Comparative Analysis of IPSS, IPSS-R, and WPSS for Predicting Survival and Leukemic Transformation in Myelodysplastic Neoplasms: A Real-World Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
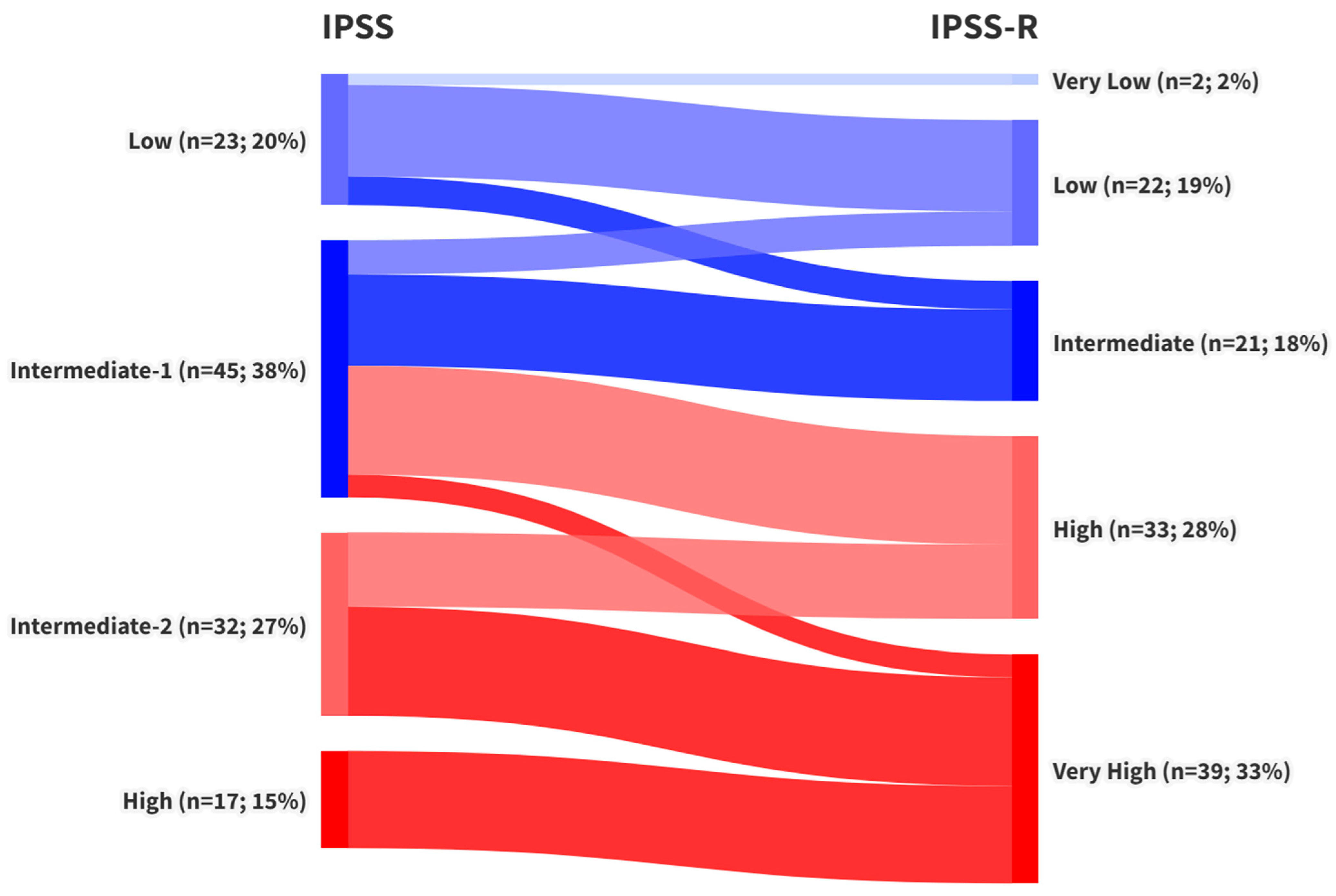
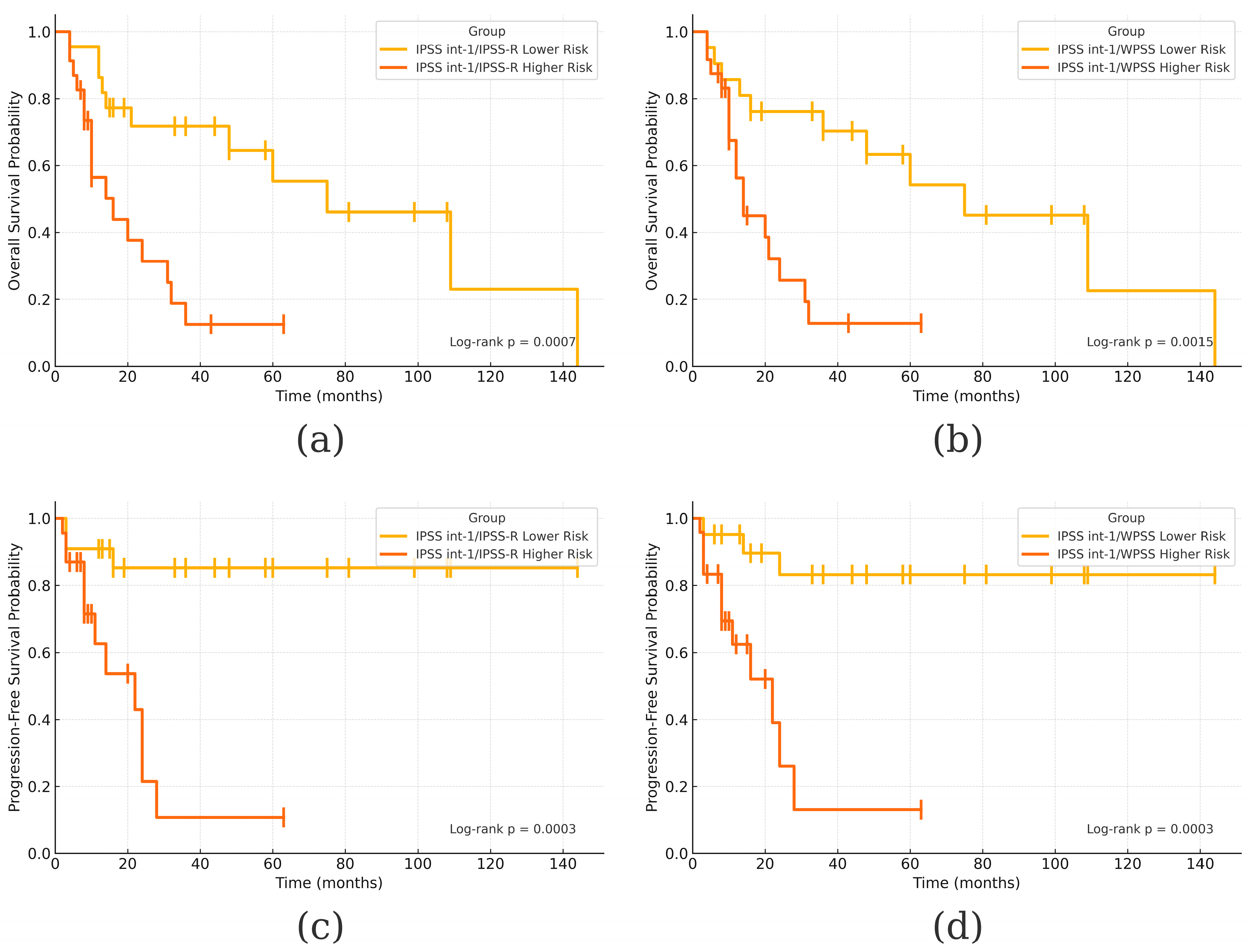
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | Acute Myeloid Leukemia |
| CI | Confidence Interval |
| Del(5q) | 5q Chromosome Deletion |
| ESAs | Erythropoietin Stimulating Agents |
| HR | Hazard Ratio |
| HR-MDS | Higher-Risk Myelodysplastic Syndromes |
| HSC | Hematopoietic Stem Cells |
| IDH1 | Isocitrate Dehydrogenase 1 |
| IPSS | International Prognostic Scoring System |
| IPSS int-1 | Intermediary-1 IPSS Group |
| IPSS-M | Molecular International Prognostic Scoring System |
| IPSS-R | International Prognostic Scoring System-Revised |
| LDH | Lactate Dehydrogenase |
| LR-MDS | Lower-Risk Myelodysplastic Syndromes |
| MDS | Myelodysplastic Syndromes |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| WHO | World Health Organization |
| WPSS | WHO Prognostic Scoring System |
References
- Sperling, A.S.; Gibson, C.J.; Ebert, B.L. The genetics of myelodysplastic syndrome: From clonal haematopoiesis to secondary leukaemia. Nat. Rev. Cancer 2017, 17, 5–19. [Google Scholar] [CrossRef]
- El Hussein, S.; Loghavi, S. The impact of clonal hierarchy and heterogeneity on phenotypic manifestations of myelodysplastic neoplasms. Cancers 2022, 14, 5690. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.J.; Shen, D.; Ding, L.; Shao, J.; Koboldt, D.C.; Chen, K.; Larson, D.E.; McLellan, M.D.; Dooling, D.; Abbott, R.; et al. Clonal architecture of secondary acute myeloid leukemia. N. Engl. J. Med. 2012, 366, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; DeZern, A.E. Therapy-related myelodysplastic syndromes and acute myeloid leukemia. Semin. Hematol. 2024, 61, 379–384. [Google Scholar] [CrossRef]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Sole, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Malcovati, L.; Germing, U.; Kuendgen, A.; Della Porta, M.G.; Pascutto, C.; Invernizzi, R.; Giagounidis, A.; Hildebrandt, B.; Bernasconi, P.; Knipp, S.; et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J. Clin. Oncol. 2007, 25, 3503–3510. [Google Scholar] [CrossRef]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Arango Ossa, J.E.; Nannya, Y.; Devlin, S.M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular international prognostic scoring system for myelodysplastic syndromes. NEJM Evid. 2022, 1, EVIDoa2200008. [Google Scholar] [CrossRef] [PubMed]
- Benton, C.B.; Khan, M.; Sallman, D.; Nazha, A.; Nogueras Gonzales, G.M.; Piao, J.; Ning, J.; Aung, F.; Al Ali, N.; Jabbour, E.; et al. Prognosis of patients with intermediate risk IPSS-R myelodysplastic syndrome indicates variable outcomes and need for models beyond IPSS-R. Am. J. Hematol. 2018, 93, 1245–1253. [Google Scholar] [CrossRef]
- Sauta, E.; Robin, M.; Bersanelli, M.; Travaglino, E.; Meggendorfer, M.; Zhao, L.-P.; Berrocal, J.C.C.; Maggioni, G.; Tentori, C.A.; Bernardi, M.; et al. Real-world validation of molecular international prognostic scoring system (IPSS-M) for myelodysplastic syndromes. Blood 2022, 140 (Suppl. S1), 1121–1124. [Google Scholar] [CrossRef]
- Aguirre, L.E.; Al Ali, N.; Ball, S.; Singh, A.M.; Jain, A.G.; Chan, O.; Schwabkey, Z.I.; Tinsley-Vance, S.M.; Kuykendall, A.; Sweet, K.; et al. Assessment of the molecular international prognostic scoring system (IPSS-M) risk stratification model in therapy-related myelodysplastic syndromes. Blood 2022, 140 (Suppl. S1), 9813–9815. [Google Scholar] [CrossRef]
- Novoa Jáuregui, S.; Palomo, L.; Pérez, A.; Tazón, B.; Montoro, M.J.; Blanco, A.; Saumell, S.; Gallur, L.; Rivero, E.; Garrido, S.; et al. IPSS-M applicability and clinical impact in decision-making process in real-life clinical practice. Blood 2022, 140 (Suppl. S1), 6966–6967. [Google Scholar] [CrossRef]
- Baer, C.; Huber, S.; Hutter, S.; Meggendorfer, M.; Nadarajah, N.; Walter, W.; Platzbecker, U.; Götze, K.S.; Kern, W.; Haferlach, T.; et al. Risk prediction in MDS: Independent validation of the IPSS-M—Ready for routine? Leukemia 2023, 37, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Qin, T.; Xu, Z.; Qu, S.; Pan, L.; Li, B.; Jia, Y.; Li, C.; Wang, H.; et al. IPSS-M has greater survival predictive accuracy compared with IPSS-R in persons ≥ 60 years with myelodysplastic syndromes. Exp. Hematol. Oncol. 2022, 11, 73. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.J.; Kim, J.; Baek, D.W.; Choi, H.; Ham, J.Y.; Chang, S.H.; Kim, J.G.; Sohn, S.K.; Moon, J.H. Prognostic impact of transfusion dependency in patients with lower-risk myelodysplastic syndrome. Clin. Lab. 2025, 71, 240820. [Google Scholar] [CrossRef]
- Braga Lemos, M.; Rodrigues, S.R.; Schroeder, T.; Kulasekararaj, A.G.; Matos, J.E.; Tang, D. Association between red blood cell transfusion dependence and burden in patients with myelodysplastic syndromes: A systematic literature review and meta-analysis. Eur. J. Haematol. 2021, 107, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, T.; Spooner, M.; Khanna, S.; Hung, K.; Toop, C.; Kutyna, M.M.; Yu, J.; Brown, A.L.; Scott, H.S.; Hahn, C.N.; et al. RBC transfusion dependency refines the molecular international prognostic scoring system for myelodysplastic syndrome. Blood Adv. 2025. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Stone, R.M.; Abaza, Y.; Al-Kali, A.; Anand, S.; Ball, B.; Bennett, J.M.; Borate, U.; Brunner, A.M.; Chai-Ho, W.; et al. Myelodysplastic syndromes, Version 2.2025 featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2025, 23, 66–75. [Google Scholar] [CrossRef]
- Fenaux, P.; Haase, D.; Santini, V.; Sanz, G.F.; Platzbecker, U.; Mey, U. Myelodysplastic syndromes: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 142–156. [Google Scholar] [CrossRef]
- Ferrara, F.; Bernardi, M. 2021 BSH guidelines for the management of adult myelodysplastic syndromes: A practical approach to a challenging disease. Br. J. Haematol. 2021, 194, 235–237. [Google Scholar] [CrossRef]
- Garcia-Manero, G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 1307–1325. [Google Scholar] [CrossRef]
- Platzbecker, U.; Santini, V.; Komrokji, R.S.; Zeidan, A.M.; Garcia-Manero, G.; Buckstein, R.; Miteva, D.; Keeperman, K.; Holot, N.; Nadal, J.A.; et al. Long-term utilization and benefit of luspatercept in transfusion-dependent, erythropoiesis-stimulating agent-refractory or -intolerant patients with lower-risk myelodysplastic syndromes with ring sideroblasts. Leukemia 2023, 37, 2314–2318. [Google Scholar] [CrossRef]
- Platzbecker, U.; Santini, V.; Fennaux, P.; Sekeres, M.A.; Savona, M.R.; Madanat, Y.F.; Díez-Campelo, M.; Valcárcel, D.; Illmer, T.; Jonášová, A.; et al. Imetelstat in patients with lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents (IMerge): A multinational, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 403, 249–260. [Google Scholar] [CrossRef]
- Symeonidis, A.; Diamantopoulos, P.; Galanopoulos, A.; Kourakli, A.; Sazakli, E.; Hatzimichael, E.; Pagoni, M.; Zikos, P.; Vassilakopoulos, T.P.; Gavrilaki, E.; et al. Lenalidomide efficacy in patients with MDS and del-5q: Real-world data from the Hellenic (Greek) National Myelodysplastic & Hypoplastic Syndromes Registry (EAKMYS). Cancers 2025, 17, 1388. [Google Scholar] [CrossRef]
- Kasprzak, A.; Kaivers, J.; Nachtkamp, K.; Haas, R.; Kobbe, G.; Gattermann, N.; Germing, U. Guidelines for myelodysplastic syndromes: Converting evidence into action? Int. J. Environ. Res. Public Health 2021, 18, 7629. [Google Scholar] [CrossRef]
- Rupp, L.; Moser, A.; Bonadies, N.; Daskalakis, M. Azacitidine treatment in MDS: A systematic literature review and meta-analysis comparing the efficacy of real world data with randomized controlled trials. Blood 2023, 142 (Suppl. S1), 6486. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellström-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- Kantarjian, H.; Issa, J.-P.J.; Rosenfeld, C.S.; Bennett, J.M.; Albitar, M.; DiPersio, J.; Klimek, V.; Slack, J.; de Castro, C.; Ravandi, F.; et al. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer 2006, 106, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; McCloskey, J.; Griffiths, E.A.; Yee, K.W.L.; Zeidan, A.M.; Al-Kali, A.; Deeg, H.J.; Patel, P.A.; Sabloff, M.; Keating, M.M.; et al. Oral decitabine–cedazuridine versus intravenous decitabine for myelodysplastic syndromes and chronic myelomonocytic leukaemia (ASCERTAIN): A registrational, randomised, crossover, pharmacokinetics, phase 3 study. Lancet Haematol. 2024, 11, e15–e26. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Loghavi, S.; Zeng, Z.; Tanaka, T.; Kim, Y.J.; Uryu, H.; Turkalj, S.; Jakobsen, N.A.; Luskin, M.R.; Duose, D.Y.; et al. A Phase Ib/II Study of Ivosidenib with Venetoclax ± Azacitidine in IDH1-Mutated Myeloid Malignancies. Blood Cancer Discov. 2023, 4, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Borate, U.; Pollyea, D.A.; Brunner, A.M.; Roncolato, F.; Garcia, J.S.; Filshie, R.; Odenike, O.; Watson, A.M.; Krishnadasan, R.; et al. A phase 1b study of venetoclax and azacitidine combination in patients with relapsed or refractory myelodysplastic syndromes. Am. J. Hematol. 2023, 98, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.C.; Mix, L.; Faustmann, P.; Weller, J.F.; Fehn, A.; Phely, L.; Riedel, A.; Vogel, W.; Faul, C.; Lengerke, C.; et al. Superior outcome of upfront allogeneic hematopoietic cell transplantation versus hypomethylating agent induction in myelodysplastic syndrome. Bone Marrow Transplant. 2024, 59, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Ciurea, S.O.; Cutler, C.; Robin, M.; Warlick, E.D.; Nakamura, R.; Brunner, A.M.; Dholaria, B.; Walker, A.R.; Kröger, N.; et al. Hematopoietic cell transplantation in the management of myelodysplastic syndrome: An evidence-based review from the American Society for Transplantation and Cellular Therapy Committee on Practice Guidelines. Transplant. Cell. Ther. 2023, 29, 71–81. [Google Scholar] [CrossRef]
- Cazzola, M. Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 383, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Germing, U.; Kobbe, G.; Haas, R.; Gattermann, N. Myelodysplastic syndromes: Diagnosis, prognosis, and treatment. Dtsch. Arztebl. Int. 2013, 110, 783–790. [Google Scholar] [CrossRef]
- Sekeres, M.A.; Taylor, J. Diagnosis and treatment of myelodysplastic syndromes: A review. JAMA 2022, 328, 872–880. [Google Scholar] [CrossRef]
- Bell, J.A.; Galaznik, A.; Blazer, M.; Farrelly, E.; Ogbonnaya, A.; Raju, A.; Eaddy, M.; Fram, R.J.; Faller, D.V. Transfusion-free interval is associated with improved survival in patients with higher-risk myelodysplastic syndromes engaged in routine care. Leuk. Lymphoma 2019, 60, 49–59. [Google Scholar] [CrossRef]
- Visconte, V.; Tiu, R.; Rogers, H. Pathogenesis of myelodysplastic syndromes: An overview of molecular and non-molecular aspects of the disease. Blood Res. 2014, 49, 216–227. [Google Scholar] [CrossRef]
- Bejar, R. Clinical and genetic predictors of prognosis in myelodysplastic syndromes. Haematologica 2014, 99, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, K.; Garcia-Manero, G.; Sardesai, S.; Oh, J.; Vigil, C.E.; Pierce, S.; Lei, X.; Shan, J.; Kantarjian, H.M.; Suarez-Almazor, M.E. Association of comorbidities with overall survival in myelodysplastic syndrome: Development of a prognostic model. J. Clin. Oncol. 2011, 29, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Rozema, J.; Hoogendoorn, M.; Kibbelaar, R.; van den Berg, E.; Veeger, N.; van Roon, E. Comorbidities and malignancies negatively affect survival in myelodysplastic syndromes: A population-based study. Blood Adv. 2021, 5, 1344–1351. [Google Scholar] [CrossRef]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the Cancer and Leukemia Group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef]
- Jain, A.G.; Ball, S.; Aguirre, L.; Al Ali, N.; Kaldas, D.; Tinsley-Vance, S.; Kuykendall, A.; Chan, O.; Sweet, K.; Lancet, J.E.; et al. Patterns of lower risk myelodysplastic syndrome progression: Factors predicting progression to high-risk myelodysplastic syndrome and acute myeloid leukemia. Haematologica 2024, 109, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Moreno Berggren, D.; Folkvaljon, Y.; Engvall, M.; Sundberg, J.; Lambe, M.; Antunovic, P.; Garelius, H.; Lorenz, F.; Nilsson, L.; Rasmussen, B.; et al. Prognostic scoring systems for myelodysplastic syndromes (MDS) in a population-based setting: A report from the Swedish MDS register. Br. J. Haematol. 2018, 181, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Bektas, Ö.; Üner, A.; Eliaçık, E.; Uz, B.; Işık, A.; Etgül, S.; Bozkurt, S.; Haznedaroğlu, İ.C.; Göker, H.; Sayınalp, N.; et al. Comparison of myelodysplastic syndrome prognostic scoring systems. Turk. J. Hematol. 2016, 33, 119–126. [Google Scholar] [CrossRef]
- Warlick, E.D.; Hirsch, B.A.; Nguyen, P.L.; Cioc, A.; Roesler, M.A.; Fonstad, R.; Miller, J.S.; Weisdorf, D.J.; Ross, J.A. Comparison of IPSS and IPSS-R scoring in a population-based myelodysplastic syndromes (MDS) study. Blood 2012, 120, 3841. [Google Scholar] [CrossRef]
- Selda, K.; Zeynep, G.; Mehmet Ali, O. The prediction of survival by standard prognostic scoring systems and comorbidity indexes in myelodysplastic syndrome: A hospital-based study. Cancer Ther. Oncol. Int. J. 2021, 19, 556024. [Google Scholar] [CrossRef]


| n | % | Median | |
|---|---|---|---|
| Age | 70 [31–89] | ||
| 30–39 | 4 | 3.5 | |
| 40–49 | 4 | 3.5 | |
| 50–59 | 6 | 5 | |
| 60–69 | 40 | 34 | |
| 70–79 | 48 | 41 | |
| ≥80 | 15 | 13 | |
| Sex | |||
| Male | 58 | 49.5 | |
| Female | 59 | 50.5 | |
| Neutrophil count (×109/L) | 1.89 [0.11–5.31] | ||
| >1.8 | 60 | 30 | |
| 0.8–1.8 | 32 | 49.5 | |
| <0.8 | 25 | 21.5 | |
| Hemoglobin (g/L) | 7.3 [4–14.5] | ||
| ≥100 | 18 | 15.5 | |
| 80–100 | 27 | 23 | |
| <80 | 72 | 61.5 | |
| Platelets (×109/L) | 113 [5–697] | ||
| >100 | 63 | 54 | |
| 50–100 | 33 | 28 | |
| <50 | 21 | 18 | |
| Transfusion dependence 1 | 86 | 73.5 | |
| Medullary Blasts (%) | |||
| 0–2 | 26 | 22 | |
| >2–<5 | 17 | 14.5 | |
| 5–10 | 36 | 31 | |
| >10 | 38 | 32.5 | |
| WHO Morphological Category 2 | |||
| RA | 4 | 3 | |
| RARS | 15 | 13 | |
| RCMD | 24 | 20.5 | |
| del5q | 8 | 7 | |
| RAEB-1 | 27 | 23 | |
| RAEB-2 | 38 | 32.5 | |
| Hypoplastic | 1 | 1 |
| Category | n | % |
|---|---|---|
| Very Good | 2 | 1.7 |
| Good | 69 | 59 |
| Intermediate | 32 | 27.3 |
| Poor | 5 | 4.3 |
| Very Poor | 9 | 7.7 |
| Category | OS Rate | OS Median | PFS Rate | PFS Median |
|---|---|---|---|---|
| Global | 42/117 (35.9%) | 20 months | 50/117 (42.7%) | 35 months |
| IPSS Lower Risk | 36/68 (53%) | 60 months | 17/67 (25%) | Not reached |
| IPSS-R Lower Risk | 29/45 (64.4%) | 109 months | 5/45 (11.1%) | Not reached |
| WPSS Lower Risk | 28/45 (62.2%) | 109 months | 6/45 (13.3%) | Not reached |
| IPSS Higher Risk | 6/49 (12.3%) | 12 months | 33/49 (67.4%) | 9 months |
| IPSS-R Higher Risk | 13/72 (18%) | 12 months | 45/72 (62.5%) | 11 months |
| WPSS Higher Risk | 14/72 (19.4%) | 12 months | 44/72 (61.1%) | 12 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapadat, M.-E.; Stanca, O.; Berbec, N.M.; Angelescu, S.; Triantafyllidis, I.N.; Ciobanu, A.M.; Negotei, C.; Barta, C.T.; Halcu, G.; Saguna, C.; et al. Comparative Analysis of IPSS, IPSS-R, and WPSS for Predicting Survival and Leukemic Transformation in Myelodysplastic Neoplasms: A Real-World Single-Center Experience. J. Clin. Med. 2025, 14, 5757. https://doi.org/10.3390/jcm14165757
Lapadat M-E, Stanca O, Berbec NM, Angelescu S, Triantafyllidis IN, Ciobanu AM, Negotei C, Barta CT, Halcu G, Saguna C, et al. Comparative Analysis of IPSS, IPSS-R, and WPSS for Predicting Survival and Leukemic Transformation in Myelodysplastic Neoplasms: A Real-World Single-Center Experience. Journal of Clinical Medicine. 2025; 14(16):5757. https://doi.org/10.3390/jcm14165757
Chicago/Turabian StyleLapadat, Mihai-Emilian, Oana Stanca, Nicoleta Mariana Berbec, Silvana Angelescu, Irina Nicoleta Triantafyllidis, Anca Mariana Ciobanu, Cristina Negotei, Cristian Tudor Barta, Georgian Halcu, Carmen Saguna, and et al. 2025. "Comparative Analysis of IPSS, IPSS-R, and WPSS for Predicting Survival and Leukemic Transformation in Myelodysplastic Neoplasms: A Real-World Single-Center Experience" Journal of Clinical Medicine 14, no. 16: 5757. https://doi.org/10.3390/jcm14165757
APA StyleLapadat, M.-E., Stanca, O., Berbec, N. M., Angelescu, S., Triantafyllidis, I. N., Ciobanu, A. M., Negotei, C., Barta, C. T., Halcu, G., Saguna, C., Popovici, C. E., Bordea, A.-M., Oprea, M. M., & Colita, A. (2025). Comparative Analysis of IPSS, IPSS-R, and WPSS for Predicting Survival and Leukemic Transformation in Myelodysplastic Neoplasms: A Real-World Single-Center Experience. Journal of Clinical Medicine, 14(16), 5757. https://doi.org/10.3390/jcm14165757






