Evaluation of Intracranial Arteriovenous Malformations Using Ischemic Stroke Color-Coded Maps Software, a New Rapid Post-Processing Tool in CT Angiography
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
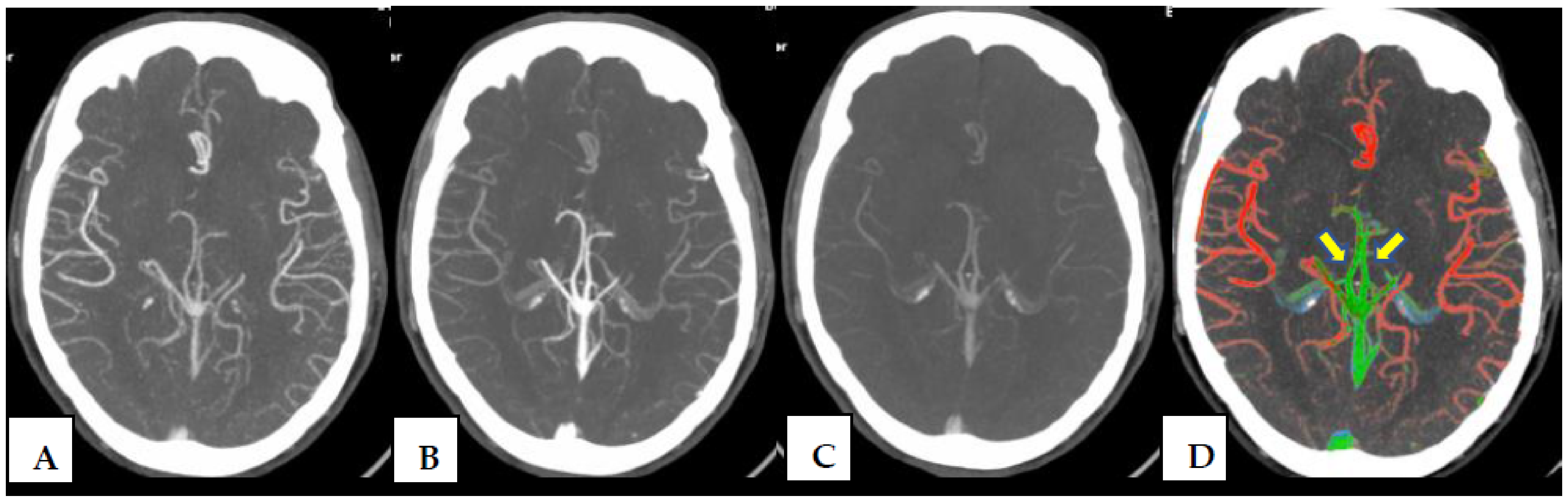
2.2. CT Protocol and Post-Processing Tool
2.3. DSA (Digital Subtraction Angiography)
2.4. Image Interpretation and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| AVM | intracranial arteriovenous malformation |
| CT | Computed Tomography |
| MCTA | multiphase CT angiography |
References
- Lawton, M.T.; Rutledge, W.C.; Kim, H.; Stapf, C.; Whitehead, K.J.; Li, D.Y.; Krings, T.; Terbrugge, K.; Kondziolka, D.; Morgan, M.K.; et al. Brain arteriovenous malformations. Nat. Rev. Dis. Prim. 2015, 1, 15008. [Google Scholar] [CrossRef] [PubMed]
- De Neuroradiologie, D.; De Rothschild, F.A.; Manin, R. Malformations Artérioveineuses Intracrâniennes: Données Épidémidémiologiques et Génétiques. J. Neuroradiol. 2004, 31, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Shankar, J.J.S.; Lum, C.; Chakraborty, S.; dos Santos, M.P. Cerebral vascular malformations: Time-resolved CT angiography compared to DSA. Neuroradiol. J. 2015, 28, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.J.; Iv, M.; Wintermark, M. Imaging of Intracranial Hemorrhage. J. Stroke 2017, 19, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Geibprasert, S.; Pongpech, S.; Jiarakongmun, P.; Shroff, M.M.; Armstrong, D.C.; Krings, T. Radiologic assessment of brain arteriovenous malformations: What clinicians need to know. Radiographics 2010, 30, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Verdolotti, T.; Pilato, F.; Cottonaro, S.; Monelli, E.; Giordano, C.; Guadalupi, P.; Benenati, M.; Ramaglia, A.; Costantini, A.M.; Alexandre, A.; et al. Colorviz, a new and rapid tool for assessing collateral circulation during stroke. Brain Sci. 2020, 10, 882. [Google Scholar] [CrossRef] [PubMed]
- D’Argento, F.; Pedicelli, A.; Ciardi, C.; Leone, E.; Scarabello, M.; Infante, A.; Alexandre, A.; Lozupone, E.; Valente, I.; Colosimo, C. Intra- and inter-observer variability in intracranial aneurysm segmentation: Comparison between CT angiography (semi-automated segmentation software stroke VCAR) and digital subtraction angiography (3D rotational angiography). Radiol. Medica 2021, 126, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Ospel, J.; Volny, O.; Qiu, W.; Najm, M.; Kashani, N.; Goyal, M.; Menon, B. Displaying multiphase CT angiography using a time-variant color map: Practical considerations and potential applications in patients with acute stroke. Am. J. Neuroradiol. 2020, 41, 200–205. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Lessons in biostatistics interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Psoter, K.J.; Roudsari, B.S.; Dighe, M.K.; Richardson, M.L.; Katz, D.S.; Bhargava, P. Biostatistics primer for the radiologist. Am. J. Roentgenol. 2014, 202, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Eddleman, C.S.; Jeong, H.J.; Hurley, M.C.; Zuehlsdorff, S.; Dabus, G.; Getch, C.G.; Batjer, H.H.; Bendok, B.R.; Carroll, T.J. 4D radial acquisition contrast-enhanced MR angiography and intracranial arteriovenous malformations: Quickly approaching digital subtraction angiography. Stroke 2009, 40, 2749–2753. [Google Scholar] [CrossRef] [PubMed]
- Ogilvy, C.S.; Stieg, P.E.; Awad, I.; Brown, R.D.; Kondziolka, D.; Rosenwasser, R.; Young, W.L.; Hademenos, G. Recommendations for the management of intracranial arteriovenous malformations: A statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Circulation 2001, 103, 2644–2657. [Google Scholar] [CrossRef] [PubMed]
- Mohr, J.P.; Parides, M.K.; Stapf, C.; Moquete, E.; Moy, C.S.; Overbey, J.R.; Salman, R.A.-S.; Vicaut, E.; Young, W.L.; Houdart, E.; et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): A multicentre, non-blinded, randomised trial. Lancet 2014, 383, 614–621. [Google Scholar] [CrossRef] [PubMed]
- ACC/AHA Task Force on Practice Guidelines. Methodologies and Policies from the ACC/AHA Task Force on Practice Guidelines. Available online: https://professional.heart.org/en/guidelines-and-statements/methodologies (accessed on 12 October 2024).
- Derdeyn, C.P.; Zipfel, G.J.; Albuquerque, F.C.; Cooke, D.L.; Feldmann, E.; Sheehan, J.P.; Torner, J.C.; American Heart Association Stroke Council. Management of Brain Arteriovenous Malformations: A Scientific Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2017, 48, e200–e224. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.J.; Huston, I.I.I.J.; Mandrekar, J.N.; Schleck, C.D.; Thielen, K.R.; Kallmes, D.F. Complications of Diagnostic Cerebral Angiography: Evaluation of 19 826 Consecutive Patients. Radiology 2007, 243, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Fifi, J.T.; Meyers, P.M.; Lavine, S.D.; Cox, V.; Silverberg, L.; Mangla, S.; Pile-Spellman, J. Complications of Modern Diagnostic Cerebral Angiography in an Academic Medical Center. J. Vasc. Interv. Radiol. 2009, 20, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Soize, S.; Bouquigny, F.; Kadziolka, K.; Portefaix, C.; Pierot, L. Value of 4D MR angiography at 3T compared with DSA for the follow-up of treated brain arteriovenous malformation. Am. J. Neuroradiol. 2014, 35, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Willinek, W.A.; Hadizadeh, D.R.; von Falkenhausen, M.; Urbach, H.; Hoogeveen, R.; Schild, H.H.; Gieseke, J. 4D time-resolved MR angiography with keyhole (4D-TRAK): More than 60 times accelerated MRA using a combination of CENTRA, keyhole, and SENSE at 3.0T. J. Magn. Reson. Imaging 2008, 27, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, D.R.; von Falkenhausen, M.; Gieseke, J.; Meyer, B.; Urbach, H.; Hoogeveen, R.; Schild, H.H.; Willinek, W.A. Cerebral Arteriovenous Malformation: Spetzler-Martin Classification at Subsecond-Temporal-Resolution Four-dimensional MR Angiography Compared with That at DSA. Radiology 2008, 246, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Schnell, S.; Carroll, T.; Vakil, P.; Hurley, M.; Wu, C.; Carr, J.; Bendok, B.; Batjer, H.; Markl, M. Intracranial 4D flow MRI: Toward individualized assessment of arteriovenous malformation hemodynamics and treatment-induced changes. Am. J. Neuroradiol. 2013, 34, 1922–1928. [Google Scholar] [CrossRef] [PubMed]


| ColorViz—Assessment of AVM Components | |
|---|---|
| Nidus Presence | Yes/No |
| Nidus type | Compact/Diffuse |
| Arterial feeder | Single/Multiple |
| Venous sinus color asymmetry | Yes/No |
| Venous drainage | Superficial veins/Deep veins/Both |
| General Criteria | ||
|---|---|---|
| ColorViz | Angiography | |
| Patients (n) | 26 (14 M; 12 F) | 21 (12 M; 9 F) |
| Age (y.o) * | 50 (±21) | 42 (±20) |
| AVM components evaluated | 5 | 4 |
| AVM site: | ||
| Supratentorial | 24 (92%) | 20 (95.3%) |
| Infratentorial | 2 (8%) | 1 (4.7%) |
| ||
| Clinical presentation | ||
| Color-Coded Map Readers’ Agreement | |||
|---|---|---|---|
| Variable | Proportion of Agreement (% of the Observations) | K Value | 95% CI |
| Nidus presence | 92% | 0.621 | 0.141–1.000 |
| Nidus type | 66.67% | −0.089 | −0.316 |
| Arterial feeder | 68% | 0.123 | −0.835 |
| Venous sinus color asymmetry | 72% | 0.434 | 0.089–0.778 |
| Venous drainage type | 80% | 0.567 | 0.232–0.903 |
| First Color-Coded Map Reader—Neurointerventional Radiologist (Angiography) Agreement | |||
|---|---|---|---|
| Variable | Proportion of Agreement (% of the Observations) | K Value | 95% CI |
| Nidus type | 66.67% | −0.119 | −0.4 |
| Arterial feeder | 58.62% | 0.144 | −0.756 |
| Venous sinus color asymmetry | 58.82% | 0 | - |
| Venous drainage type | 87.50% | 0.758 | 0.468–1 |
| Second Color-Coded Map Reader—Neurointerventional Radiologist (Angiography) Agreement | |||
|---|---|---|---|
| Variable | Proportion of Agreement (% of the Observations) | K Value | 95% CI |
| Nidus type | 73.33% | 0.412 | −0.926 |
| Arterial feeder | 72.22% | 0.444 | −0.889 |
| Venous sinus color asymmetry | 38.89% | 0 | - |
| Venous drainage type | 88.89% | 0.746 | 0.424–1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Argento, F.; Verdolotti, T.; D’Abronzo, R.; De Leoni, D.; Ferravante, E.; Arbia, F.; Iacobucci, M.; Gaudino, S.; Mancino, M.; Schiarelli, C.; et al. Evaluation of Intracranial Arteriovenous Malformations Using Ischemic Stroke Color-Coded Maps Software, a New Rapid Post-Processing Tool in CT Angiography. J. Clin. Med. 2025, 14, 5833. https://doi.org/10.3390/jcm14165833
D’Argento F, Verdolotti T, D’Abronzo R, De Leoni D, Ferravante E, Arbia F, Iacobucci M, Gaudino S, Mancino M, Schiarelli C, et al. Evaluation of Intracranial Arteriovenous Malformations Using Ischemic Stroke Color-Coded Maps Software, a New Rapid Post-Processing Tool in CT Angiography. Journal of Clinical Medicine. 2025; 14(16):5833. https://doi.org/10.3390/jcm14165833
Chicago/Turabian StyleD’Argento, Francesco, Tommaso Verdolotti, Rosa D’Abronzo, Davide De Leoni, Emanuele Ferravante, Francesco Arbia, Marta Iacobucci, Simona Gaudino, Matteo Mancino, Chiara Schiarelli, and et al. 2025. "Evaluation of Intracranial Arteriovenous Malformations Using Ischemic Stroke Color-Coded Maps Software, a New Rapid Post-Processing Tool in CT Angiography" Journal of Clinical Medicine 14, no. 16: 5833. https://doi.org/10.3390/jcm14165833
APA StyleD’Argento, F., Verdolotti, T., D’Abronzo, R., De Leoni, D., Ferravante, E., Arbia, F., Iacobucci, M., Gaudino, S., Mancino, M., Schiarelli, C., Garignano, G., & Pedicelli, A. (2025). Evaluation of Intracranial Arteriovenous Malformations Using Ischemic Stroke Color-Coded Maps Software, a New Rapid Post-Processing Tool in CT Angiography. Journal of Clinical Medicine, 14(16), 5833. https://doi.org/10.3390/jcm14165833






