Ischemia with Non-Obstructive Coronary Artery Disease: Sex-Based Differences in Pathophysiology, Clinical Presentation, and Prognosis
Abstract
1. Introduction
2. INOCA Overview
2.1. Prevalence
2.2. Pathophysiology
2.3. Phenotypes
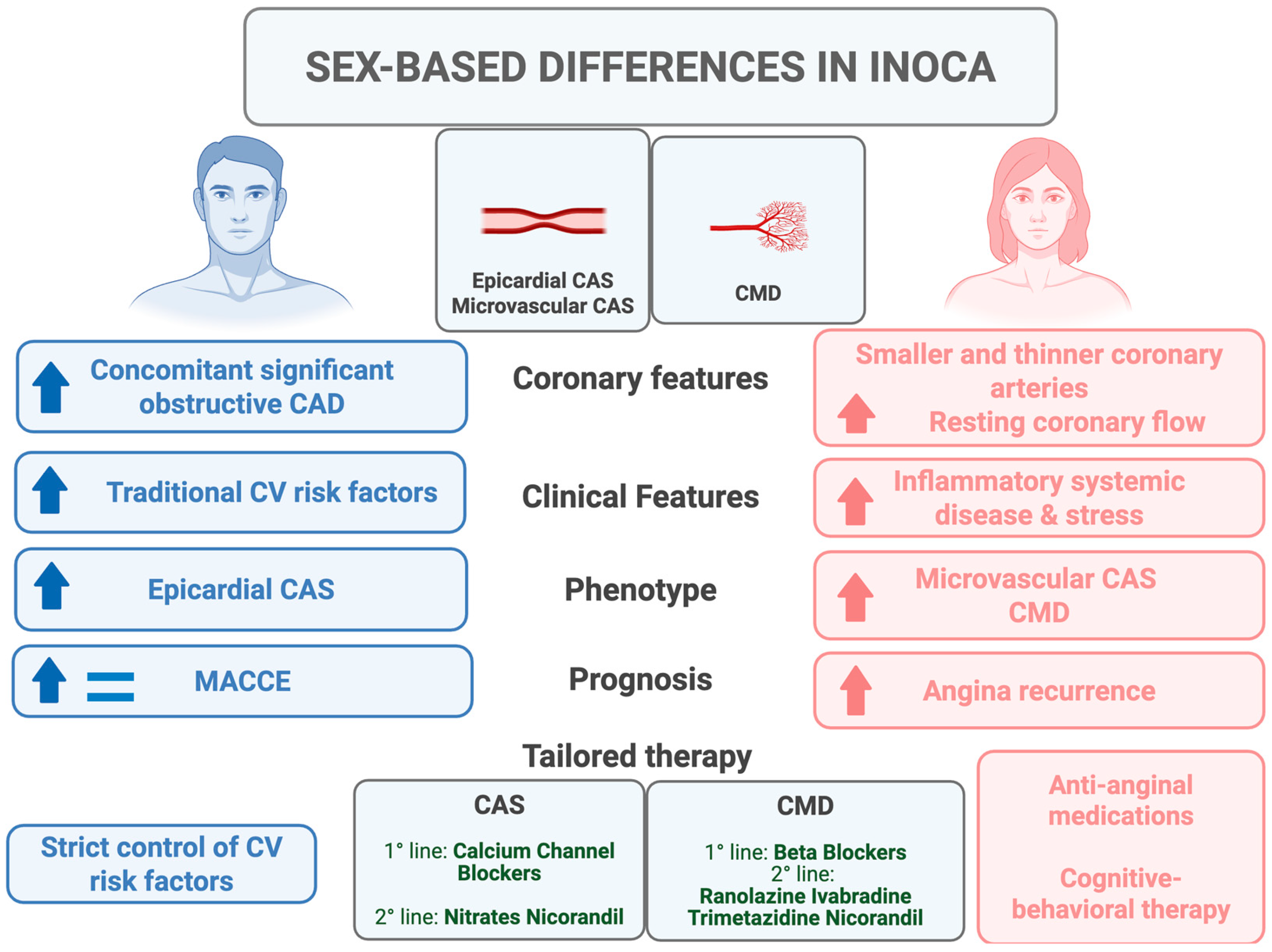
3. Pathophysiology of INOCA in Women
4. CMD in Women vs. Men: Prevalence and Prognosis
5. Vasomotor Disorders in Women vs. Men: Prevalence and Prognosis
6. Potential Therapeutic Implications and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| CAD | Coronary artery disease |
| CAS | Coronary artery spasm |
| CFR | Coronary flow reserve |
| CMD | Coronary microvascular dysfunction |
| COVADIS | Coronary Vasomotion Disorders International Study |
| IMR | Index of microvascular resistance |
| INOCA | Ischemia with non-obstructive coronary artery disease |
| MACE | Major adverse cardiovascular events |
| MVA | Microvascular angina |
| NTCVRF | Non-traditional cardiovascular risk factors |
| VSMC | Vascular smooth muscle cell |
| WISE | Women’s Ischemia Syndrome Evaluation |
References
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low diagnostic yield of elective coronary angiography. N. Engl. J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention 2021, 16, 1049–1069. [Google Scholar] [CrossRef]
- Gurgoglione, F.L.; Benatti, G.; Denegri, A.; Donelli, D.; Covani, M.; De Gregorio, M.; Dallaglio, G.; Navacchi, R.; Niccoli, G. Coronary Microvascular Dysfunction: Insights on Prognosis and Future Perspectives. Rev. Cardiovasc. Med. 2025, 26, 25757. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Campo, G.; DISerafino, L.; Zanon, S.; Rubino, F.; Monizzi, G.; Biscaglia, S.; Ancona, M.; Polimeni, A.; Niccoli, G.; et al. #FullPhysiology: A systematic step-by-step guide to implement intracoronary physiology in daily practice. Minerva Cardiol. Angiol. 2023, 71, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Ghizzoni, G.; Di Serafino, L.; Botti, G.; Galante, D.; D’Amario, D.; Benenati, S.; Gurgoglione, F.L.; Laborante, R.; Pompei, G.; Porto, I.; et al. L’ischemia miocardica in assenza di coronaropatia ostruttiva: Stato dell’arte [Ischemia with non-obstructive coronary artery disease: State-of-the-art review]. G. Ital. Cardiol. 2023, 24 (Suppl. S2), 5S–20S. (In Italian) [Google Scholar] [CrossRef]
- Montone, R.A.; Rinaldi, R.; Niccoli, G.; Andò, G.; Gragnano, F.; Piccolo, R.; Pelliccia, F.; Moscarella, E.; Zimarino, M.; Fabris, E.; et al. Optimizing Management of Stable Angina: A Patient-Centered Approach Integrating Revascularization, Medical Therapy, and Lifestyle Interventions. J. Am. Coll. Cardiol. 2024, 84, 744–760. [Google Scholar] [CrossRef]
- Leone, A.M.; Galante, D.; Viceré, A.; Marrone, A.; Verardi, F.M.; Giuliana, C.; Pollio Benvenuto, C.; Viccaro, V.; Todisco, S.; Erriquez, A.; et al. Functional coronary assessment in angina with intermediate coronary stenosis: The #FullPhysiology approach. Eur. Heart J. 2025, 46, 978–980. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll. Cardiol. 2018, 72 Pt A, 2841–2855. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Diaz, A.; Cyr, D.D.; Shaw, L.J.; Mancini, G.B.J.; Leipsic, J.; Budoff, M.J.; Min, J.K.; Hague, C.J.; Berman, D.S.; et al. Ischemia with Nonobstructive Coronary Arteries: Insights from the ISCHEMIA Trial. JACC Cardiovasc. Imaging 2023, 16, 63–74. [Google Scholar] [CrossRef]
- Avran, A.; Zuffi, A.; Gobbi, C.; Gasperetti, A.; Schiavone, M.; Werner, G.S.; Kambis, M.; Boudou, N.; Galassi, A.R.; Sianos, G.; et al. Gender differences in percutaneous coronary intervention for chronic total occlusions from the ERCTO study. Catheter Cardiovasc. Interv. 2023, 101, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Caffè, A.; Yasumura, K.; Kini, A. Routine diagnosis of ANOCA/INOCA: Pros and cons. EuroIntervention 2025, 21, e293–e295. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Mehta, P.K.; Huang, J.; Levit, R.D.; Malas, W.; Waheed, N.; Bairey Merz, C.N. Ischemia and no obstructive coronary arteries (INOCA): A narrative review. Atherosclerosis 2022, 363, 8–21. [Google Scholar] [CrossRef]
- Gurgoglione, F.L.; Vignali, L.; Montone, R.A.; Rinaldi, R.; Benatti, G.; Solinas, E.; Leone, A.M.; Galante, D.; Campo, G.; Biscaglia, S.; et al. Coronary Spasm Testing with Acetylcholine: A Powerful Tool for a Personalized Therapy of Coronary Vasomotor Disorders. Life 2024, 14, 292. [Google Scholar] [CrossRef]
- Prescott, E.; Bove, K.B.; Bechsgaard, D.F.; Shafi, B.H.; Lange, T.; Schroder, J.; Suhrs, H.E.; Nielsen, R.L. Biomarkers and Coronary Microvascular Dysfunction in Women with Angina and No Obstructive Coronary Artery Disease. JACC Adv. 2023, 2, 100264. [Google Scholar] [CrossRef]
- Camilli, M.; Russo, M.; Rinaldi, R.; Caffè, A.; La Vecchia, G.; Bonanni, A.; Iannaccone, G.; Basile, M.; Vergallo, R.; Aurigemma, C.; et al. Air Pollution and Coronary Vasomotor Disorders in Patients with Myocardial Ischemia and Unobstructed Coronary Arteries. J. Am. Coll. Cardiol. 2022, 80, 1818–1828. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Iannaccone, G.; Meucci, M.C.; Rinaldi, R.; D’Amario, D.; Niccoli, G. Diagnostic work-up and therapeutic implications in MINOCA: Need for a personalized approach. Future Cardiol. 2021, 17, 149–154. [Google Scholar] [CrossRef]
- Montone, R.A.; Cosentino, N.; Graziani, F.; Gorla, R.; Del Buono, M.G.; La Vecchia, G.; Rinaldi, R.; Marenzi, G.; Bartorelli, A.L.; De Marco, F.; et al. Precision medicine versus standard of care for patients with myocardial infarction with non-obstructive coronary arteries (MINOCA): Rationale and design of the multicentre, randomised PROMISE trial. EuroIntervention 2022, 18, e933–e939. [Google Scholar] [CrossRef]
- Montone, R.A.; Jang, I.K.; Beltrame, J.F.; Sicari, R.; Meucci, M.C.; Bode, M.; Gaibazzi, N.; Niccoli, G.; Bucciarelli-Ducci, C.; Crea, F. The evolving role of cardiac imaging in patients with myocardial infarction and non-obstructive coronary arteries. Prog. Cardiovasc. Dis. 2021, 68, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Rinaldi, R.; Del Buono, M.G.; Gurgoglione, F.; La Vecchia, G.; Russo, M.; Caffè, A.; Burzotta, F.; Leone, A.M.; Romagnoli, E.; et al. Safety and prognostic relevance of acetylcholine testing in patients with stable myocardial ischaemia or myocardial infarction and non-obstructive coronary arteries. EuroIntervention 2022, 18, e666–e676. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Niccoli, G.; Fracassi, F.; Russo, M.; Gurgoglione, F.; Cammà, G.; Lanza, G.A.; Crea, F. Patients with acute myocardial infarction and non-obstructive coronary arteries: Safety and prognostic relevance of invasive coronary provocative tests. Eur. Heart J. 2018, 39, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Montone, R.A.; Rinaldi, R. Pathophysiology of Coronary Microvascular Dysfunction. Circ. J. 2022, 86, 1319–1328. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef]
- Chen, M.T.; Chang, J.; Manchanda, A.S.; Cook-Wiens, G.; Shufelt, C.L.; Anderson, R.D.; Petersen, J.W.; Naik, D.R.; Thomson, L.E.J.; Berman, D.S.; et al. Autoimmune rheumatic diseases in women with coronary microvascular dysfunction: A report from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (WISE-CVD) project. Front. Cardiovasc. Med. 2023, 10, 1155914. [Google Scholar] [CrossRef]
- Hiteshi, A.K.; Li, D.; Gao, Y.; Chen, A.; Flores, F.; Mao, S.S.; Budoff, M.J. Gender differences in coronary artery diameter are not related to body habitus or left ventricular mass. Clin. Cardiol. 2014, 37, 605–609. [Google Scholar] [CrossRef]
- Corban, M.T.; Prasad, A.; Gulati, R.; Lerman, L.O.; Lerman, A. Sex-specific differences in coronary blood flow and flow velocity reserve in symptomatic patients with non-obstructive disease. EuroIntervention 2021, 16, 1079–1084. [Google Scholar] [CrossRef]
- A Montone, R.; Camilli, M.; Calvieri, C.; Magnani, G.; Bonanni, A.; Bhatt, D.L.; Rajagopalan, S.; Crea, F.; Niccoli, G. Exposome in ischaemic heart disease: Beyond traditional risk factors. Eur. Heart J. 2024, 45, 419–438. [Google Scholar] [CrossRef]
- Gurgoglione, F.L.; Rizzello, D.; Giacalone, R.; Ferretti, M.; Vezzani, A.; Pfleiderer, B.; Pelà, G.; De Panfilis, C.; Cattabiani, M.A.; Benatti, G.; et al. Precipitating factors in patients with spontaneous coronary artery dissection: Clinical, laboratoristic and prognostic implications. Int. J. Cardiol. 2023, 385, 1–7. [Google Scholar] [CrossRef]
- Kim, H.L. Differences in Risk Factors for Coronary Atherosclerosis According to Sex. J. Lipid Atheroscler. 2024, 13, 97–110. [Google Scholar] [CrossRef]
- Pepine, C.J.; Anderson, R.D.; Sharaf, B.L.; Reis, S.E.; Smith, K.M.; Handberg, E.M.; Johnson, B.D.; Sopko, G.; Merz, C.N.B. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J. Am. Coll. Cardiol. 2010, 55, 2825–2832. [Google Scholar] [CrossRef]
- Tarhouni, K.; Guihot, A.; Vessieres, E.; Procaccio, V.; Grimaud, L.; Abraham, P.; Lenfant, F.; Arnal, J.; Favre, J.; Loufrani, L.; et al. Estrogens are needed for the improvement in endothelium-mediated dilation induced by a chronic increase in blood flow in rat mesenteric arteries. Vasc. Pharmacol. 2016, 80, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Tunc, E.; Eve, A.A.; Madak-Erdogan, Z. Coronary Microvascular Dysfunction and Estrogen Receptor Signaling. Trends Endocrinol. Metab. 2020, 31, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Faccini, A.; Kaski, J.C.; Camici, P.G. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur. Heart J. 2016, 37, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Quesada, O.; Cook-Wiens, G.; Wei, J.; Minissian, M.; Handberg, E.M.; Merz, N.B.; Pepine, C.J. Adverse Pregnancy Outcomes Are Associated with Reduced Coronary Flow Reserve in Women With Signs and Symptoms of Ischemia Without Obstructive Coronary Artery Disease: A Report from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Study. J. Women’s Health 2020, 29, 487–492. [Google Scholar] [CrossRef]
- Gitto, M.; Gentile, F.; Nowbar, A.N.; Chieffo, A.; Al-Lamee, R. Gender-Related Differences in Clinical Presentation and Angiographic Findings in Patients with Ischemia and No Obstructive Coronary Artery Disease (INOCA): A Single-Center Observational Registry. Int. J. Angiol. 2020, 29, 250–255. [Google Scholar] [CrossRef]
- Dal Lin, C.; Tona, F.; Osto, E. The crosstalk between the cardiovascular and the immune system. Vasc. Biol. 2019, 1, H83–H88. [Google Scholar] [CrossRef]
- Wei, J.; Barsky, L.; Jalnapurkar, S.; Zarrini, P.; Cook-Wiens, G.; AlBadri, A.; Nelson, M.D.; Shufelt, C.; Sharif, B.; Berman, D.; et al. Cold Pressor Testing and Sympathetic Nervous System Contribution to Ischemia with No Obstructive Coronary Artery Disease: Results from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Project. Am. Heart J. Plus 2022, 13, 100080. [Google Scholar] [CrossRef]
- Roy, R.; Aldiwani, H.; Darouian, N.; Sharma, S.; Torbati, T.; Wei, J.; Nelson, M.D.; Shufelt, C.; Minissian, M.B.; Li, L.; et al. Ambulatory and silent myocardial ischemia in women with coronary microvascular dysfunction: Results from the Cardiac Autonomic Nervous System study (CANS). Int. J. Cardiol. 2020, 316, 1–6. [Google Scholar] [CrossRef]
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, E.; Kelbaek, H.; Prescott, E.; Kelbæk, H. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012, 33, 734–744. [Google Scholar] [CrossRef]
- Kenkre, T.S.; Malhotra, P.; Johnson, B.D.; Handberg, E.M.; Thompson, D.V.; Marroquin, O.C.; Rogers, W.J.; Pepine, C.J.; Bairey Merz, C.N.; Kelsey, S.F. Ten-Year Mortality in the WISE Study (Women’s Ischemia Syndrome Evaluation). Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003863. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Fearon, W.F.; Honda, Y.; Tanaka, S.; Pargaonkar, V.; Fitzgerald, P.J.; Lee, D.P.; Stefanick, M.; Yeung, A.C.; Tremmel, J.A. Effect of Sex Differences on Invasive Measures of Coronary Microvascular Dysfunction in Patients with Angina in the Absence of Obstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2015, 8, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.; Lennon, R.J.; Ackerman, M.J.; Friedman, P.A.; Noseworthy, P.A.; Lerman, A. Coronary microvascular dysfunction is associated with baseline QTc prolongation amongst patients with chest pain and non-obstructive coronary artery disease. J. Electrocardiol. 2016, 49, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Yii, E.; Sidik, N.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; et al. Ischemia and No Obstructive Coronary Artery Disease: Prevalence and Correlates of Coronary Vasomotion Disorders. Circ. Cardiovasc. Interv. 2019, 12, e008126. [Google Scholar] [CrossRef]
- Pargaonkar, V.S.; Kobayashi, Y.; Kimura, T.; Schnittger, I.; Chow, E.K.H.; Froelicher, V.F.; Rogers, I.S.; Lee, D.P.; Fearon, W.F.; Yeung, A.C.; et al. Accuracy of non-invasive stress testing in women and men with angina in the absence of obstructive coronary artery disease. Int. J. Cardiol. 2019, 282, 7–15. [Google Scholar] [CrossRef]
- Rahman, H.; Ryan, M.; Lumley, M.; Modi, B.; McConkey, H.; Ellis, H.; Scannell, C.; Clapp, B.; Marber, M.; Webb, A.; et al. Coronary Microvascular Dysfunction Is Associated with Myocardial Ischemia and Abnormal Coronary Perfusion During Exercise. Circulation 2019, 140, 1805–1816. [Google Scholar] [CrossRef]
- Kumar, S.; Mehta, P.K.; Eshtehardi, P.; Hung, O.Y.; Koh, J.S.; Kumar, A.; Al-Badri, A.; Rabah, R.; D’Souza, M.; Gupta, S.; et al. Functional coronary angiography in symptomatic patients with no obstructive coronary artery disease. Catheter. Cardiovasc. Interv. 2021, 98, 827–835. [Google Scholar] [CrossRef]
- Chung, J.H.; Lee, K.E.; Lee, J.M.; Her, A.Y.; Kim, C.H.; Choi, K.H.; Song, Y.B.; Hahn, J.Y.; Kim, H.Y.; Choi, J.H.; et al. Effect of Sex Difference of Coronary Microvascular Dysfunction on Long-Term Outcomes in Deferred Lesions. JACC Cardiovasc. Interv. 2020, 13, 1669–1679. [Google Scholar] [CrossRef]
- Mileva, N.; Nagumo, S.; Mizukami, T.; Sonck, J.; Berry, C.; Gallinoro, E.; Monizzi, G.; Candreva, A.; Munhoz, D.; Vassilev, D.; et al. Prevalence of Coronary Microvascular Disease and Coronary Vasospasm in Patients with Nonobstructive Coronary Artery Disease: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e023207. [Google Scholar] [CrossRef]
- Vink, C.E.; Woudstra, J.; Lee, J.M.; Boerhout, C.K.; Cook, C.M.; Hoshino, M.; Mejia-Renteria, H.; Lee, S.H.; Jung, J.-H.; Echavarria-Pinto, M.; et al. Sex differences in prevalence and outcomes of the different endotypes of chronic coronary syndrome in symptomatic patients undergoing invasive coronary angiography: Insights from the global ILIAS invasive coronary physiology registry. Atherosclerosis 2023, 384, 117167. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Cooper-DeHoff, R.M.; McClure, C.; Johnson, B.D.; Shaw, L.J.; Handberg, E.M.; Zineh, I.; Kelsey, S.F.; Arnsdorf, M.F.; Black, H.R.; et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: A report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch. Intern. Med. 2009, 169, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Radico, F.; Zimarino, M.; Fulgenzi, F.; Ricci, F.; Di Nicola, M.; Jespersen, L.; Chang, S.M.; Humphries, K.H.; Marzilli, M.; De Caterina, R. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: A systematic review and meta-analysis. Eur. Heart J. 2018, 39, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Merz, C.N.B.; Pepine, C.J.; Reis, S.E.; Bittner, V.; Kip, K.E.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Mankad, S.; et al. The economic burden of angina in women with suspected ischemic heart disease: Results from the National Institutes of Health–National Heart, Lung, and Blood Institute--sponsored Women’s Ischemia Syndrome Evaluation. Circulation 2006, 114, 894–904. [Google Scholar] [CrossRef]
- Odanović, N.; Schwann, A.N.; Zhang, Z.; Kapadia, S.S.; Kunnirickal, S.J.; Parise, H.; Tirziu, D.; Ilic, I.; Lansky, A.J.; Pietras, C.G.; et al. Long-term outcomes of ischaemia with no obstructive coronary artery disease (INOCA): A systematic review and meta-analysis. Open Heart 2024, 11, e002852. [Google Scholar] [CrossRef]
- Shaw, L.J.; Olson, M.B.; Kip, K.; Kelsey, S.F.; Johnson, B.D.; Mark, D.B.; Reis, S.E.; Mankad, S.; Rogers, W.J.; Pohost, G.M.; et al. The value of estimated functional capacity in estimating outcome: Results from the NHBLI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J. Am. Coll. Card. 2006, 47, S36–S43. [Google Scholar] [CrossRef]
- Potts, S.G.; Bass, C.M. Psychological morbidity in patients with chest pain and normal or near-normal coronary arteries: A long-term follow-up study. Psychol. Med. 1995, 25, 339–347. [Google Scholar] [CrossRef]
- Humphreys, H.; Paddock, D.; Brown, S.; Berry, C.; Cowie, A.; Dawkes, S.; Nichols, S. Living with myocardial ischaemia and no obstructive coronary arteries: A qualitative study. Open Heart 2024, 11, e002569. [Google Scholar] [CrossRef]
- Ong, P.; Hubert, A.; Schwidder, M.; Beltrame, J.F. Coronary Spasm: Ethnic and Sex Differences. Eur. Cardiol. 2023, 18, e43. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.; Bae, M.H.; Kwon, Y.S.; Lee, J.H.; Ryu, H.M.; Park, Y.; Yang, D.H.; Park, H.S.; Cho, Y.; et al. Gender differences among korean patients with coronary spasm. Korean Circ. J. 2009, 39, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Kawana, A.; Takahashi, J.; Takagi, Y.; Yasuda, S.; Sakata, Y.; Tsunoda, R.; Ogata, Y.; Seki, A.; Sumiyoshi, T.; Matsui, M.; et al. Gender differences in the clinical characteristics and outcomes of patients with vasospastic angina—A report from the Japanese Coronary Spasm Association. Circ. J. 2013, 77, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Hansen, H.S.; Sechtem, U.; Prescott, E.; Ong, P. Sex-Related Differences in Vasomotor Function in Patients with Angina and Unobstructed Coronary Arteries. J. Am. Coll. Cardiol. 2017, 70, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takahashi, J.; Odaka, Y.; Suda, A.; Sueda, S.; Teragawa, H.; Ishii, K.; Kiyooka, T.; Hirayama, A.; Sumiyoshi, T.; et al. Clinical characteristics and long-term prognosis of contemporary patients with vasospastic angina: Ethnic differences detected in an international comparative study. Int. J. Cardiol. 2019, 291, 13–18. [Google Scholar] [CrossRef]
- Kim, H.L.; Jo, S.H.; Kim, H.J.; Lee, M.H.; Seo, W.W.; Baek, S.H. Sex differences in clinical characteristics and long-term outcomes in patients with vasospastic angina: Results from the VA-Korea registry, a prospective multi-center cohort. Biol. Sex Differ. 2020, 11, 66. [Google Scholar] [CrossRef]
- Ohba, K.; Sugiyama, S.; Sumida, H.; Nozaki, T.; Matsubara, J.; Matsuzawa, Y.; Konishi, M.; Akiyama, E.; Kurokawa, H.; Maeda, H.; et al. Microvascular coronary artery spasm presents distinctive clinical features with endothelial dysfunction as nonobstructive coronary artery disease. J. Am. Heart Assoc. 2012, 1, e002485. [Google Scholar] [CrossRef]
- Jansen, T.P.J.; Elias-Smale, S.E.; Oord, S.v.D.; Gehlmann, H.; Dimitiriu-Leen, A.; Maas, A.H.E.M.; Konst, R.E.; van Royen, N.; Damman, P. Sex Differences in Coronary Function Test Results in Patient with Angina and Nonobstructive Disease. Front. Cardiovasc. Med. 2021, 8, 750071. [Google Scholar] [CrossRef]
- Sueda, S.; Sakaue, T. Sex-related Differences in Patients with Positive Coronary Spasm as Identified by Acetylcholine Testing. Intern. Med. 2021, 60, 2357–2365. [Google Scholar] [CrossRef]
- Saito, Y.; Saito, Y.; Kato, K.; Kobayashi, Y. Gender differences in factors associated with vasospastic angina. Int. J. Cardiol. 2022, 349, 7–11. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, S.Y.; Rha, S.W.; Choi, B.G.; Noh, Y.K.; Kim, Y.H. Sex Difference in Coronary Artery Spasm Tested by Intracoronary Acetylcholine Provocation Test in Patients with Nonobstructive Coronary Artery Disease. J. Interv. Cardiol. 2022, 2022, 5289776. [Google Scholar] [CrossRef]
- Rinaldi, R.; Russo, M.; Occhipinti, G.; Laudani, C.; Torre, I.; Colucci, M.; Gurgoglione, F.L.; Animati, F.M.; Lenkowicz, J.; Tudor, A.M.; et al. Sex-Related Differences in the Prognostic Role of Acetylcholine Provocation Testing. J. Am. Heart Assoc. 2025, 14, e037942. [Google Scholar] [CrossRef]
- Gurgoglione, F.L.; Solinas, E.; Pfleiderer, B.; Vezzani, A.; Niccoli, G. Coronary atherosclerotic plaque phenotype and physiopathologic mechanisms: Is there an influence of sex? Insights from intracoronary imaging. Atherosclerosis 2023, 384, 117273. [Google Scholar] [CrossRef]
- Montone, R.A.; Pitocco, D.; Gurgoglione, F.L.; Rinaldi, R.; Del Buono, M.G.; Camilli, M.; Rizzi, A.; Tartaglione, L.; Rizzo, G.E.; Di Leo, M.; et al. Microvascular complications identify a specific coronary atherosclerotic phenotype in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2022, 21, 211. [Google Scholar] [CrossRef]
- Ong, P.; Athanasiadis, A.; Perne, A.; Mahrholdt, H.; Schäufele, T.; Hill, S.; Sechtem, U. Coronary vasomotor abnormalities in patients with stable angina after successful stent implantation but without in-stent restenosis. Clin. Res. Cardiol. 2014, 103, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Gurgoglione, F.L.; Del Buono, M.G.; Rinaldi, R.; Meucci, M.C.; Iannaccone, G.; La Vecchia, G.; Camilli, M.; D’aMario, D.; Leone, A.M.; et al. Interplay Between Myocardial Bridging and Coronary Spasm in Patients with Myocardial Ischemia and Non-Obstructive Coronary Arteries: Pathogenic and Prognostic Implications. J. Am. Heart Assoc. 2021, 10, e020535. [Google Scholar] [CrossRef] [PubMed]
- Sueda, S.; Miyoshi, T.; Sasaki, Y.; Sakaue, T.; Habara, H.; Kohno, H. Gender differences in sensitivity of acetylcholine and ergonovine to coronary spasm provocation test. Heart Vessel. 2016, 31, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, J.; Shimokawa, H.; Mukai, Y.; Ichiki, T.; Takeshita, A. Divergent effects of estrogen and nicotine on Rho-kinase expression in human coronary vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2005, 326, 154–159. [Google Scholar] [CrossRef]
- Cleary, M.P.; Grossmann, M.E. Minireview: Obesity and breast cancer: The estrogen connection. Endocrinology 2009, 150, 2537–2542. [Google Scholar] [CrossRef]
- Schindler, T.H.; Fearon, W.F.; Pelletier-Galarneau, M.; Ambrosio, G.; Sechtem, U.; Ruddy, T.D.; Patel, K.K.; Bhatt, D.L.; Bateman, T.M.; Gewirtz, H.; et al. Myocardial perfusion PET for the detection and reporting of coronary microvascular dysfunction: A JACC: Cardiovascular imaging expert panel statement. JACC Cardiovasc. Imaging 2023, 16, 536–548. [Google Scholar] [CrossRef]
- Kotecha, T.; Martinez-Naharro, A.; Boldrini, M.; Knight, D.; Hawkins, P.; Kalra, S.; Patel, D.; Coghlan, G.; Moon, J.; Plein, S.; et al. Automated pixel-wise quantitative myocardial perfusion mapping by cmr to detect obstructive coronary artery disease and coronary microvascular dysfunction: Validation against invasive coronary physiology. JACC Cardiovasc. Imaging 2019, 12, 1958–1969. [Google Scholar] [CrossRef]
- Cevik, E.; Tas, A.; Demirtakan, Z.G.; Damman, P.; Alan, Y.; Broyd, C.J.; Ozcan, A.; Simsek, D.H.; Sonsoz, M.R.; van Royen, N.; et al. Intracoronary electrocardiogram detects coronary microvascular dysfunction and ischemia in patients with no obstructive coronary arteries disease. Am. Heart J. 2024, 270, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gong, Y.; Xu, L.; Xia, L.; Zhang, J.; Zheng, D.; Yao, Z.; Zhang, X.; Wei, H.; Jiang, J.; et al. Entropy-based reliable non-invasive detection of coronary microvascular dysfunction using machine learning algorithm. Math. Biosci. Eng. 2023, 20, 13061–13085. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, K.; Pyrpyris, N.; Sakalidis, A.; Dri, E.; Iliakis, P.; Tsioufis, P.; Tatakis, F.; Beneki, E.; Fragkoulis, C.; Aznaouridis, K.; et al. ANOCA updated: From pathophysiology to modern clinical practice. Cardiovasc. Revasc Med. 2025, 71, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reriani, M.; Raichlin, E.; Prasad, A.; Mathew, V.; Pumper, G.M.; Nelson, R.E.; Lennon, R.; Rihal, C.; Lerman, L.O.; Lerman, A. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation 2010, 122, 958–966. [Google Scholar] [CrossRef]
- Henry, T.D.; Merz, C.N.B.; Wei, J.; Corban, M.T.; Quesada, O.; Joung, S.; Kotynski, C.L.; Wang, J.; Lewis, M.; Schumacher, A.M.; et al. Autologous CD34+ Stem Cell Therapy Increases Coronary Flow Reserve and Reduces Angina in Patients with Coronary Microvascular Dysfunction. Circ. Cardiovasc. Interv. 2022, 15, e010802. [Google Scholar] [CrossRef]
- Corban, M.T.; Toya, T.; Albers, D.; Sebaali, F.; Lewis, B.R.; Bois, J.; Gulati, R.; Prasad, A.; Best, P.J.; Bell, M.R.; et al. IMPROvE-CED Trial: Intracoronary Autologous CD34+ Cell Therapy for Treatment of Coronary Endothelial Dysfunction in Patients with Angina and Nonobstructive Coronary Arteries. Circ. Res. 2022, 130, 326–338. [Google Scholar] [CrossRef]
- Foley, M.J.; Rajkumar, C.A.; Ahmed-Jushuf, F.; Simader, F.A.; Chotai, S.; Pathimagaraj, R.H.; Mohsin, M.; Salih, A.; Wang, D.; Dixit, P.; et al. Coronary sinus reducer for the treatment of refractory angina (ORBITA-COSMIC): A randomised, placebo-controlled trial. Lancet 2024, 403, 1543–1553. [Google Scholar] [CrossRef]
- Tebaldi, M.; Campo, G.; Ugo, F.; Guarracini, S.; Marrone, A.; Clò, S.; Abdirashid, M.; Di Mauro, M.; Rametta, F.; Di Marco, M.; et al. Coronary Sinus Narrowing Improves Coronary Microcirculation Function in Patients with Refractory Angina: A Multicenter Prospective INROAD Study. Circ. Cardiovasc. Interv. 2024, 17, e013481. [Google Scholar] [CrossRef]
- Cunningham, C.; Brown, S.; Kaski, J.C. Effects of transcendental meditation on symptoms and electrocardiographic changes in patients with cardiac syndrome X. Am. J. Cardiol. 2000, 85, 653–655. [Google Scholar] [CrossRef]
| First Author, Year, Date, Reference | Study Population | Proportion of Females | Endpoints | Results [Women vs. Men] |
|---|---|---|---|---|
| Kobayashi et al., 2015 [44] | 157 ANOCA patients | 74.5% | CMD: CFR < 2.0 and/or IMR ≥ 25 | Abnormal CFR: 8.6% vs. 0% * CFR: 3.8 ± 1.6 vs. 4.8 ± 1.9 * Abnormal IMR: 28.2% vs. 15% |
| Sara et al., 2016 [45] | 926 ANOCA/INOCA patients | 74.7% | CMD: CFR ≤ 2.5 | CMD: 30.6% vs. 29.9% |
| Ford et al., 2019 [46] | 187 INOCA patients | 67.9% | MVA: IMR ≥ 25 and/or CFR < 2.0 and/or microvascular spasm | MVA: 74.8% vs. 65% |
| Pargaonkar et al., 2019 [47] | 155 INOCA patients | 76.8% | CMD: IMR ≥ 25 | CMD: 21% vs. 19.4% |
| Rahman et al., 2019 [48] | 85 ANOCA patients | 77.7% | CMD: CFR < 2.5 | CMD: 59% vs. 31.6% |
| Kumar et al., 2020 [49] | 163 INOCA and UA patients | 48.5% | CMD: CFR < 2.5 and/or HMR ≥ 2 and/or ≤50% change in coronary blood flow with Ach | CFR: 2.2 ± 0.8 vs. 2.5 ± 1.1 * |
| Chung et al., 2020 [50] | 434 ANOCA patients | 30.7% | CMD: CFR ≤ 2.0 and/or IMR ≥ 23 | CMD: 46.6% vs. 39.5% CFR: 2.69 vs. 3.20 * IMR: 17.9 vs. 17.1 5-year MACE rate:1.1% vs. 5.5% * |
| First Author, Date, Reference | Study Population | Proportion of Females | Endpoints | Results |
|---|---|---|---|---|
| Lee et al., 2009 [61] | 104 ANOCA patients | 20.2% | Epicardial CAS: >95% diameter reduction + chest pain + ischemic ECG changes | Men have more CAS in the RCA, women in the LAD. |
| Ohba et al., 2012 [66] | 370 ANOCA patients | 57.0% | Epicardial CAS: >90% diameter reduction + chest pain + ischemic ECG changes. Microvascular CAS: transcardiac lactate production + decreased quantitative coronary blood flow | Female sex was independently correlated with the presence of microvascular CAS. Patients with microvascular CAS exhibited a good prognosis after treatment with CCBs. |
| Kawana et al., 2013 [62] | 1429 VSA patients | 23.7% | Epicardial CAS: >90% spasm + chest pain + ischemic ECG changes | No significant sex-based differences in 5-year MACE-free survival. The long-term prognosis was lowest in the young female group. |
| Aziz et al., 2017 [63] | 1379 ANOCA patients | 42% | Epicardial CAS: >75% diameter reduction + chest pain + ischemic ECG changes. Microvascular CAS: <75% diameter reduction + chest pain + ischemic ECG changes | Women had a higher prevalence of both epicardial and microvascular CAS. |
| Sueda et al., 2021 [68] | 917 VSA patients | 80.4% | Epicardial coronary spasm: >90% spasm + typical chest pain + ECG changes | Women had a higher prevalence of diffuse and combined CAS. Prognosis was similar between sexes. |
| Jansen et al., 2021 [67] | 266 ANOCA patients | 85.7% | Epicardial CAS: >90% diameter reduction + chest pain + ischemic ECG changes. Microvascular CAS: <90% diameter reduction + chest pain + ischemic ECG changes | Men had more epicardial CAS and less microvascular CAS than women. |
| Park et al., 2022 [70] | 5491 ANOCA patients | 54.4% | Epicardial coronary spasm: >70% spasm + typical chest pain + ECG changes | 5-year major clinical outcomes were similar between men and women. |
| Saito et al., 2022 [69] | 797 ANOCA patients | 46.4% | Epicardial CAS: angiographic spasm + chest pain + ischemic ECG changes | Men were more likely to have positive ACh provocation test as compared to women. |
| Rinaldi et al., 2025 [71] | 519 patients with suspected CAS | 53.0% | Epicardial CAS: >90% diameter reduction + typical chest pain + ischemic ECG changes. Microvascular CAS: <90% diameter reduction + typical chest pain + ischemic ECG changes | Women exhibited a higher incidence of microvascular CAS. In female patients, a positive ACh test was associated with a higher rate of angina recurrence. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurgoglione, F.L.; Benatti, G.; Denegri, A.; Solinas, E.; Tadonio, I.; De Gregorio, M.; Triglia, L.T.; Donelli, D.; Covani, M.; Dallaglio, G.; et al. Ischemia with Non-Obstructive Coronary Artery Disease: Sex-Based Differences in Pathophysiology, Clinical Presentation, and Prognosis. J. Clin. Med. 2025, 14, 5764. https://doi.org/10.3390/jcm14165764
Gurgoglione FL, Benatti G, Denegri A, Solinas E, Tadonio I, De Gregorio M, Triglia LT, Donelli D, Covani M, Dallaglio G, et al. Ischemia with Non-Obstructive Coronary Artery Disease: Sex-Based Differences in Pathophysiology, Clinical Presentation, and Prognosis. Journal of Clinical Medicine. 2025; 14(16):5764. https://doi.org/10.3390/jcm14165764
Chicago/Turabian StyleGurgoglione, Filippo Luca, Giorgio Benatti, Andrea Denegri, Emilia Solinas, Iacopo Tadonio, Mattia De Gregorio, Laura Torlai Triglia, Davide Donelli, Marco Covani, Gabriella Dallaglio, and et al. 2025. "Ischemia with Non-Obstructive Coronary Artery Disease: Sex-Based Differences in Pathophysiology, Clinical Presentation, and Prognosis" Journal of Clinical Medicine 14, no. 16: 5764. https://doi.org/10.3390/jcm14165764
APA StyleGurgoglione, F. L., Benatti, G., Denegri, A., Solinas, E., Tadonio, I., De Gregorio, M., Triglia, L. T., Donelli, D., Covani, M., Dallaglio, G., Barocelli, F., Magnani, G., Russo, M., Vignali, L., & Niccoli, G. (2025). Ischemia with Non-Obstructive Coronary Artery Disease: Sex-Based Differences in Pathophysiology, Clinical Presentation, and Prognosis. Journal of Clinical Medicine, 14(16), 5764. https://doi.org/10.3390/jcm14165764











