Comparison of Safety and Effectiveness Between Direct Oral Anticoagulants and Vitamin K Antagonists in Dementia Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approvals and Registrations
2.2. Data Sources, Searches, and Study Selection
2.3. Quality Control, Bias Assessment, and Data Extraction
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Selection and Characteristics
3.2. Outcomes
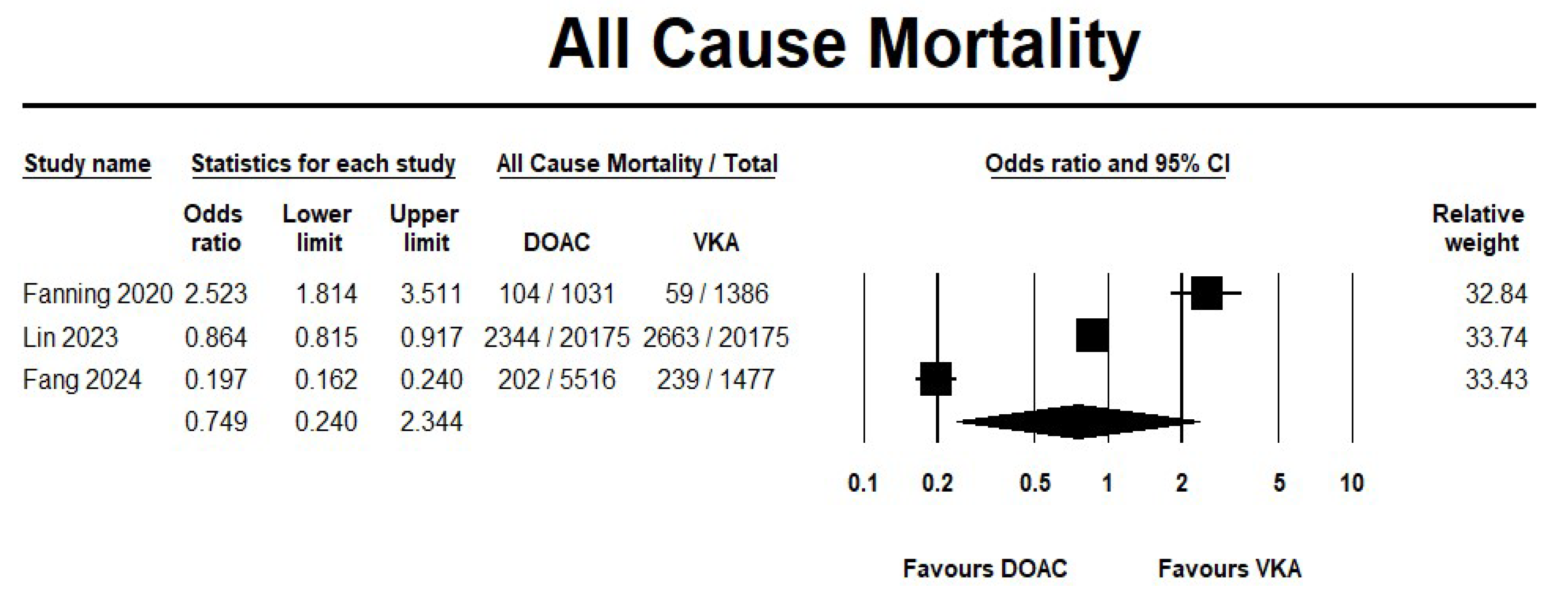
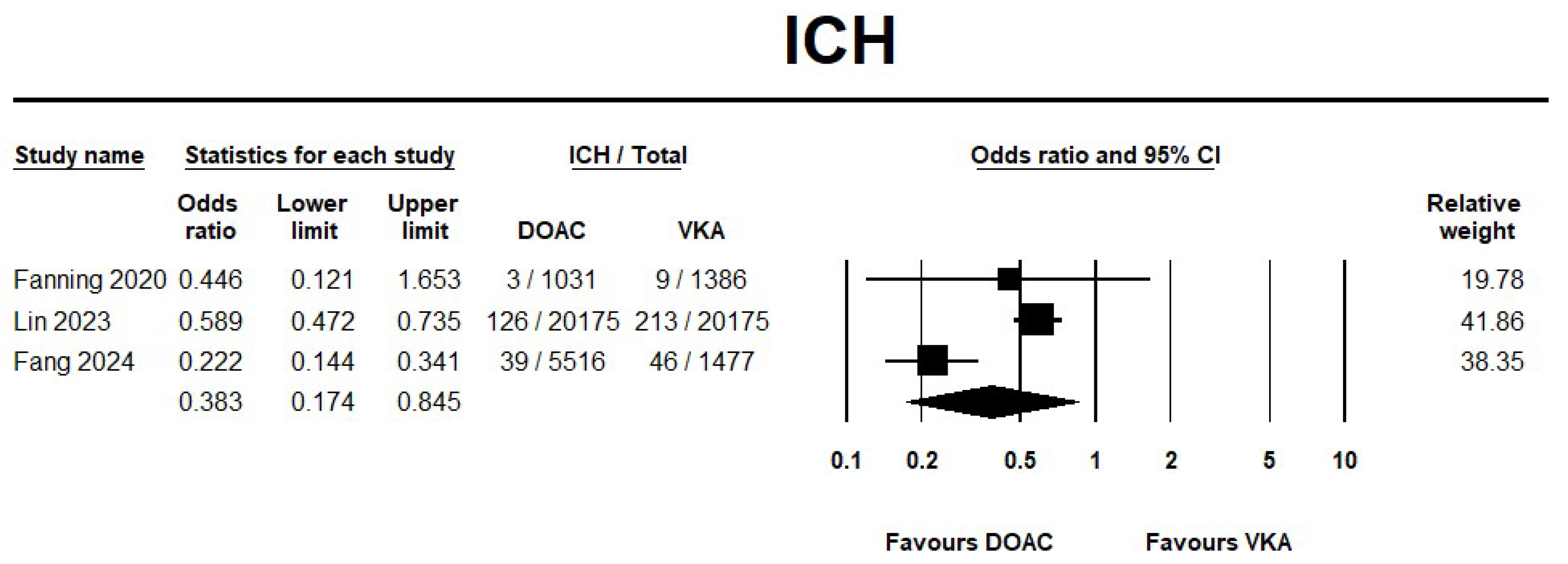
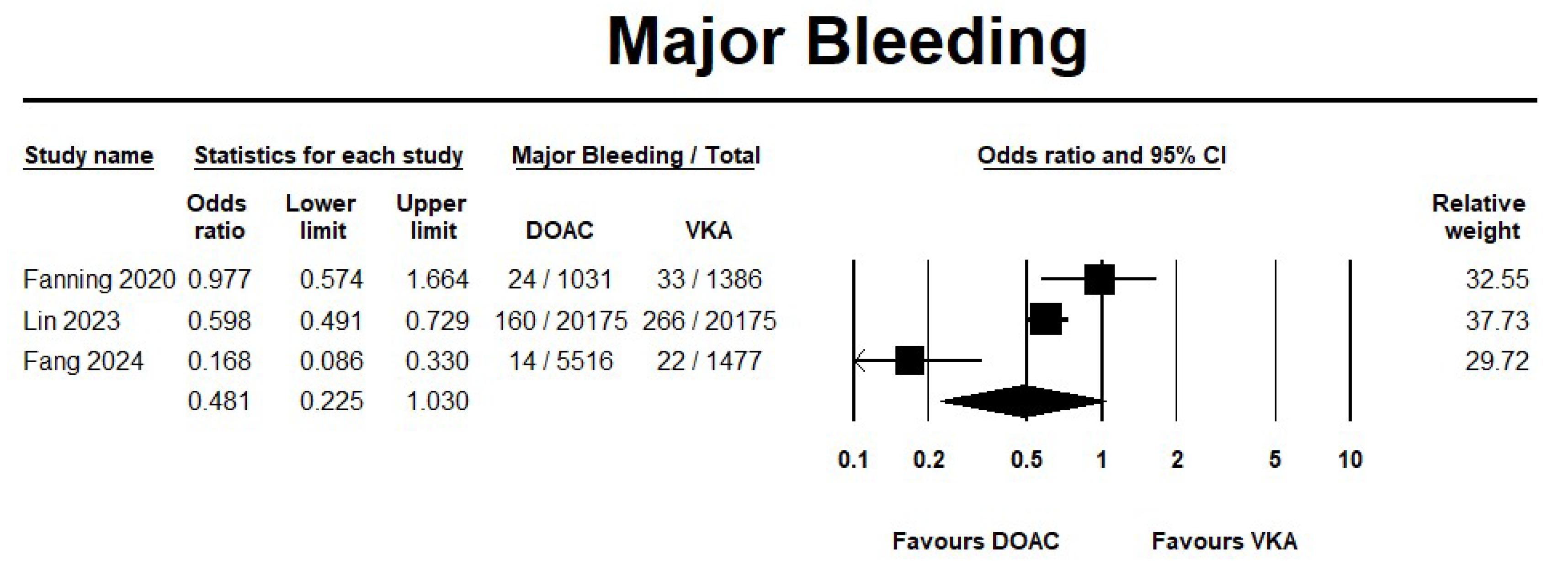
3.2.1. DOAC Versus VKA
Effectiveness Outcomes
Safety Outcomes
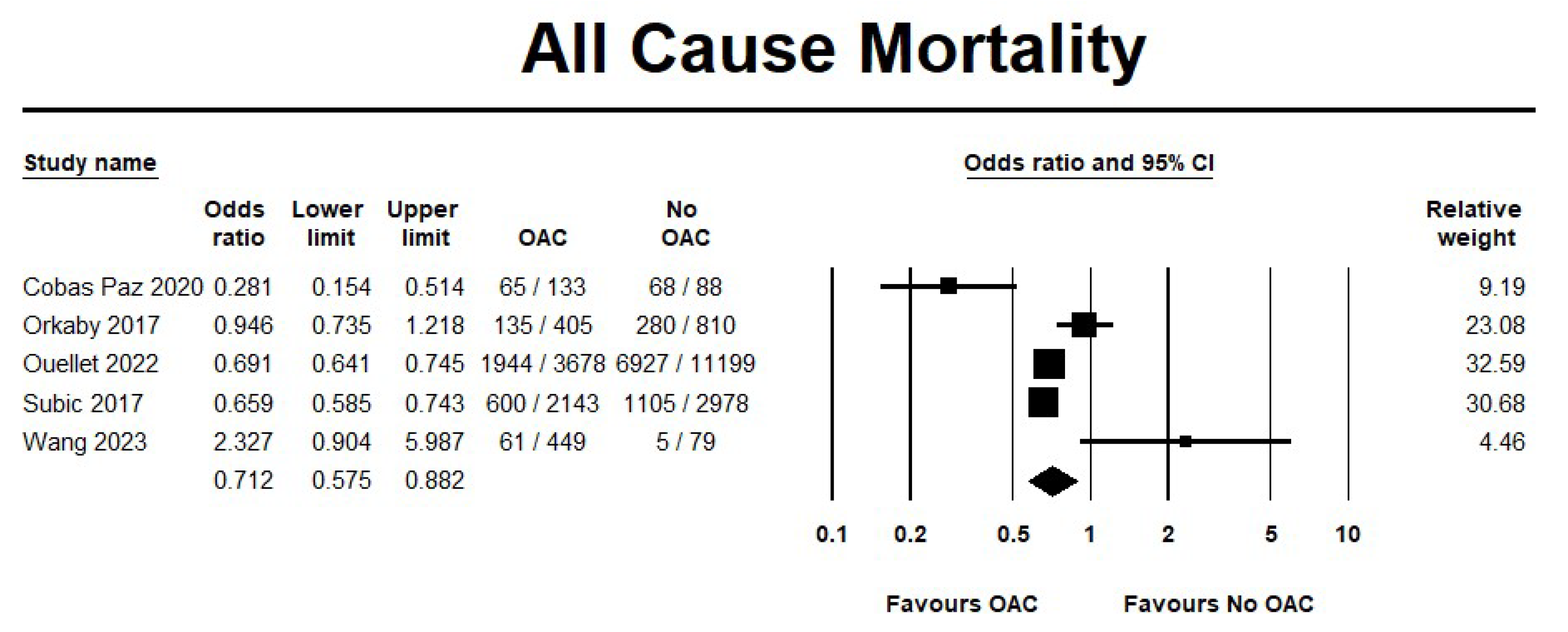
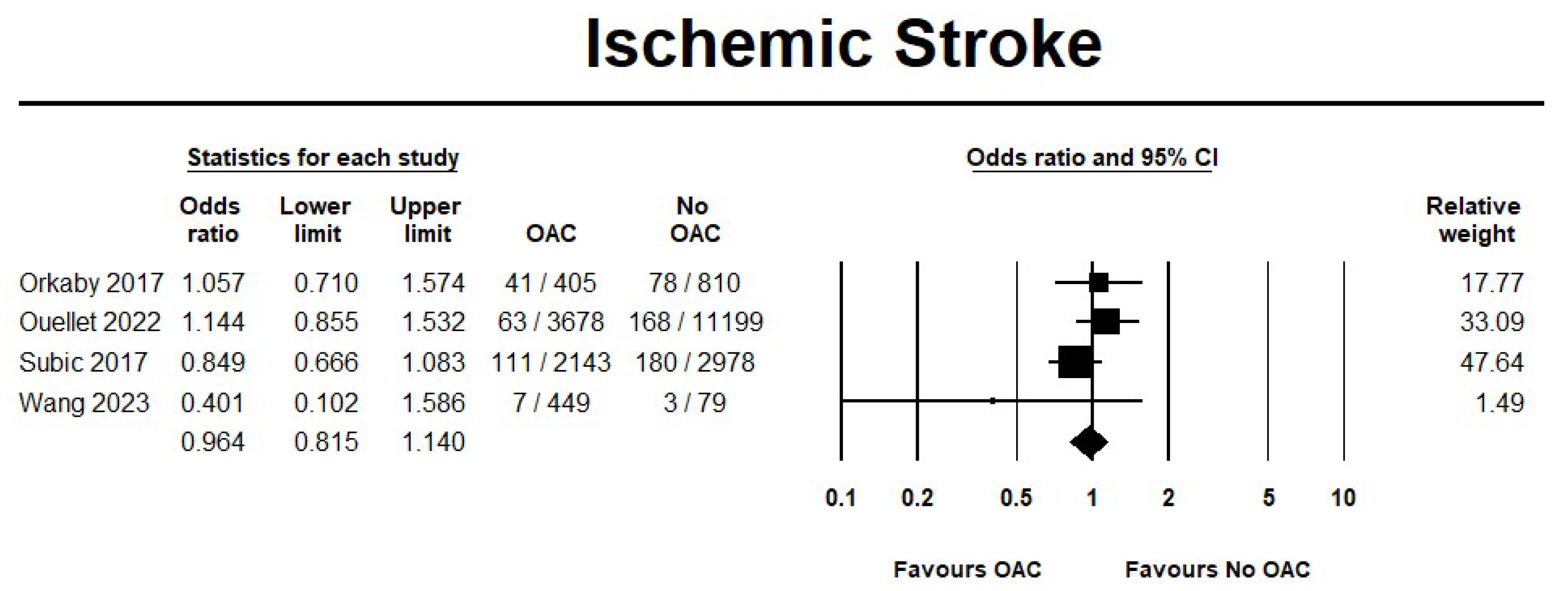
3.2.2. OAC Versus No OAC
Effectiveness Outcomes
Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Ashburner, J.M.; Ellinor, P.T.; McManus, D.D.; Atlas, S.J.; Singer, D.E.; Lubitz, S.A. Prevalence and Incidence of Atrial Fibrillation Among Older Primary Care Patients. JAMA Netw. Open 2023, 6, e2255838. [Google Scholar] [CrossRef] [PubMed]
- Rosas Diaz, A.N.; Troy, A.L.; Kaplinskiy, V.; Pritchard, A.; Vani, R.; Ko, D.; Orkaby, A.R. Assessment and Management of Atrial Fibrillation in Older Adults with Frailty. Geriatrics 2024, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.H.; Lew, L.Z.W.; Franke, K.B.; Elliott, A.D.; Lau, D.H.; Thiyagarajah, A.; Linz, D.; Arstall, M.; Tully, P.J.; Baune, B.T.; et al. Predictive role of atrial fibrillation in cognitive decline: A systematic review and meta-analysis of 2.8 million individuals. Europace 2022, 24, 1229–1239. [Google Scholar] [CrossRef]
- Papanastasiou, C.A.; Theochari, C.A.; Zareifopoulos, N.; Arfaras-Melainis, A.; Giannakoulas, G.; Karamitsos, T.D.; Palaiodimos, L.; Ntaios, G.; Avgerinos, K.I.; Kapogiannis, D.; et al. Atrial Fibrillation Is Associated with Cognitive Impairment, All-Cause Dementia, Vascular Dementia, and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Gen. Intern. Med. 2021, 36, 3122–3135. [Google Scholar] [CrossRef]
- Giannone, M.E.; Filippini, T.; Whelton, P.K.; Chiari, A.; Vitolo, M.; Boriani, G.; Vinceti, M. Atrial Fibrillation and the Risk of Early-Onset Dementia: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e025653. [Google Scholar] [CrossRef]
- Dodson, J.A.; Petrone, A.; Gagnon, D.R.; Tinetti, M.E.; Krumholz, H.M.; Gaziano, J.M. Incidence and Determinants of Traumatic Intracranial Bleeding Among Older Veterans Receiving Warfarin for Atrial Fibrillation. JAMA Cardiol. 2016, 1, 65–72. [Google Scholar] [CrossRef]
- Jankowska-Polańska, B.; Katarzyna, L.; Lidia, A.; Joanna, J.; Dudek, K.; Izabella, U. Cognitive function and adherence to anticoagulation treatment in patients with atrial fibrillation. J. Geriatr. Cardiol. 2016, 13, 559–565. [Google Scholar] [CrossRef]
- Nishimura, S.; Kumamaru, H.; Shoji, S.; Nakatani, E.; Yamamoto, H.; Ichihara, N.; Sandhu, A.T.; Miyachi, Y.; Miyata, H.; Kohsaka, S. Frailty and subsequent adverse outcomes in older patients with atrial fibrillation treated with oral anticoagulants: The Shizuoka study. Res. Pract. Thromb. Haemost. 2023, 7, 100129. [Google Scholar] [CrossRef]
- Cobas Paz, R.; Raposeiras Roubín, S.; Abu Assi, E.; Pardal, C.B.; Comesaña, J.G.; López, A.G.-C.; Queija, B.C.; Fernández, M.C.; Pousa, I.M.; Erquicia, P.D.; et al. Impact of anticoagulation in patients with dementia and atrial fibrillation. Results of the CardioCHUVI-FA registry. Rev. Esp. Cardiol. (Engl. Ed.) 2020, 73, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Orkaby, A.R.; Ozonoff, A.; Reisman, J.I.; Miller, D.R.; Zhao, S.; Rose, A.J. Continued Use of Warfarin in Veterans with Atrial Fibrillation After Dementia Diagnosis. J. Am. Geriatr. Soc. 2017, 65, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, G.M.; O’Leary, J.R.; Leggett, C.G.; Skinner, J.; Tinetti, M.E.; Cohen, A.B. Benefits and harms of oral anticoagulants for atrial fibrillation in nursing home residents with advanced dementia. J. Am. Geriatr. Soc. 2023, 71, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Subic, A.; Cermakova, P.; Religa, D.; Han, S.; von Euler, M.; Kåreholt, I.; Johnell, K.; Fastbom, J.; Bognandi, L.; Winblad, B.; et al. Treatment of Atrial Fibrillation in Patients with Dementia: A Cohort Study from the Swedish Dementia Registry. J. Alzheimers. Dis. 2018, 61, 1119–1128. [Google Scholar] [CrossRef]
- Wang, W.; Lessard, D.; Kiefe, C.I.; Goldberg, R.J.; Parish, D.; Helm, R.; Trymbulak, K.; Mehawej, J.; Abu, H.; Bamgbade, B.A.; et al. Differential effect of anticoagulation according to cognitive function and frailty in older patients with atrial fibrillation. J. Am. Geriatr. Soc. 2023, 71, 394–403. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Fang, C.-W.; Hsieh, C.-Y.; Yang, H.-Y.; Tsai, C.-F.; Sung, S.-F. Comparative effectiveness and safety of direct oral anticoagulants and warfarin in atrial fibrillation patients with dementia. Eur. Stroke J. 2024, 10, 128–136. [Google Scholar] [CrossRef]
- Fanning, L.; Lau, W.C.Y.; Mongkhon, P.; Man, K.K.C.; Bell, J.S.; Ilomäki, J.; Dārziņš, P.; Lau, K.K.; Wei, L.; Wong, L.C.K. Safety and effectiveness of direct oral anticoagulants vs. warfarin in people with atrial fibrillation and dementia. J. Am. Med. Dir. Assoc. 2020, 21, 1058–1064. [Google Scholar] [CrossRef]
- Lin, K.J.; Singer, D.E.; Bykov, K.; Bessette, L.G.; Mastrorilli, J.M.; Cervone, A.; Kim, D.H. Comparative effectiveness and safety of oral anticoagulants by dementia status in older patients with atrial fibrillation. JAMA Netw. 2023, 6, e234086. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ott. Hosp. Res. Inst. 2014. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 November 2024).
- Bruggemann, P.; Rajguru, K. Comprehensive meta-analysis (CMA) 3.0: A software review. J. Market. Anal. 2022, 10, 425–429. [Google Scholar] [CrossRef]
- Wang, D.; Xu, X.; Han, X.; Xie, J.; Zhou, H.; Peng, W.; Pan, G. Clinical benefits of oral anticoagulants in atrial fibrillation patients with dementia: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1265331. [Google Scholar] [CrossRef]
- Yang, L.; Brooks, M.M.; Glynn, N.W.; Zhang, Y.; Saba, S.; Hernandez, I. Real-World Direct Comparison of the Effectiveness and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Medicare Beneficiaries with Atrial Fibrillation. Am. J. Cardiol. 2020, 126, 29–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xian, Y.; Xu, H.; O’Brien, E.C.; Shah, S.; Thomas, L.; Pencina, M.J.; Fonarow, G.C.; Olson, D.M.; Schwamm, L.H.; Bhatt, D.L.; et al. Clinical Effectiveness of Direct Oral Anticoagulants vs Warfarin in Older Patients With Atrial Fibrillation and Ischemic Stroke: Findings From the Patient-Centered Research Into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) Study. JAMA Neurol. 2019, 76, 1192–1202. [Google Scholar] [CrossRef]
- Ruiz Vargas, E.; Sposato, L.A.; Lee, S.A.W.; Hachinski, V.; Cipriano, L.E. Anticoagulation Therapy for Atrial Fibrillation in Patients With Alzheimer’s Disease. Stroke 2018, 49, 2844–2850. [Google Scholar] [CrossRef]
- Pendlebury, S.T. Direct Oral Anticoagulants and Prevention of Dementia in Nonvalvular Atrial Fibrillation. Stroke 2021, 52, 3469–3471. [Google Scholar] [CrossRef]
- Latif, F.; Nasir, M.M.; Meer, K.K.; Farhan, S.H.; Cheema, H.A.; Khan, A.B.; Umer, M.; Rehman, W.U.; Ahmad, A.; Khan, M.A.; et al. The effect of oral anticoagulants on the incidence of dementia in patients with atrial fibrillation: A systematic review and meta-analysis. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 21, 200282. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Zhao, X.; Jiang, D.; Liu, X.; Liang, Y. Association of direct oral anticoagulants and warfarin with incidence of dementia in atrial fibrillation patients: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2024, 54, 101401. [Google Scholar] [CrossRef]
- Fusco, L.; Palamà, Z.; Scarà, A.; Borrelli, A.; Robles, A.G.; Luca, G.D.M.D.; Romano, S.; Sciarra, L. Management of cerebral amyloid angiopathy and atrial fibrillation: We are still far from precision medicine. World J. Cardiol. 2024, 16, 231–239. [Google Scholar] [CrossRef]



| Study | Country | Year | Population | Design | Sample Size | Intervention | Comparator | Follow-Up (Years) | Female (%) | Dementia Diagnosis | Ischemic Stroke (%) | DM (%) | HTN (%) | HF (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cobas Paz 2020 [11] | Spain | 2020 | Common population | Retrospective | 221 | VKA/DOAC | OAC withdrawal | 2.8 | 69.7 | RGDS (5–7) | 18.1 | 18.1 | 65.6 | 18.1 |
| Orkaby 2017 [12] | US | 2017 | Veterans | Retrospective | 1215 | VKA | OAC withdrawal | 4 | 1.4 | ICD-9 code | 25.4 | 45.2 | 95 | 59.2 |
| Ouellet 2022 [13] | US | 2022 | Nursing home residents | Retrospective | 14,877 | VKA/DOAC | OAC withdrawal | 1 | 72 | CPS (5–6) | 55.2 | 38 | 89.7 | 42.5 |
| Subic 2017 [14] | Sweden | 2017 | Common population | Retrospective | 5121 | VKA | OAC withdrawal | 1.7 | 47.2 | ICD-10 code | 22.7 | 19.4 | 62.8 | 37.2 |
| Wang 2023 [15] | US | 2023 | Patients with contraindications to anticoagulation were excluded | Retrospective | 528 | VKA/DOAC | OAC withdrawal | 2 | 49.9 | MoCA (<23) | 9.8 | 27.8 | 90.9 | 37.2 |
| Fanning 2020 [21] | UK | 2020 | Common population | Retrospective | 2399 | DOAC | VKA | 0.8 | 54 | Not mentioned | 27.3 | 22.2 | 68.3 | 13.6 |
| Lin 2023 [22] | US | 2023 | Common population | Retrospective | 40,350 | DOAC | VKA | 0.5 | 59.5 | ICD-9/10 code | 42.5 | 42.9 | 92.2 | 83.5 |
| Fang 2024 [20] | Taiwan | 2024 | Common population | Retrospective | 6993 | DOAC | VKA | NR | 56.8 | ICD-9/10 code | 1.95 | 12.4 | 31.7 | 74.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, A.M.; Alfehaid, L.; Alhawas, S.; Alsuwaylihi, A.; Alarifi, A.; Al Yami, M.S. Comparison of Safety and Effectiveness Between Direct Oral Anticoagulants and Vitamin K Antagonists in Dementia Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 5758. https://doi.org/10.3390/jcm14165758
Alshehri AM, Alfehaid L, Alhawas S, Alsuwaylihi A, Alarifi A, Al Yami MS. Comparison of Safety and Effectiveness Between Direct Oral Anticoagulants and Vitamin K Antagonists in Dementia Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(16):5758. https://doi.org/10.3390/jcm14165758
Chicago/Turabian StyleAlshehri, Abdulmajeed M., Lama Alfehaid, Solaiman Alhawas, Abdulmajeed Alsuwaylihi, Abdulaziz Alarifi, and Majed S. Al Yami. 2025. "Comparison of Safety and Effectiveness Between Direct Oral Anticoagulants and Vitamin K Antagonists in Dementia Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 16: 5758. https://doi.org/10.3390/jcm14165758
APA StyleAlshehri, A. M., Alfehaid, L., Alhawas, S., Alsuwaylihi, A., Alarifi, A., & Al Yami, M. S. (2025). Comparison of Safety and Effectiveness Between Direct Oral Anticoagulants and Vitamin K Antagonists in Dementia Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(16), 5758. https://doi.org/10.3390/jcm14165758






