Clinical Effectiveness and Safety of Reduced-Dose Prasugrel in Asian Patients: The PROMISE-TW Registry
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Clinical Outcomes
2.3. Data Collection
2.4. Calculation of Sample Size
2.5. Statistical Analysis
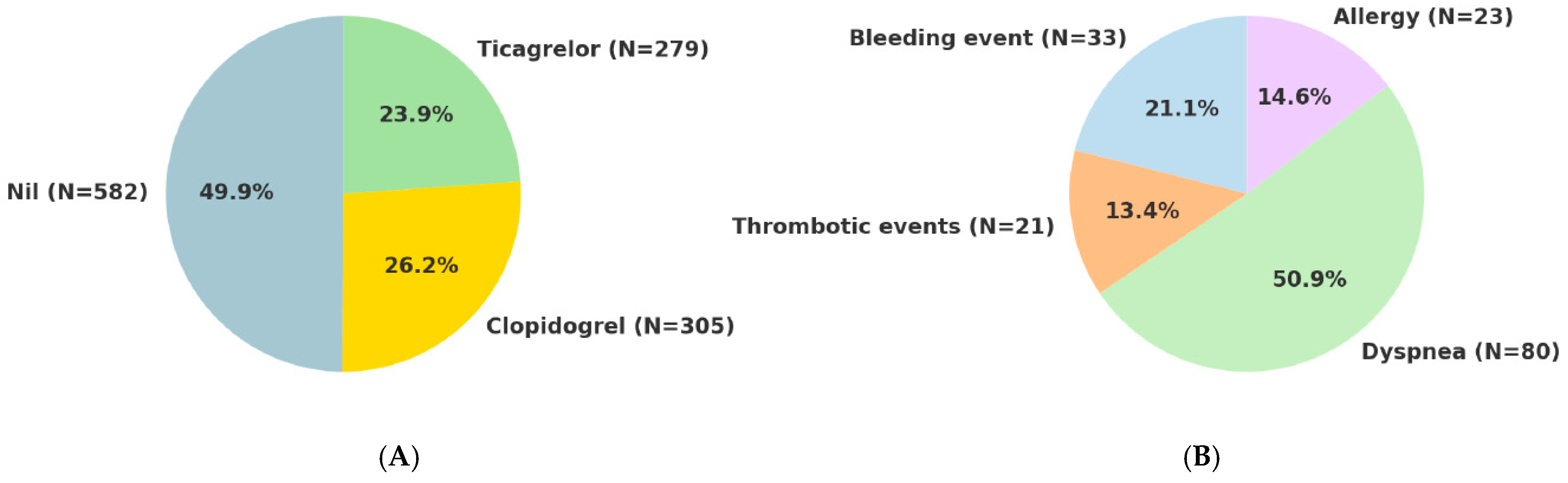
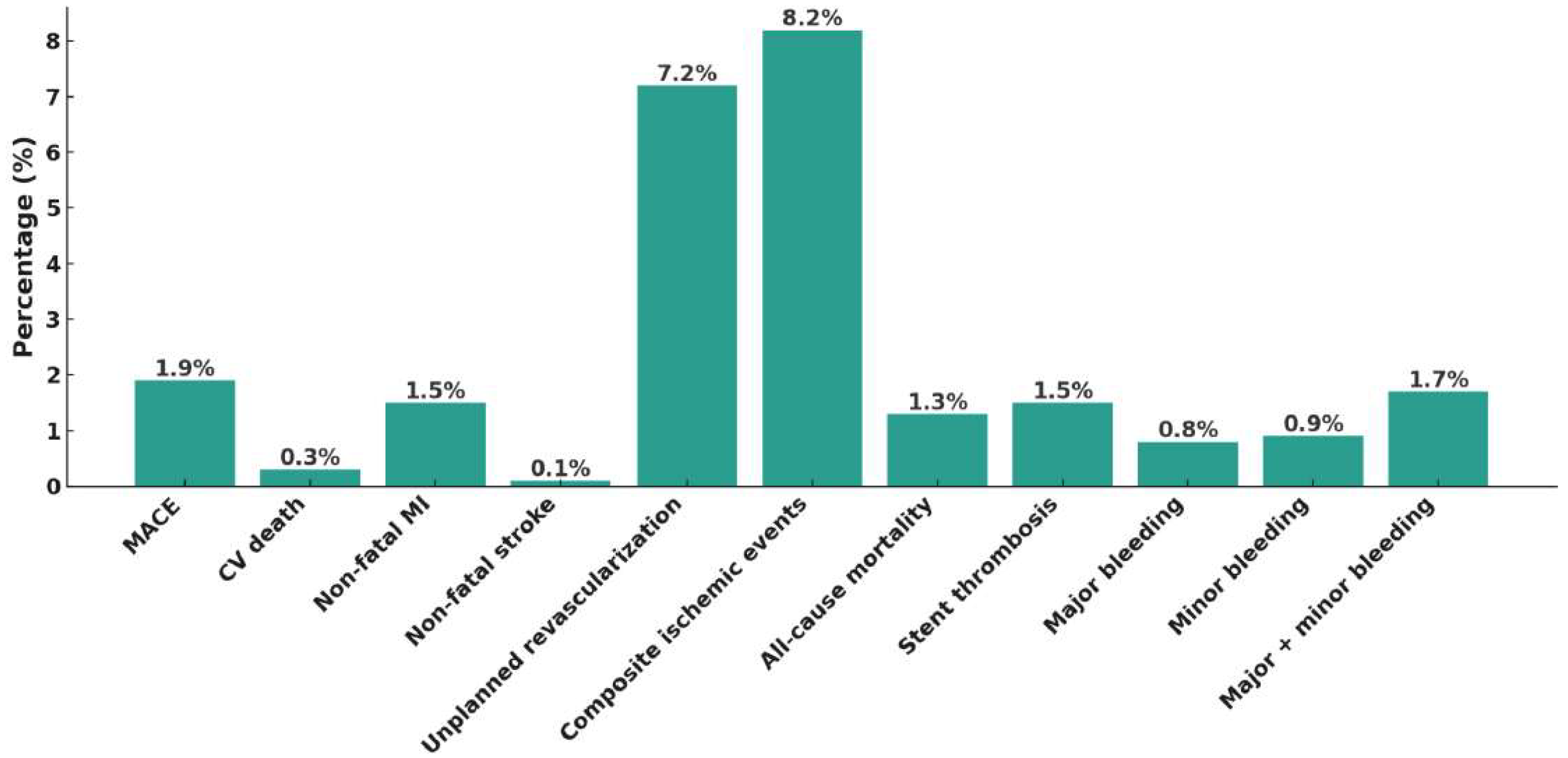
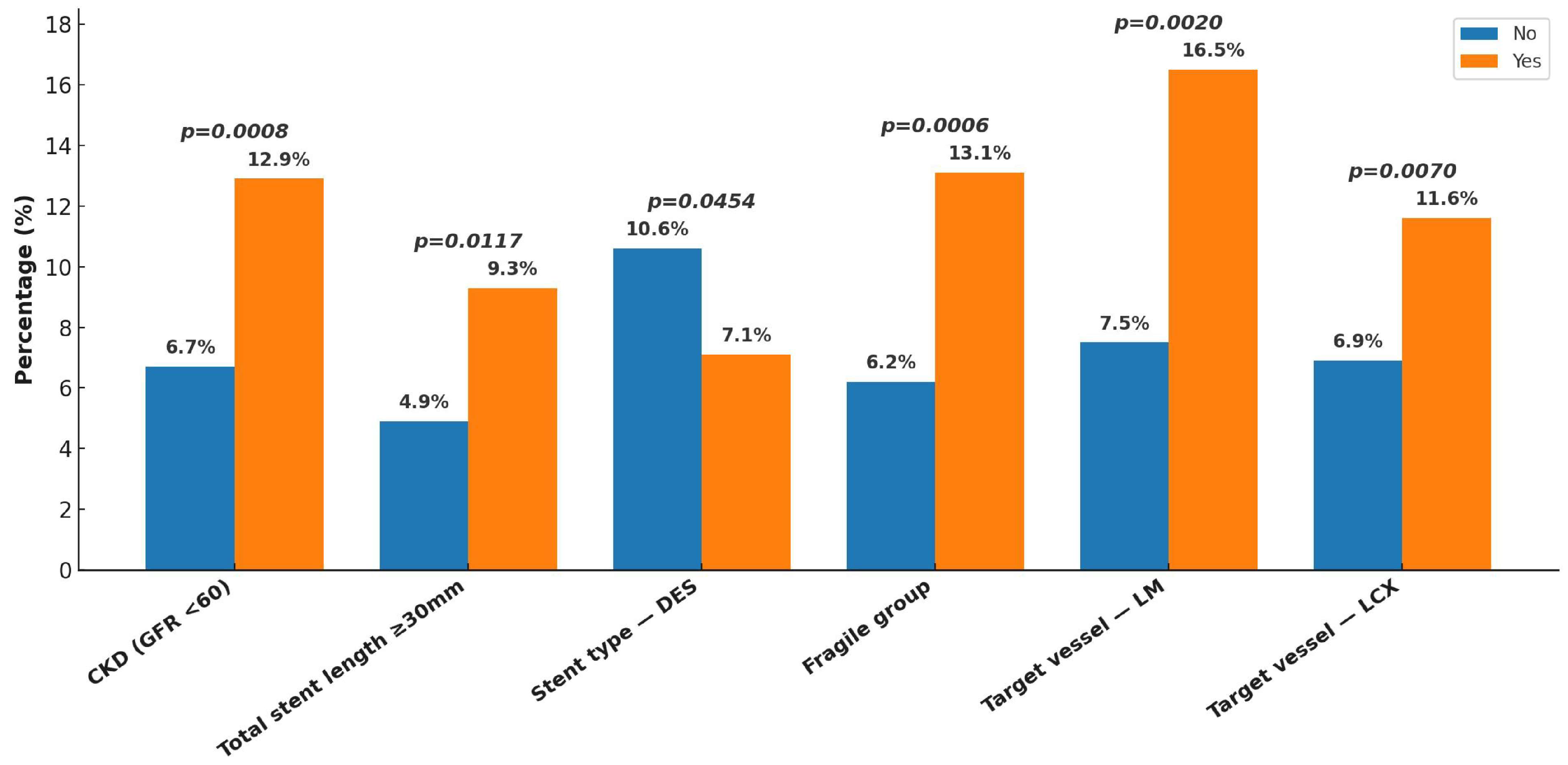
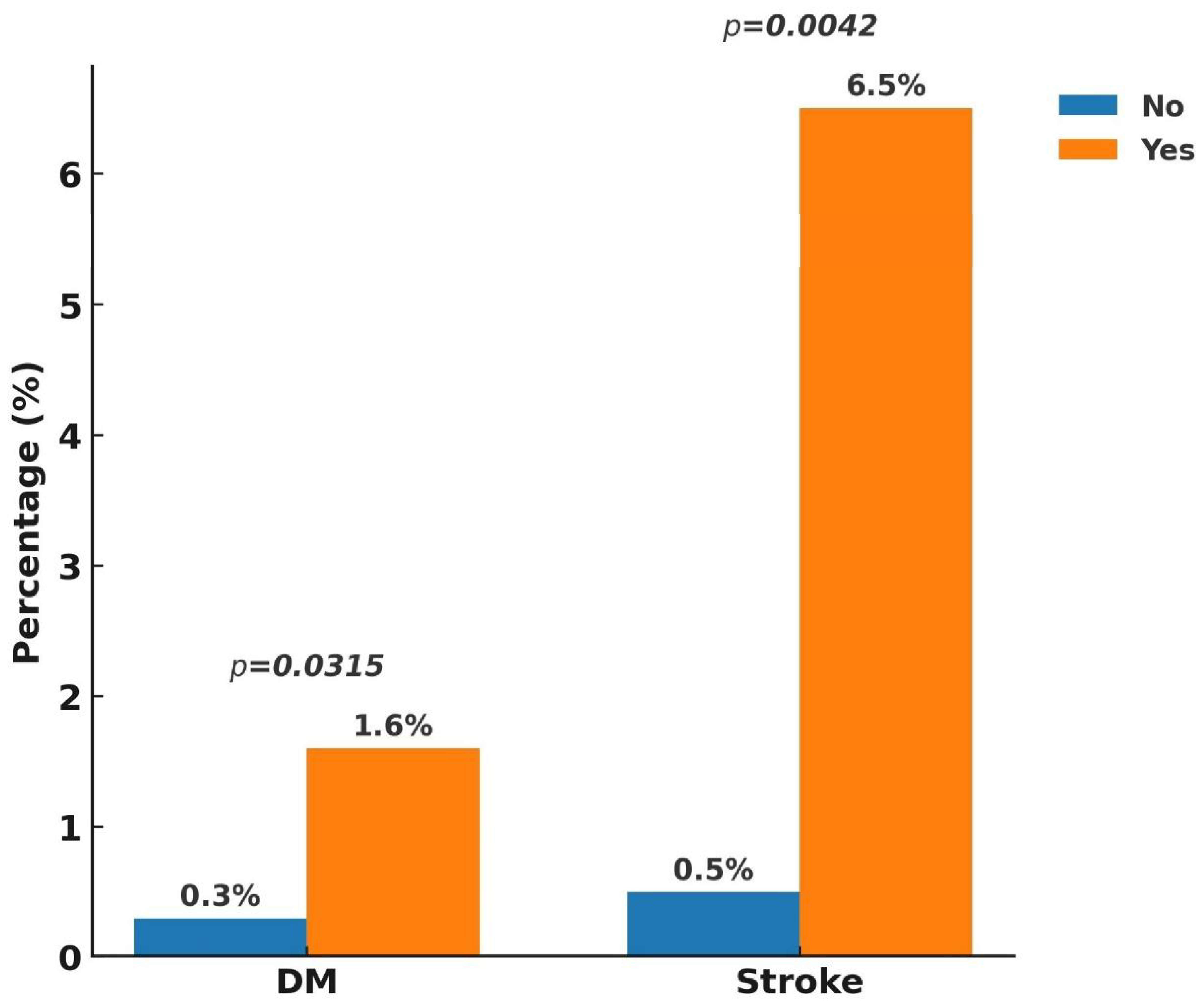
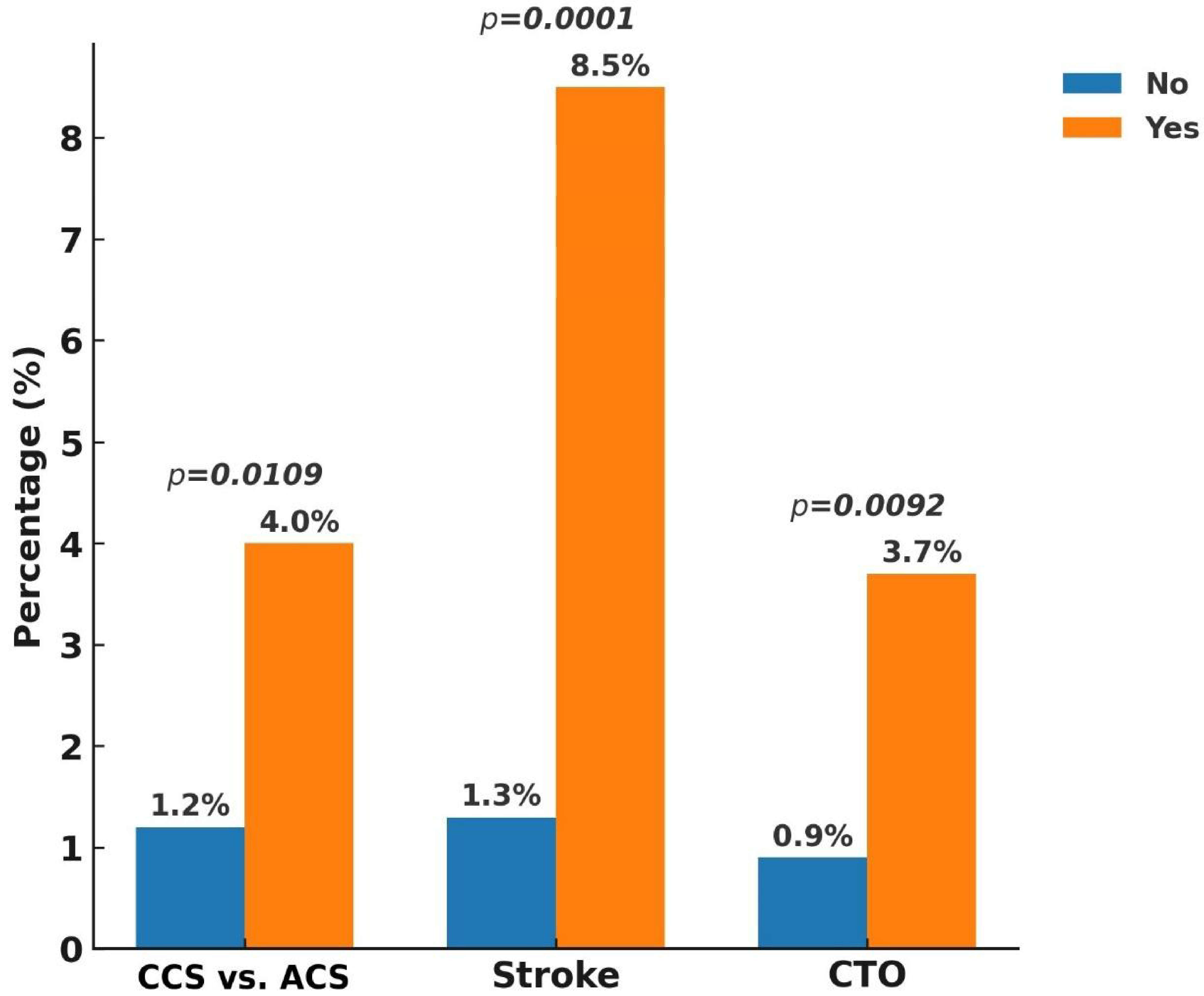
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [PubMed]
- Steinhubl, S.R.; Berger, P.B.; Mann, J.T., 3rd; DeLago, A.; Wilmer, C.; Topol, E.J.; CREDO Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trial. JAMA 2002, 288, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Yusuf, S.; Peters, R.J.; Bertrand, M.E.; Lewis, B.S.; Natarajan, M.K.; Malmberg, K.; Rupprecht, H.-J.; Zhao, F.; Chrolavicius, S.; et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001, 358, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Isshiki, T.; Kimura, T.; Ogawa, H.; Yokoi, H.; Nanto, S.; Takayama, M.; Kitagawa, K.; Nishikawa, M.; Miyazaki, S.; et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: The PRASFIT-ACS study. Circ. J. 2014, 78, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Iizuka, T.; Sagawa, K.; Abe, K.; Chikada, S.; Arai, M. Prasugrel for Japanese patients with acute coronary syndrome in short-term clinical practice (PRASFIT-Practice I): A postmarketing observational study. Cardiovasc. Interv. Ther. 2018, 33, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kitazono, T.; Kozuma, K.; Sekine, T.; Nakamura, S.; Shiosakai, K.; Iizuka, T. Prasugrel for Japanese Patients With Ischemic Heart Disease in Long-Term Clinical Practice (PRASFIT-Practice II)—1-Year Follow-up Results of a Postmarketing Observational Study. Circ. J. 2019, 83, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Akita, K.; Inohara, T.; Yamaji, K.; Kohsaka, S.; Numasawa, Y.; Ishii, H.; Amano, T.; Kadota, K.; Nakamura, M.; Maekawa, Y. Impact of reduced-dose prasugrel vs. standard-dose clopidogrel on in-hospital outcomes of percutaneous coronary intervention in 62737 patients with acute coronary syndromes: A nationwide registry study in Japan. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Sawano, M.; Sandhu, A.T.; Heidenreich, P.A.; Shiraishi, Y.; Ikemura, N.; Ueno, K.; Suzuki, M.; Numasawa, Y.; Fukuda, K.; et al. Ischemic and Bleeding Events Among Patients with Acute Coronary Syndrome Associated With Low-Dose Prasugrel vs Standard-Dose Clopidogrel Treatment. JAMA Netw. Open 2020, 3, e202004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C. Study Design and Rationale for the Prasugrel Reduced-dose Observation for Measuring Improvement in Safety and Effectiveness in Taiwan (PROMISE-TW) Registry. J. Taiwan Cardiovasc. Interv. 2024, 15, 63–72. [Google Scholar]
- Chiang, F.-T.; Shyu, K.-G.; Wu, C.-J.; Mar, G.-Y.; Hou, C.J.-Y.; Li, A.-H.; Wen, M.-S.; Lai, W.-T.; Lin, S.-J.; Kuo, C.-T.; et al. Predictors of 1-year outcomes in the Taiwan Acute Coronary Syndrome Full Spectrum Registry. J. Formos. Med. Assoc. 2014, 113, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Lee, S.; Cho, K.H.; Takegami, M.; Nishihira, K.; Kojima, S.; Asaumi, Y.; Saji, M.; Yamashita, J.; Hibi, K.; et al. Clinical outcomes of adjusted-dose versus standard-dose prasugrel in East Asian patients with acute myocardial infarction. Int. J. Cardiol. 2024, 410, 132197. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Park, D.-W. Antithrombotic Therapy After Acute Coronary Syndromes or Percutaneous Coronary Interventions in East Asian Populations. JACC Asia 2022, 2, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-H.; Hung, C.-F.; Chen, Y.-J.; Fang, C.-C. Clinical Efficacy and Safety of Reduced-Dose Prasugrel After Percutaneous Coronary Intervention for Taiwanese Patients with Acute Coronary Syndromes. J. Clin. Med. 2024, 13, 7221. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-Y.; Su, C.-H.; Kuo, F.-Y.; Lee, W.-L.; Wang, Y.-C.; Lin, W.-S.; Chu, P.-H.; Lu, T.-M.; Lo, P.-H.; Lee, C.-H.; et al. Prasugrel switching from clopidogrel after percutaneous coronary intervention for acute coronary syndrome in Taiwanese patients: An analysis of safety and efficacy. Cardiovasc. Interv. Ther. 2022, 37, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.; Coughlan, J.J.; Rossello, X.; Ibanez, B.; Members of the Task Force for the 2023 ESC Guidelines for the management of acute coronary syndromes; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | All N (=1167) |
|---|---|
| Age | 64.6 (15.5) |
| >75 | 162 (13.9) |
| Male | 948 (81.2) |
| ACS vs. CCS | |
| STEMI | 336 (28.8) |
| NSTEMI | 314 (26.9) |
| Unstable angina | 268 (23.0) |
| Chronic stable phase after ACS | 45 (3.9) |
| CCS | 198 (17.0) |
| Concomitant anti-thrombotic agents during the index event | |
| Nil | 185 (15.9) |
| Aspirin | 951 (81.5) |
| Warfarin | 3 (0.3) |
| DOAC | 14 (1.2) |
| Aspirin + warfarin | 2 (0.2) |
| Aspirin + DOAC | 12 (1.0) |
| PCI | 1060 (90.8) |
| Undergoing coronary stent implantation | 941 (80.6) |
| Drug-eluting stent | 813 (69.7) |
| Bare-metal stent | 156 (13.4) |
| Complex PCI | 819 (70.2) |
| Left main | 97 (8.3) |
| True bifurcation lesion | 400 (34.2) |
| CTO | 187 (16.0) |
| Stenting longer than 30 mm | 566 (48.5) |
| ≥3 stents | 123 (10.5) |
| Syntax score ≥ 33 | 73 (6.3) |
| Calcified plaque requiring atherectomy | 105 (9.0) |
| Mechanical support | 148 (12.7) |
| Vascular access | |
| Radial arteries | 845 (72.4) |
| Femoral arteries | 260 (22.3) |
| The target coronary arteries during PCI | |
| Left main | 97 (8.3) |
| LAD | 653 (56.0) |
| LCX | 335 (28.7) |
| RCA | 373 (32.0) |
| SVG | 2 (0.2) |
| 3-vessel CAD | 326 (27.9) |
| Comorbidities | |
| Hypertension | 709 (60.8) |
| Diabetes | 446 (38.2) |
| Hyperlipidemia | 320 (27.4) |
| Chronic kidney disease | 222 (19.0) |
| Stroke | 49 (4.2) |
| Fragile population | 8 (0.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Lu, C.-R.; Shiao, Y.-T.; Chang, K.-C.; Su, C.-H.; Chiu, Y.-W.; Huang, C.-L.; Liu, W.-S.; Yu, C.-L.; Hsieh, M.-J.; et al. Clinical Effectiveness and Safety of Reduced-Dose Prasugrel in Asian Patients: The PROMISE-TW Registry. J. Clin. Med. 2025, 14, 5791. https://doi.org/10.3390/jcm14165791
Wang Y-C, Lu C-R, Shiao Y-T, Chang K-C, Su C-H, Chiu Y-W, Huang C-L, Liu W-S, Yu C-L, Hsieh M-J, et al. Clinical Effectiveness and Safety of Reduced-Dose Prasugrel in Asian Patients: The PROMISE-TW Registry. Journal of Clinical Medicine. 2025; 14(16):5791. https://doi.org/10.3390/jcm14165791
Chicago/Turabian StyleWang, Yu-Chen, Chiung-Ray Lu, Yi-Tzone Shiao, Kuan-Cheng Chang, Chun-Hung Su, Yu-Wei Chiu, Chien-Lung Huang, Wei-Shin Liu, Ching-Lung Yu, Ming-Jer Hsieh, and et al. 2025. "Clinical Effectiveness and Safety of Reduced-Dose Prasugrel in Asian Patients: The PROMISE-TW Registry" Journal of Clinical Medicine 14, no. 16: 5791. https://doi.org/10.3390/jcm14165791
APA StyleWang, Y.-C., Lu, C.-R., Shiao, Y.-T., Chang, K.-C., Su, C.-H., Chiu, Y.-W., Huang, C.-L., Liu, W.-S., Yu, C.-L., Hsieh, M.-J., Lu, Y.-H., Su, H.-M., Lin, P.-C., Leu, H.-B., & Lee, W.-L. (2025). Clinical Effectiveness and Safety of Reduced-Dose Prasugrel in Asian Patients: The PROMISE-TW Registry. Journal of Clinical Medicine, 14(16), 5791. https://doi.org/10.3390/jcm14165791








