Neurological Complications Following Temporomandibular Joint Injections in Patients with Temporomandibular Disorders: A Systematic Review of Reported Adverse Events
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection and Data Processing
2.4. Study Risk of Bias Assessment
3. Results
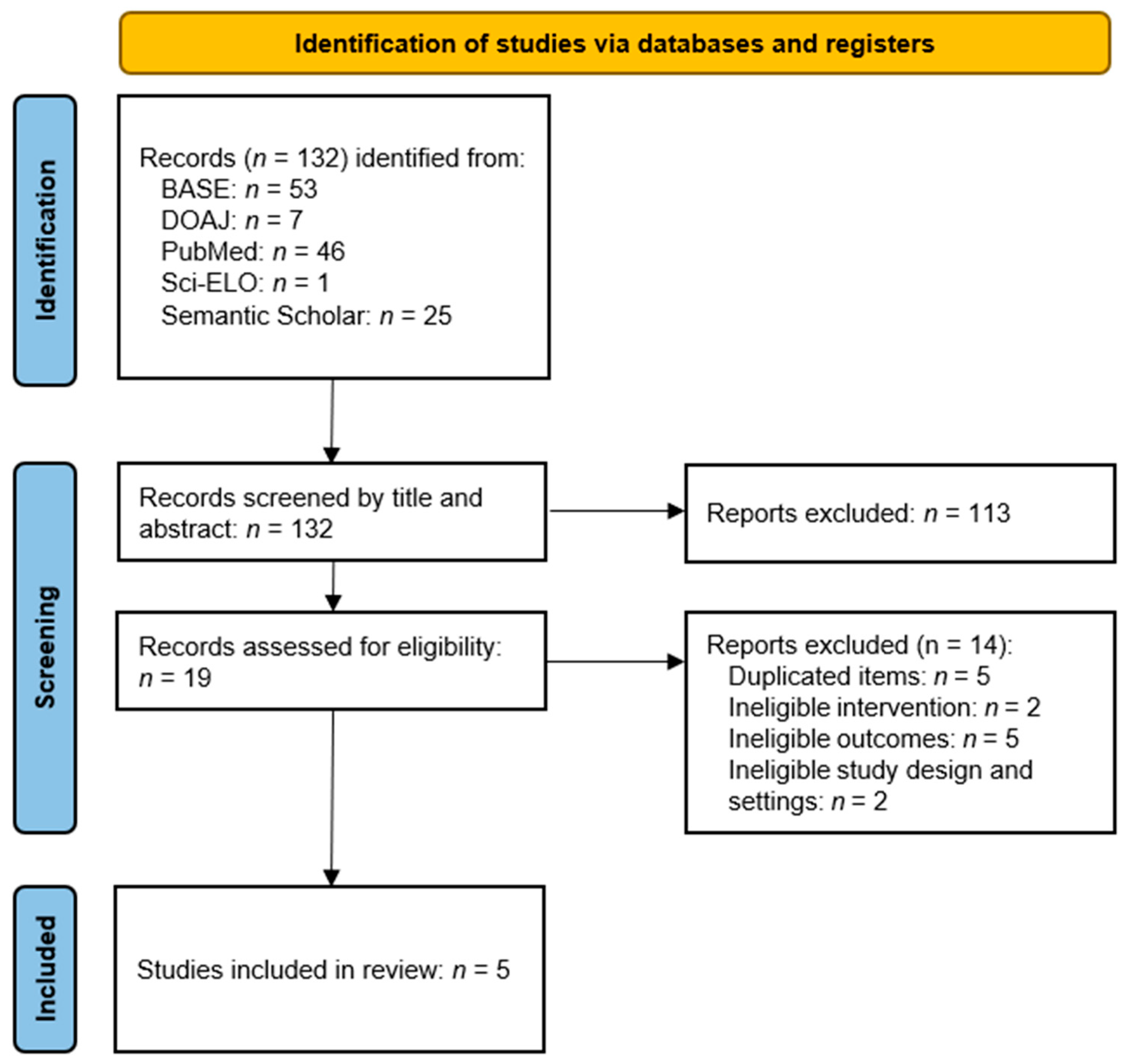
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Studies
3.4. Results of Individual Studies
3.5. Results of Syntheses
4. Discussion
4.1. General Interpretation
4.2. Future Perspectives
- (1)
- Minimize the risk of complications by administering a smaller volume—choose injection rather than arthrocentesis if possible.
- (2)
- Choose substances safer with regard to deposition in an unintended location, including extra-articular or even intravascular.
- (3)
4.3. Strengths
4.4. Limitations
5. Conclusions
- (1)
- Neurological complications following intra-articular injections for TMD are rarely described but potentially common, especially when local anesthesia and/or joint irrigation with 60 mL or more of fluid using the double-needle technique are used.
- (2)
- The most common are transient facial nerve palsies (temporal and zygomatic branches), resulting from the spread of the anesthetic agent or pressure from fluid leakage outside the joint cavity.
- (3)
- These mechanisms may result from an imprecise technique, suggesting the need for careful deposition and developing navigation to optimize clinical safety.
- (4)
- Although most complications are mild and self-limiting, one case of epidural hematoma has been reported.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TMDs | Temporomandibular disorders |
| TMJ | Temporomandibular joint |
Appendix A
| Search Engine | Total Retrieval Range | Identified Records |
|---|---|---|
| Bielefeld Academic Search Engine (BASE) | 423,038,726 | 53 |
| Directory of Open Access Journals (DOAJ) | 11,166,729 | 7 |
| National Library of Medicine (PubMed) | Over 38,000,000 | 46 |
| Scientific Electronic Library Online (SciELO) | 14,388,173 | 1 |
| Semantic Scholar | 226,698,359 | 25 |
| First Author, Publication Year | Title | Digital Object Identifier (DOI) or Alternative | Reason for Exclusion |
|---|---|---|---|
| Huang, 2024 [39] | A clinical trial of ropivacaine in arthrocentesis for TMD | 10.1186/s12903-024-04606-x | No neurological adverse events reported |
| Talaat, 2022 [40] | Minimally invasive surgeries for the treatment of temporomandibular disorders: Prognostic indicators and persistence of treatment outcomes over a 5-year follow-up | 10.4103/abhs.abhs_14_21 | Concomitant invasive co-intervention (arthroscopy) |
| Vervaeke, 2022 [41] | Correlation of MRI and arthroscopic findings with clinical outcome in temporomandibular joint disorders: a retrospective cohort study | 10.1186/s13005-021-00305-y | No neurological adverse events reported |
| Ferreira, 2021 [42] | Clinical outcomes in TMD patients after arthrocentesis with lysis, lavage, and viscossupplementation | 10.1080/07853890.2021.1897446 | Conference proceeding; no neurological adverse events reported |
| Zhang, 2020 [43] | Gradient sequential treatment of temporomandibular joint disorders | 10.12016/j.issn.2096-1456.2020.01.002 | Review article |
| Patel, 2017 [44] | Clinical and radiological outcome of arthrocentesis followed by autologous blood injection for treatment of chronic recurrent temporomandibular joint dislocation | 10.4317/jced.53812 | No neurological adverse events reported |
| Bayoumi, 2014 [45] | Arthrocentesis followed by intra-articular autologous blood injection for the treatment of recurrent temporomandibular joint dislocation | 10.1016/j.ijom.2014.05.004 | No neurological adverse events reported |
| Etoz, 2010 [46] | Accidental use of alcohol during arthrocentesis of the temporomandibular joint | 10.1016/j.bjoms.2010.11.010 | Unintentional use of an inappropriate substance (alcohol) |
| Simpson, 1968 [47] | Auriculotemporal syndrome after injection into the temporomandibular joint: report of case | PMID: 4299382 | Full text not retrieved |
| First Author, Publication Year | Title | Digital Object Identifier (DOI) or Alternative | Study Design |
|---|---|---|---|
| Baş, 2025 [27] | What Are the Complications of Temporomandibular Joint Arthrocentesis? | 10.1016/j.joms.2024.12.012 | Retrospective study |
| Carroll, 2000 [28] | Extradural haematoma following temporomandibular joint arthrocentesis and lavage | 10.1080/02688690050004633 | Case report |
| Aliyev, 2019 [29] | An unusual complication during arthrocentesis: N. facialis paralysis, with N. lingualis and N. alveolaris inferior anesthesia | 10.17245/jdapm.2019.19.2.115 | Case report |
| Isacsson, 2019 [30] | Pain relief following a single-dose intra-articular injection of methylprednisolone in the temporomandibular joint arthralgia—A multicentre randomised controlled trial | 10.1111/joor.12718 | Randomized controlled trial |
| Vaira, 2018 [31] | Complications and post-operative sequelae of temporomandibular joint arthrocentesis | 10.1080/08869634.2017.1341138 | Retrospective study |
| JBI Critical Appraisal Criteria | Response |
|---|---|
| Was true randomization used for the assignment of participants to treatment groups? | Yes |
| Was allocation to treatment groups concealed? | Yes |
| Were treatment groups similar at the baseline? | Yes |
| Were participants blind to treatment assignment? | No |
| Were those delivering the treatment blind to treatment assignment? | Yes |
| Were treatment groups treated identically, other than the intervention of interest? | Yes |
| Were outcome assessors blind to treatment assignment? | Yes |
| Were outcomes measured in the same way for treatment groups? | Yes |
| Were outcomes measured in a reliable way? | Yes |
| Was the follow-up complete, and if not, were the differences between groups in terms of their follow-up adequately described and analyzed? | Yes |
| Were participants analyzed in the groups to which they were randomized? | Yes |
| Was an appropriate statistical analysis used? | Yes |
| Was the trial design appropriate, and were any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial? | Yes |
| First Author, Publication Year | Were the Criteria for Inclusion in the Sample Clearly Defined? | Were the Study Subjects and the Setting Described in Detail? | Was the Exposure Measured in a Valid and Reliable Way? | Were Objective, Standard Criteria Used for Measurement of the Condition? | Were Confounding Factors Identified? | Were Strategies to Deal with Confounding Factors Stated? | Were the Outcomes Measured in a Valid and Reliable Way? | Was Appropriate Statistical Analysis Used? |
|---|---|---|---|---|---|---|---|---|
| Baş, 2025 [27] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Vaira, 2018 [31] | Yes | Yes | Yes | Yes | No | No | Yes | Unclear |
| First Author, Publication Year | Were the Patient’s Demographic Characteristics Clearly Described? | Was the Patient’s History Clearly Described and Presented as a Timeline? | Was the Current Clinical Condition of the Patient on Presentation Clearly Described? | Were Diagnostic Tests or Assessment Methods and the Results Clearly Described? | Was the Intervention(s) or Treatment Procedure(s) Clearly Described? | Was the Post-Intervention Clinical Condition Clearly Described? | Were Adverse Events (Harms) or Unanticipated Events Identified and Described? | Does the Case Report Provide Takeaway Lessons? |
|---|---|---|---|---|---|---|---|---|
| Carroll, 2000 [28] | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes |
| Aliyev, 2019 [29] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
References
- Ferreira, N.D.R.; Sanz, C.K.; Raybolt, A.; Pereira, C.M.; DosSantos, M.F. Action of Hyaluronic Acid as a Damage-Associated Molecular Pattern Molecule and Its Function on the Treatment of Temporomandibular Disorders. Front. Pain Res. 2022, 3, 852249. [Google Scholar] [CrossRef] [PubMed]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7405. [Google Scholar] [CrossRef]
- Elledge, R.O.C. Classifications for the Temporomandibular Joint (TMJ): A Systematic Review of the Literature. J. Cranio-Maxillofac. Surg. 2024, 52, 890–894. [Google Scholar] [CrossRef]
- Warzocha, J.; Gadomska-Krasny, J.; Mrowiec, J. Etiologic Factors of Temporomandibular Disorders: A Systematic Review of Literature Containing Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) and Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) from 2018 to 2022. Healthcare 2024, 12, 575. [Google Scholar] [CrossRef]
- Kukreja, P.; Kukreja, B.J.; Marrapodi, M.M.; Ronsivalle, V.; Cicciù, M.; Minervini, G. Efficacy of Hyaluronic Acid in Temporomandibular Disorders Evaluated with Diagnostic Criteria for Temporomandibular Disorders (DC/TMD). J. Oral Rehabil. 2025, 52, 254–265. [Google Scholar] [CrossRef]
- Siewert-Gutowska, M.; Pokrowiecki, R.; Kamiński, A.; Zawadzki, P.; Stopa, Z. State of the Art in Temporomandibular Joint Arthrocentesis-A Systematic Review. J. Clin. Med. 2023, 12, 4439. [Google Scholar] [CrossRef] [PubMed]
- Piech, J.; Pihut, M.; Kulesa-Mrowiecka, M. Physiotherapy in Hypomobility of Temporomandibular Joints. Folia Medica Cracoviensia 2020, 60, 123–134. [Google Scholar] [CrossRef]
- Pyne, J.M.; Davis, C.M.; Kelm, R.; Bussolaro, C.; Dobrovolsky, W.; Seikaly, H. Advanced Mandibular Reconstruction with Fibular Free Flap and Alloplastic TMJ Prosthesis with Digital Planning. J. Otolaryngol. Head Neck Surg. 2023, 52, s40463-023. [Google Scholar] [CrossRef]
- Niezen, E.T.; van Minnen, B.; Bos, R.R.M.; Dijkstra, P.U. Temporomandibular Joint Prosthesis as Treatment Option for Mandibular Condyle Fractures: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2022, 52, 88–97. [Google Scholar] [CrossRef]
- Penlington, C.; Bowes, C.; Taylor, G.; Otemade, A.A.; Waterhouse, P.; Durham, J.; Ohrbach, R. Psychological Therapies for Temporomandibular Disorders (TMDs). Cochrane Database Syst. Rev. 2022, 2022. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013515.pub2/full (accessed on 14 August 2025.). [CrossRef]
- Christidis, N.; Al-Moraissi, E.A.; Al-Ak’hali, M.S.; Minarji, N.; Zerfu, B.; Grigoriadis, A.; Schibbye, R.; Christidis, M. Psychological Treatments for Temporomandibular Disorder Pain—A Systematic Review. J. Oral Rehabil. 2024, 51, 1320–1336. [Google Scholar] [CrossRef]
- Sielski, M.; Chęcińska, K.; Turosz, N.; Chęciński, M.; Sikora, M. Single Intra-Articular Administration of Injectable Platelet-Rich Fibrin (I-PRF) in Alleviating Temporomandibular Joint Pain: A Pilot Clinical Trial. Dent. Med. Probl. 2025, 62, 187–192. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, J.; Di, W.; Teo, K.Y.W.; Toh, W.S. Mesenchymal Stem Cell-Based Therapies for Temporomandibular Joint Repair: A Systematic Review of Preclinical Studies. Cells 2024, 13, 990. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xue, Y.; Liu, P. Application of Auriculotemporal Nerve Block and Dextrose Prolotherapy in Exercise Therapy of TMJ Closed Lock in Adolescents and Young Adults. Head Face Med. 2021, 17, 11. [Google Scholar] [CrossRef]
- Kucukguven, A.; Demiryurek, M.D.; Vargel, I. Temporomandibular Joint Innervation: Anatomical Study and Clinical Implications. Ann. Anat. Anat. Anz. 2022, 240, 151882. [Google Scholar] [CrossRef]
- Chowdhury, S.K.R.; Saxena, V.; Rajkumar, K.; Shadamarshan, R.A. Complications of Diagnostic TMJ Arthroscopy: An Institutional Study. J. Oral Maxillofac. Surg. 2019, 18, 531–535. [Google Scholar] [CrossRef]
- Hunt, B.R.; Johson, S.A.; Klukkert, Z.S. Distribution, Scaling, and Depiction of the Temporal Branches of the Facial Nerve. Sci. Rep. 2025, 15, 11350. [Google Scholar] [CrossRef]
- Pauwels, A.; Lozano, C.; López, J.P. The Facial Nerve Injury After Temporomandibular Joint Surgery after Endaural Approach with Sharp Dissection. J. Oral Maxillofac. Surg. 2022, 21, 957–960. [Google Scholar] [CrossRef]
- Peres Lima, F.G.G.; Rios, L.G.C.; Bianchi, J.; Gonçalves, J.R.; Paranhos, L.R.; Vieira, W.A.; Zanetta-Barbosa, D. Complications of Total Temporomandibular Joint Replacement: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2023, 52, 584–594. [Google Scholar] [CrossRef]
- Jurek-Matusiak, O.; Brożek-Mądry, E.; Jastrzębska, H.; Krzeski, A. Orbital Decompression for Thyroid Eye Disease: Surgical Treatment Outcomes in Endocrinological Assessment. Endokrynol. Pol. 2021, 72, 609–617. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Chen, H.-T.; Yang, F.-S.; Hsu, C.-C.; Yin, T.-C.; Wu, R.-W.; Chen, S.-H.; Lu, M.-L. Optimal Timing for Decompression in Post-Operative Epidural Hematoma: A Retrospective Analysis and Treatment Flowchart. Eur. Spine J. 2025, 34, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.A.; Gout, T. Orbital Decompression: Conceptual Approach for Orbital Volume Expansion. Ophthalmic Plast. Reconstr. Surg. 2023, 39, S105–S111. [Google Scholar] [CrossRef] [PubMed]
- Turosz, N.; Chęcińska, K.; Chęciński, M.; Michcik, A.; Chlubek, D.; Sikora, M. Adverse Events of Intra-Articular Temporomandibular Joint Injections: A Systematic Search and Review. Pomeranian J. Life Sci. 2023, 69, 48–54. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- PROSPERO—International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 14 November 2024).
- JBI Critical Appraisal Tools | JBI. Available online: https://jbi.global/critical-appraisal-tools (accessed on 14 June 2025).
- Baş, B.; Singer, E.A.; Çankaya, R.T.A. What Are the Complications of Temporomandibular Joint Arthrocentesis? J. Oral Maxillofac. Surg. 2025, 83, 414–420. [Google Scholar] [CrossRef]
- Carroll, T.A.; Smith, K.; Jakubowski, J. Extradural Haematoma Following Temporomandibular Joint Arthrocentesis and Lavage. Br. J. Neurosurg. 2000, 14, 152–154. [Google Scholar] [CrossRef]
- Aliyev, T.; Berdeli, E.; Şahin, O. An Unusual Complication during Arthrocentesis: N. Facialis Paralysis, with N. Lingualis and N. Alveolaris Inferior Anesthesia. J. Dent. Anesth. Pain Med. 2019, 19, 115. [Google Scholar] [CrossRef]
- Isacsson, G.; Schumann, M.; Nohlert, E.; Mejersjö, C.; Tegelberg, Å. Pain Relief Following a Single-dose Intra-articular Injection of Methylprednisolone in the Temporomandibular Joint Arthralgia—A Multicentre Randomised Controlled Trial. J. Oral Rehabil. 2019, 46, 5–13. [Google Scholar] [CrossRef]
- Vaira, L.A.; Raho, M.T.; Soma, D.; Salzano, G.; Dell’aversana Orabona, G.; Piombino, P.; De Riu, G. Complications and Post-Operative Sequelae of Temporomandibular Joint Arthrocentesis. CRANIO® 2018, 36, 264–267. [Google Scholar] [CrossRef]
- Wittig, J.; Borumandi, F.; Gaggl, A.; Hachleitner, J. Septic Arthritis of the Temporomandibular Joint Leading to an Epidural Abscess. BMJ Case Rep. 2018, 2018, bcr-2017-223563. [Google Scholar] [CrossRef]
- Mehra, P.; Arya, V. Temporomandibular Joint Arthrocentesis: Outcomes Under Intravenous Sedation Versus General Anesthesia. J. Oral Maxillofac. Surg. 2015, 73, 834–842. [Google Scholar] [CrossRef]
- Atalı, O.; Özçelik, E.; Gönül, O.; Garip, H. Evaluation of Patient Comfort and Impact of Different Anesthesia Techniques on the Temporomandibular Joint Arthrocentesis Applications by Comparing Gow-Gates Mandibular Block Anesthesia with Auriculotemporal Nerve Block. Pain Res. Manag. 2022, 2022, 4206275. [Google Scholar] [CrossRef]
- Steć, Z.; Burska, Z.; Brożek-Mądry, E.; Straburzyński, M.; Waliszewska-Prosół, M.; Krzeski, A. Clinical Characteristics of Acute Rhinosinusitis in COVID-19 a Post Hoc Analysis of a Longitudinal Study. Otolaryngol. Pol. 2022, 77, 12–18. [Google Scholar] [CrossRef]
- Tsui, H.C.; Lam, C.M.; Leung, Y.Y.; Li, K.Y.; Wong, N.S.M.; Li, D.T.S. Lavage Volume of Arthrocentesis in the Management of Temporomandibular Disorders: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2622. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Wu, F.H.W.; Chan, H.H. Ultrasonography-Guided Arthrocentesis versus Conventional Arthrocentesis in Treating Internal Derangement of Temporomandibular Joint: A Systematic Review. Clin. Oral Investig. 2020, 24, 3771–3780. [Google Scholar] [CrossRef]
- De Nordenflycht, D.; Tesch, R. Advantages of Ultrasound Guidance for TMJ Arthrocentesis and Intra-Articular Injection: A Narrative Review. Dent. Med. Probl. 2022, 59, 647–656. [Google Scholar] [CrossRef]
- Huang, L.; Huang, Z.; Bi, S.; Mai, H. A Clinical Trial of Ropivacaine in Arthocentesis for TMD. BMC Oral Health 2024, 24, 1311. [Google Scholar] [CrossRef]
- Talaat, W.M.; Hamdoon, Z.; Ghoneim, M.M. Minimally Invasive Surgeries for the Treatment of Temporomandibular Disorders: Prognostic Indicators and Persistence of Treatment Outcomes over a 5-Year Follow-Up. Adv. Biomed. Health Sci. 2022, 1, 34–44. [Google Scholar] [CrossRef]
- Vervaeke, K.; Verhelst, P.-J.; Orhan, K.; Lund, B.; Benchimol, D.; Van Der Cruyssen, F.; De Laat, A.; Jacobs, R.; Politis, C. Correlation of MRI and Arthroscopic Findings with Clinical Outcome in Temporomandibular Joint Disorders: A Retrospective Cohort Study. Head Face Med. 2022, 18, 2. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Nunes, M.A.; Salvado, F. Clinical Outcomes in TMD Patients after Arthrocentesis with Lysis, Lavage and Viscossuplementation. Ann. Med. 2021, 53, S87–S88. [Google Scholar] [CrossRef]
- Zhang, Q.; Guan, H. Gradient sequential treatment of temporomandibular joint disorders. Kouqiang Jibing Fangzhi 2020, 28, 11–15. [Google Scholar] [CrossRef]
- Patel, J.; Nilesh, K.; Parkar, M.; Vaghasiya, A. Clinical and Radiological Outcome of Arthrocentesis Followed by Autologous Blood Injection for Treatment of Chronic Recurrent Temporomandibular Joint Dislocation. J. Clin. Exp. Dent. 2017, 9, e962. [Google Scholar] [CrossRef]
- Bayoumi, A.M.; Al-Sebaei, M.O.; Mohamed, K.M.; Al-Yamani, A.O.; Makrami, A.M. Arthrocentesis Followed by Intra-Articular Autologous Blood Injection for the Treatment of Recurrent Temporomandibular Joint Dislocation. Int. J. Oral Maxillofac. Surg. 2014, 43, 1224–1228. [Google Scholar] [CrossRef]
- Etoz, O.; Er, N.; Alkan, A. Accidental Use of Alcohol during Arthrocentesis of the Temporomandibular Joint. Br. J. Oral Maxillofac. Surg. 2011, 49, e1–e2. [Google Scholar] [CrossRef]
- Simpson, W. Auriculotemporal Syndrome after Injection into the Temporomandibular Joint: Report of Case. J. Oral Surg. 1968, 26, 602–603. [Google Scholar] [PubMed]
| Framework Component | Criteria for Inclusion | Criteria for Exclusion |
|---|---|---|
| Patient population | Patient with TMDs | Cadaver or animal studies |
| Index intervention | TMJ intra-articular injection | More invasive concurrent procedure |
| Comparator (included in selected analyses only) | Placebo, active substance injection, or arthrocentesis | Not applicable |
| Outcomes | Neurological adverse effects related to intra-articular injections | Non-specific symptoms or unrelated systemic conditions |
| Study design and settings | Articles on primary studies | No English abstract available |
| First Author, Publication Year | Diagnosis per Authors | Substance and Intervention | Intra-Articular Injection Volume | Neuro Complications (n/N) | Type and Course of Complication | Duration (Until Resolution or End of Observation) |
|---|---|---|---|---|---|---|
| Baş, 2025 [27] | Painful intra-articular temporomandibular disorders | Lidocaine local anesthesia and lactated Ringer solution two-needle arthrocentesis | 60–100 mL in one session | 30/210 | Transient facial nerve paralysis (mainly temporal and zygomatic branches) | Resolved within a few hours |
| Carroll, 2000 [28] | TMJ dysfunction | Lactated Ringer solution arthrocentesis | 60–80 mL in one session | 1/1 | Right-sided extradural haematoma with third nerve palsy and left hemiparesis; required craniotomy and clot evacuation; full neurological recovery | Neurological symptoms resolved within 2 weeks after craniotomy; 3-month follow-up was uneventful |
| Aliyev, 2019 [29] | Severe bruxism causing painful jaw locking and trismus in the right TMJ | Intra-articular articaine injection and two-needle arthrocentesis with lactated Ringer solution | 2.5 mL articaine hydrochloride, 102–202 mL lactated Ringer solution (administration stopped during the second 100 mL portion) | 1/1 | Transient right-sided facial nerve paralysis; anesthesia of the inferior alveolar and lingual nerves | Resolved within 24 h (symptoms observed for ~2 h post-op; no complications on next-day follow-up) |
| Isacsson, 2019 [30] | Unilateral TMJ arthralgia without sounds in the affected joint | Auriculotemporal nerve block with 1.8 mL prilocaine-felypressin and intra-articular methylprednisolone 40 mg/mL | 1 mL in one session | 3/27 cases of eyelid paresthesia and 2/27 cases of numbness (overlap between cases not reported) | Transient paresthesias and numbness | Resolved during 4-week follow-up (exact timing not specified) |
| Vaira, 2018 [31] | Temporomandibular disorders | Auriculotemporal nerve block with mepivacaine with adrenaline; two-needle arthrocentesis with lactated Ringer solution; intra-articular sodium hyaluronate | 100 mL lactated Ringer solution and 1.5–2 mL sodium hyaluronate | 282/433 procedures in 315 patients | Transient frontalis and orbicularis oculis paresis (facial nerve) | Fully resolved with the end of the local anaesthesia effect |
| Cranial Nerve | Number of Cases | Assumed Mechanism |
|---|---|---|
| III (Oculomotor) | 1 | (1) Extradural hematoma due to vascular injuryor or (2) Solution leakage |
| V1 (Ophthalmic branch of trigeminal) | 3 | (1) Local anesthetic effect causing sensory disturbances (2) Administration of corticosteroids |
| V3 (Mandibular branch of trigeminal) | 3 | (1) Local anesthetic effect causing sensory disturbances (2) Administration of corticosteroids |
| VII (Facial)—temporal and zygomatic branches | 313 | (1) Diffusion of local anesthetic resulting in transient motor blockadeor or (2) Extravasation of irrigation fluid causing compressive neurapraxia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chęciński, M.; Chęcińska, K.; Chyży, I.; Walkowiak, K.; Turosz, N.; Kosiński, B.; Zduński, S.; Chlubek, D.; Sikora, M. Neurological Complications Following Temporomandibular Joint Injections in Patients with Temporomandibular Disorders: A Systematic Review of Reported Adverse Events. J. Clin. Med. 2025, 14, 5770. https://doi.org/10.3390/jcm14165770
Chęciński M, Chęcińska K, Chyży I, Walkowiak K, Turosz N, Kosiński B, Zduński S, Chlubek D, Sikora M. Neurological Complications Following Temporomandibular Joint Injections in Patients with Temporomandibular Disorders: A Systematic Review of Reported Adverse Events. Journal of Clinical Medicine. 2025; 14(16):5770. https://doi.org/10.3390/jcm14165770
Chicago/Turabian StyleChęciński, Maciej, Kamila Chęcińska, Izabella Chyży, Kamila Walkowiak, Natalia Turosz, Bartosz Kosiński, Sebastian Zduński, Dariusz Chlubek, and Maciej Sikora. 2025. "Neurological Complications Following Temporomandibular Joint Injections in Patients with Temporomandibular Disorders: A Systematic Review of Reported Adverse Events" Journal of Clinical Medicine 14, no. 16: 5770. https://doi.org/10.3390/jcm14165770
APA StyleChęciński, M., Chęcińska, K., Chyży, I., Walkowiak, K., Turosz, N., Kosiński, B., Zduński, S., Chlubek, D., & Sikora, M. (2025). Neurological Complications Following Temporomandibular Joint Injections in Patients with Temporomandibular Disorders: A Systematic Review of Reported Adverse Events. Journal of Clinical Medicine, 14(16), 5770. https://doi.org/10.3390/jcm14165770









