 0.84 | 0.78–0.89 |
| 90-day survival | 0.84 | 0.79–0.90 | ||
| 1-year survival | 0.77 | 0.73–0.80 | ||
| Pan et al. (2024) [28] | 42-day survival | Machine Learning Algorithm (SORG-MLA) | 0.69 | 0.63–0.74 |
| 90-day survival | 0.72 | 0.66–0.77 | ||
| 1-year survival | 0.70 | 0.61–0.78 | ||
| Shi et al. (2024) [15] | Massive intraoperative blood loss for spinal metastases | XGBoosting machine (XGBM; Machine Learning) | 0.809 | 0.778–0.860 |
| Li et al. (2023) [29] | 90-day survival | Machine Learning Algorithm (SORG-MLA) | 0.743 | 0.666–0.817 |
| 180-day survival | Machine Learning Algorithm (Revised Katagiri) | 0.761 | 0.696–0.826 | |
| 1-year survival | Machine Learning Algorithm (SORG-MLA) | 0.787 | 0.730–0.838 | |
| 2-year survival | Machine Learning Algorithm (Revised Katagiri) | 0.779 | 0.747–0.811 | |
| Su et al. (2023) [30] | 6-week survival after RT 1 only | Machine Learning Algorithm (SORG-MLA) | 0.77 | 0.74–0.79 |
| 6-week survival after surgery | 0.84 | 0.79–0.90 | ||
| Zhong et al. (2023) [31] | 90-day mortality | Machine Learning Algorithm (SORG-MLA) | 0.714 | 0.589–0.839 |
| 1-year mortality | 0.832 | 0.758–0.906 | ||
| Karhade et al. (2022) [21] | 6-week mortality | Elastic-net penalized logistic regression | 0.82 | 0.78–0.85 |
| Yen et al. (2022) [32] | 90-day survival | Machine Learning Algorithm (SORG-MLA) | 0.78 | 0.76–0.80 |
| 1-year survival | 0.76 | 0.74–0.78 | ||
| Shah et al. (2021) [33] | 90-day mortality | Machine Learning Algorithm (SORG-MLA) | 0.84 | 0.79–0.89 |
| 1-year mortality | 0.90 | 0.86–0.93 | ||
| Yang et al. (2021) [34] | 90-day mortality | Machine Learning Algorithm (SORG-MLA) | 0.73 | 0.67–0.78 |
| 1-year mortality | 0.74 | 0.69–0.79 | ||
| Bongers et al. (2020) [35] | 90-day mortality | Machine Learning Algorithm (SORG-MLA) | 0.81 | 0.74–0.87 |
| 1-year mortality | 0.84 | 0.77–0.89 | ||
| Karhade et al. (2020) [36] | 90-day mortality | Machine Learning Algorithm (SORG-MLA) | 0.81 | 0.70–0.89 |
| 1-year mortality | 0.78 | 0.67–0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanker, V.; Dawer, P.; Thaller, A.; Li, Z.; Heesen, P.; Hariharan, S.; Nordin, E.O.R.; Cavagnaro, M.J.; Ratliff, J.; Desai, A. Artificial Intelligence Models for Predicting Outcomes in Spinal Metastasis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 5885. https://doi.org/10.3390/jcm14165885
Sanker V, Dawer P, Thaller A, Li Z, Heesen P, Hariharan S, Nordin EOR, Cavagnaro MJ, Ratliff J, Desai A. Artificial Intelligence Models for Predicting Outcomes in Spinal Metastasis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(16):5885. https://doi.org/10.3390/jcm14165885
Chicago/Turabian StyleSanker, Vivek, Prachi Dawer, Alexander Thaller, Zhikai Li, Philip Heesen, Srinath Hariharan, Emil O. R. Nordin, Maria Jose Cavagnaro, John Ratliff, and Atman Desai. 2025. "Artificial Intelligence Models for Predicting Outcomes in Spinal Metastasis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 16: 5885. https://doi.org/10.3390/jcm14165885
APA StyleSanker, V., Dawer, P., Thaller, A., Li, Z., Heesen, P., Hariharan, S., Nordin, E. O. R., Cavagnaro, M. J., Ratliff, J., & Desai, A. (2025). Artificial Intelligence Models for Predicting Outcomes in Spinal Metastasis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(16), 5885. https://doi.org/10.3390/jcm14165885







