Coronary Artery Bypass Grafting Versus Percutaneous Coronary Intervention for Left Main Coronary Artery Disease—Long-Term Outcomes
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
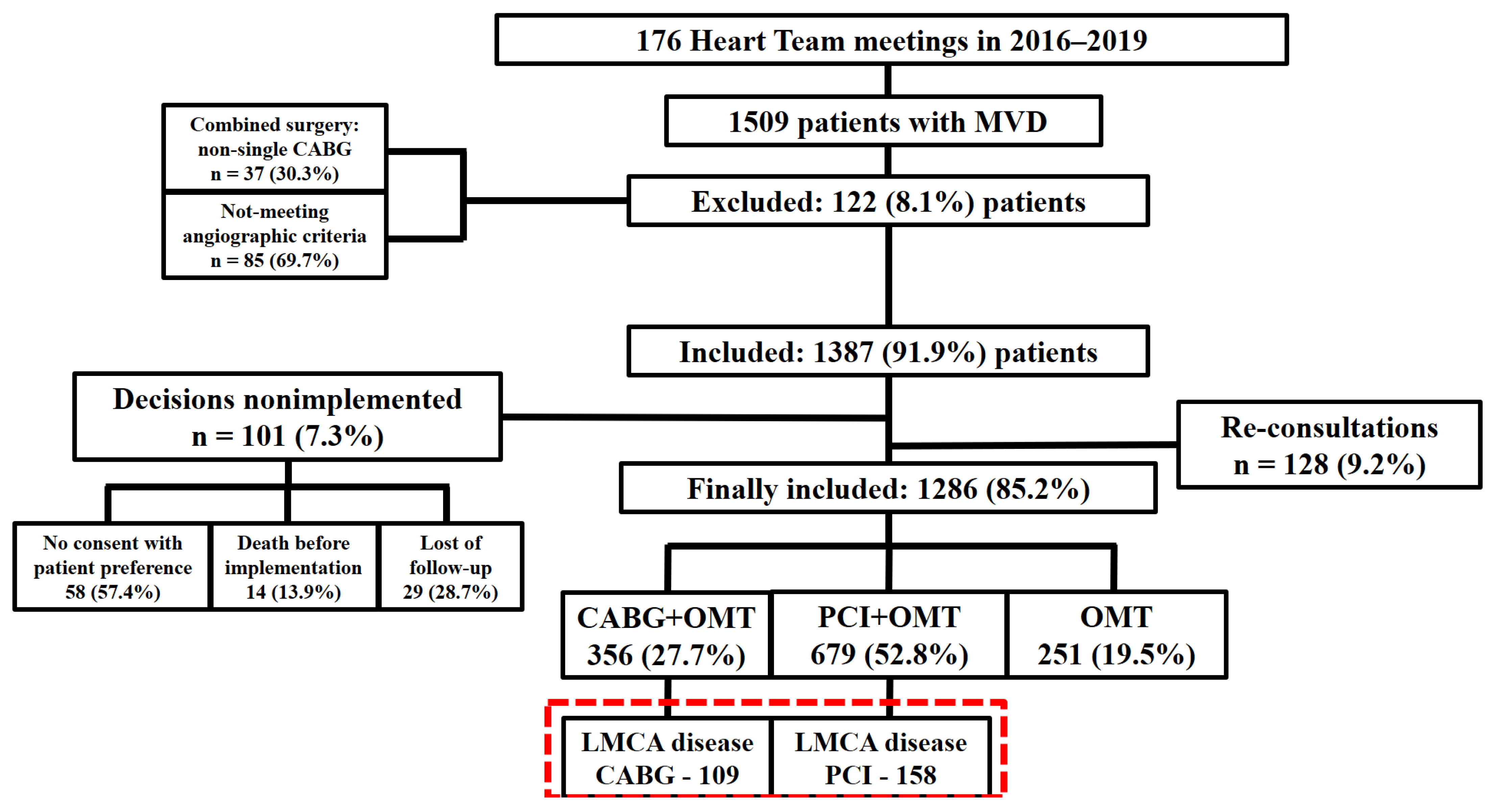
3. Results Study Population
3.1. Angiographic Parameters
3.2. Medications on Admission and at Discharge
3.3. Outcomes
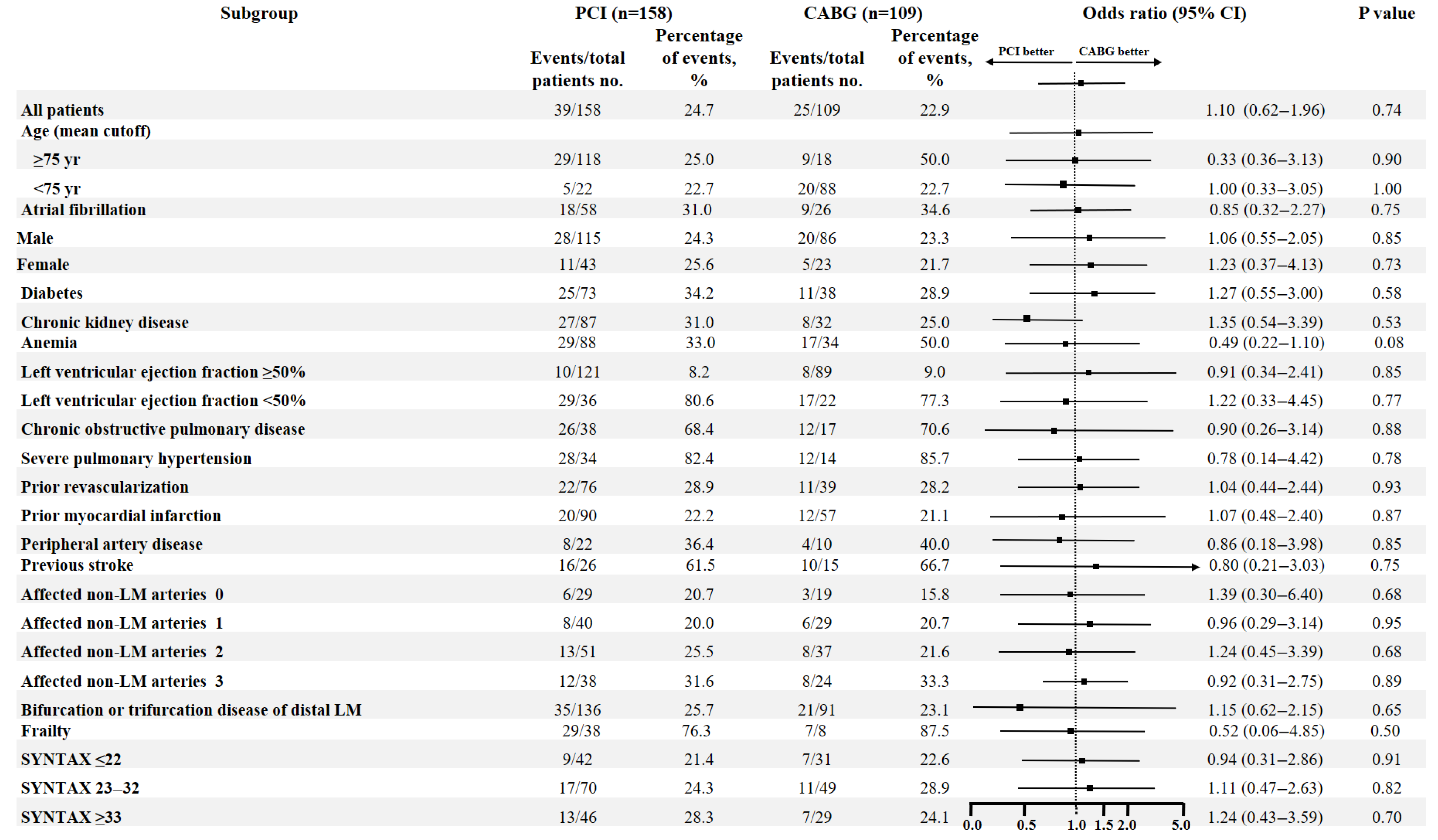
3.4. Propensity Score Matching Analysis
3.5. Multivariable Cox Proportional Hazards Model
4. Discussion
4.1. Main Findings
4.2. Interpretation of Main Findings
4.3. Other Studies in the Field and Novelty of the Study
4.4. Interpretation of the Main Findings
4.5. Limitations and Conclusions
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collet, C.; Capodanno, D.; Onuma, Y.; Banning, A.; Stone, G.W.; Taggart, D.P.; Sabik, J.; Serruys, P.W. Left main coronary artery disease: Pathophysiology, diagnosis, and treatment. Nat. Rev. Cardiol. 2018, 15, 321–331. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Presutti, D.G.; Picardi, E.; Moretti, C.; Omedè, P.; Sciuto, F.; Novara, M.; Yan, A.T.; Goodman, S.; Mahajan, N.; et al. Prevalence and non-invasive predictors of left main or three-vessel coronary disease: Evidence from a collaborative international meta-analysis including 22,740 patients. Heart 2012, 98, 914–919. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; A Byrne, R.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Hoole, S.P.; Bambrough, P. Recent advances in percutaneous coronary intervention. Heart 2020, 106, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Spirito, A.; Cangialosi, P.; Cao, D.; Nicolas, J.; Mehran, R. Recent Advances in Antiplatelet Therapy in Complex Percutaneous Coronary Intervention. Interv. Cardiol. Clin. 2022, 11, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.-C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown, W.M.; Lembo, N.J.; et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N. Engl. J. Med. 2019, 381, 1820–1830. [Google Scholar] [CrossRef]
- Wang, R.; Serruys, P.W.; Gao, C.; Hara, H.; Takahashi, K.; Ono, M.; Kawashima, H.; O’lEary, N.; Holmes, D.R.; Witkowski, A.; et al. Ten-year all-cause death after percutaneous or surgical revascularization in diabetic patients with complex coronary artery disease. Eur. Heart J. 2021, 43, 56–67. [Google Scholar] [CrossRef]
- Park, D.W.; Ahn, J.M.; Park, H.; Yun, S.C.; Kang, D.Y.; Lee, P.H.; Kim, Y.H.; Lim, D.S.; Rha, S.W.; Park, G.M.; et al. Ten-Year Outcomes After Drug-Eluting Stents Versus Coronary Artery Bypass Grafting for Left Main Coronary Disease: Extended Follow-Up of the PRECOMBAT Trial. Circulation 2020, 141, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Thuijs, D.; Kappetein, A.P.; Serruys, P.W.; Mohr, F.-W.; Morice, M.-C.; Curzen, N.; Davierwala, P.; Noack, T.; Milojevic, M.; Dawkins, K.D.; et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019, 394, 1325–1334. [Google Scholar] [CrossRef]
- Gallo, M.; Blitzer, D.; Laforgia, P.L.; Doulamis, I.P.; Perrin, N.; Bortolussi, G.; Guariento, A.; Putzu, A. Percutaneous coronary intervention versus coronary artery bypass graft for left main coronary artery disease: A meta-analysis. J. Thorac. Cardiovasc. Surg. 2022, 163, 94–105.e15. [Google Scholar] [CrossRef]
- Watanabe, H.; Shiomi, H.; Morimoto, T.; Furukawa, Y.; Nakagawa, Y.; Ando, K.; Kadota, K.; Tazaki, J.; Natsuaki, M.; Minatoya, K.; et al. Percutaneous coronary intervention versus coronary arterial bypass grafting in patients with multi-vessel coronary revascularization (from the CREDO-Kyoto PCI/CABG registry/cohort-2). Catheter. Cardiovasc. Interv. 2020, 96, 42–51. [Google Scholar] [CrossRef]
- Tam, D.Y.; Fang, J.; Rocha, R.V.; Rao, S.V.; Dzavik, V.; Lawton, J.; Austin, P.C.; Gaudino, M.; Fremes, S.E.; Lee, D.S. Real-World Examination of Revascularization Strategies for Left Main Coronary Disease in Ontario, Canada. JACC Cardiovasc. Interv. 2023, 16, 277–288. [Google Scholar] [CrossRef]
- Palmerini, T.; Serruys, P.; Kappetein, A.P.; Genereux, P.; Della Riva, D.; Reggiani, L.B.; Christiansen, E.H.; Holm, N.R.; Thuesen, L.; Makikallio, T.; et al. Clinical outcomes with percutaneous coronary revascularization vs coronary artery bypass grafting surgery in patients with unprotected left main coronary artery disease: A meta-analysis of 6 randomized trials and 4,686 patients. Am. Heart J. 2017, 190, 54–63. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Bergmark, B.A.; Murphy, S.A.; O’GAra, P.T.; Smith, P.K.; Serruys, P.W.; Kappetein, A.P.; Park, S.-J.; Park, D.-W.; Christiansen, E.H.; et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease: An individual patient data meta-analysis. Lancet 2021, 398, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.J.; Chinnakondepalli, K.; Magnuson, E.A.; Kandzari, D.E.; Puskas, J.D.; Ben-Yehuda, O.; van Es, G.A.; Taggart, D.P.; Morice, M.C.; Lembo, N.J.; et al. Quality-of-Life After Everolimus-Eluting Stents or Bypass Surgery for Left-Main Disease: Results From the EXCEL Trial. J. Am. Coll. Cardiol. 2017, 70, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.R.; Makikallio, T.; Lindsay, M.M.; Spence, M.S.; Erglis, A.; A Menown, I.B.; Trovik, T.; Kalinauskas, G.; Mogensen, L.J.H.; Nielsen, P.H.; et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: Updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet 2020, 395, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, J.A.; Kunutsor, S.K.; Niemela, M.; Kervinen, K.; Thuesen, L.; Mäkikallio, T.H. All-cause mortality and major cardiovascular outcomes comparing percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: A meta-analysis of short-term and long-term randomised trials. Open Heart 2017, 4, e000638. [Google Scholar] [CrossRef]
- Bangalore, S.; Guo, Y.; Samadashvili, Z.; Blecker, S.; Xu, J.; Hannan, E.L. Everolimus-eluting stents or bypass surgery for multivessel coronary disease. N. Engl. J. Med. 2015, 372, 1213–1222. [Google Scholar] [CrossRef]
- Stone, G.W.; Sabik, J.F.; Serruys, P.W.; Simonton, C.A.; Généreux, P.; Puskas, J.; Kandzari, D.E.; Morice, M.-C.; Lembo, N.; Brown, W.M.I.; et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N. Engl. J. Med. 2016, 375, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Milojevic, M.; Head, S.J.; Parasca, C.A.; Serruys, P.W.; Mohr, F.W.; Morice, M.C.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; Dawkins, K.D.; et al. Causes of Death Following PCI Versus CABG in Complex CAD: 5-Year Follow-Up of SYNTAX. J. Am. Coll. Cardiol. 2016, 67, 42–55. [Google Scholar] [CrossRef]
- Serruys, P.W.; Morice, M.C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; Van Den Brand, M.; Bass, E.J.; et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef]
- Ahn, J.M.; Roh, J.H.; Kim, Y.H.; Park, D.W.; Yun, S.C.; Lee, P.H.; Chang, M.; Park, H.W.; Lee, S.W.; Lee, C.W.; et al. Randomized Trial of Stents Versus Bypass Surgery for Left Main Coronary Artery Disease: 5-Year Outcomes of the PRECOMBAT Study. J. Am. Coll. Cardiol. 2015, 65, 2198–2206. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Genereux, P.; Chen, S.; Dressler, O.; Ben-Yehuda, O.; Morice, M.-C.; Puskas, J.D.; Taggart, D.P.; Kandzari, D.E.; Crowley, A.; et al. Left Main Coronary Artery Disease Revascularization According to the SYNTAX Score. Circ. Cardiovasc. Interv. 2019, 12, e008007. [Google Scholar] [CrossRef]
- Head, S.J.; Milojevic, M.; Daemen, J.; Ahn, J.-M.; Boersma, E.; Christiansen, E.H.; Domanski, M.J.; E Farkouh, M.; Flather, M.; Fuster, V.; et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: A pooled analysis of individual patient data. Lancet 2018, 391, 939–948. [Google Scholar] [CrossRef]
- Park, D.W.; Ahn, J.M.; Yun, S.C.; Yoon, Y.-H.; Kang, D.-Y.; Lee, P.H.; Lee, S.-W.; Park, S.-W.; Seung, K.B.; Gwon, H.-C.; et al. 10-Year Outcomes of Stents Versus Coronary Artery Bypass Grafting for Left Main Coronary Artery Disease. J. Am. Coll. Cardiol. 2018, 72, 2813–2822. [Google Scholar] [CrossRef]
- Paszek, E.; Zajdel, W.; Musialek, P.; Sokołowski, A.; Guzik, B.; Kabłak-Ziembicka, A.; Niewiara, Ł.; Pankowska, M.; Mielimonka, A.; Żmudka, K. Percutaneous management of long and diffused coronary lesions using newer generation drug-eluting stents in routine clinical practice: Long-term outcomes and complication predictors. Pol. Arch. Intern. Med. 2019, 129, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Parasca, C.A.; Head, S.J.; Milojevic, M.; Mack, M.J.; Serruys, P.W.; Morice, M.C.; Mohr, F.W.; Feldman, T.E.; Colombo, A.; Dawkins, K.D.; et al. Incidence, Characteristics, Predictors, and Outcomes of Repeat Revascularization After Percutaneous Coronary Intervention and Coronary Artery Bypass Grafting: The SYNTAX Trial at 5 Years. JACC Cardiovasc. Interv. 2016, 9, 2493–2507. [Google Scholar] [CrossRef] [PubMed]
- Jonik, S.; Marchel, M.; Pedzich-Placha, E.; Pietrasik, A.; Rdzanek, A.; Huczek, Z.; Kochman, J.; Budnik, M.; Piątkowski, R.; Scisło, P.; et al. Optimal Management of Patients with Severe Coronary Artery Disease following Multidisciplinary Heart Team Approach-Insights from Tertiary Cardiovascular Care Center. Int. J. Environ. Res. Public Health 2022, 19, 3933. [Google Scholar] [CrossRef]
- Tanaka, A.; Giustino, G.; Briede, I.; Sawaya, F.J.; Daemen, J.; Kawamoto, H.; Meliga, E.; D’AScenzo, F.; Cerrato, E.; Stefanini, G.G.; et al. New-generation drug-eluting stents for left main coronary artery disease according to the EXCEL trial enrollment criteria: Insights from the all-comers, international, multicenter DELTA-2 registry. Int. J. Cardiol. 2019, 280, 30–37. [Google Scholar] [CrossRef]
- Head, S.J.; Kaul, S.; Mack, M.J.; Serruys, P.W.; Taggart, D.P.; Holmes, D.R.; Leon, M.B.; Marco, J.; Bogers, A.J.J.C.; Kappetein, A.P. The rationale for Heart Team decision-making for patients with stable, complex coronary artery disease. Eur. Heart J. 2013, 34, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Serruys, P.W.; Sabik, J.F., 3rd; Mehran, R.; Maehara, A.; Puskas, J.D.; Simonton, C.A.; Lembo, N.J.; Kandzari, D.E.; Morice, M.C.; et al. Mortality After Repeat Revascularization Following PCI or CABG for Left Main Disease: The EXCEL Trial. JACC Cardiovasc. Interv. 2020, 13, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, L.; Pastena, P.; Cacciatore, S.; Betti, M.; Biondi-Zoccai, G.; D’aScenzo, F.; De Ferrari, G.M.; De Filippo, O.; Versaci, F.; Sciarretta, S.; et al. One-Year Prognostic Differences and Management Strategies between ST-Elevation and Non-ST-Elevation Myocardial Infarction: Insights from the PRAISE Registry. Am. J. Cardiovasc. Drugs 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, M.; Alsabbagh, A.; Kuntze, T.; Lauer, B.; Ohlow, M.A. Impact of Hierarchy on Multidisciplinary Heart-Team Recommendations in Patients with Isolated Multivessel Coronary Artery Disease. J. Clin. Med. 2019, 8, 1490. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Overall (267) | CABG (109) | PCI (158) | p Value | |
|---|---|---|---|---|---|
| Preprocedural | Age, years; median (Q1; Q3) | 74 (67; 78) | 71 (62; 76) | 76 (69; 79) | <0.001 |
| Gender, male; n (%) | 201 (75.3) | 86 (78.9) | 115 (72.8) | 0.26 | |
| BMI, kg/m2; median (Q1; Q3) | 28.2 (26.3; 29.7) | 28.2 (26.8; 29.0) | 28.1 (25.6; 30.1) | 0.92 | |
| Frailty; n (%) | 46 (17.2) | 8 (7.3) | 38 (24.1) | <0.001 | |
| Current smoking; n (%) | 68 (25.5) | 24 (22.0) | 44 (27.8) | 0.28 | |
| COPD; n (%) | 55 (20.6) | 17 (15.6) | 38 (24.1) | 0.09 | |
| Diabetes; n (%) | 111 (41.6) | 38 (34.9) | 73 (46.2) | 0.07 | |
| with insulin; n (%) | 51 (19.1) | 16 (14.7) | 35 (22.2) | 0.13 | |
| Hypertension; n (%) | 237 (88.8) | 92 (84.4) | 145 (91.8) | 0.06 | |
| Severe PH; n (%) | 48 (18.0) | 14 (12.8) | 34 (21.5) | 0.07 | |
| Dyslipidemia; n (%) | 214 (80.1) | 80 (73.4) | 134 (84.8) | 0.02 | |
| Congestive heart failure; n (%) | 58 (21.7) | 22 (20.2) | 36 (22.8) | 0.61 | |
| LVEF, %; median (Q1; Q3) | 34.0 (30.0; 50.0) | 34.0 (31.0; 50.0) | 33.0 (29.0; 50.0) | 0.24 | |
| CKD; n (%) | 119 (44.6) | 32 (29.4) | 87 (55.1) | <0.001 | |
| Atrial fibrillation; n (%) | 84 (31.5) | 26 (23.9) | 58 (36.7) | 0.03 | |
| Anemia; n (%) | 120 (44.9) | 34 (31.2) | 88 (55.7) | <0.001 | |
| Prior MI; n (%) | 147 (55.1) | 57 (52.3) | 90 (57.0) | 0.45 | |
| Prior revascularization; n (%) | 115 (43.1) | 39 (35.8) | 76 (48.1) | 0.05 | |
| Indication; n (%) | |||||
| CCS | 93 (34.8) | 43 (39.4) | 50 (31.6) | 0.19 | |
| ACS | 174 (65.2) | 66 (60.6) | 108 (68.4) | 0.19 | |
| STEMI | 11 (4.1) | 2 (1.8) | 9 (5.7) | 0.12 | |
| NSTEMI/UA | 163 (61.0) | 64 (58.7) | 99 (62.7) | 0.52 | |
| PAD; n (%) | 32 (12.0) | 10 (9.2) | 22 (13.9) | 0.24 | |
| Prior stroke/TIA; n (%) | 41 (15.4) | 15 (13.8) | 26 (16.5) | 0.55 | |
| Active cancer; n (%) | 15 (5.6) | 0 (0.0) | 15 (9.5) | <0.001 | |
| EuroSCORE II, %; median (Q1; Q3) | 5.8 (3.6; 9.9) | 3.8 (3.2; 5.3) | 9.8 (6.0; 10.5) | <0.001 | |
| STS score, %; median (Q1; Q3) | 3.7 (2.3; 6.4) | 2.5 (2.1; 3.3) | 6.1 (3.9; 6.7) | <0.001 | |
| Procedural | Complete revascularization; n (%) | 149 (55.8) | 77 (70.6) | 72 (45.6) | <0.001 |
| Angiographic Parameters | Overall (267) | CABG (109) | PCI (158) | p Value |
|---|---|---|---|---|
| Qualifying LM lesion | ||||
| LM coronary segment | 259 (97.0) | 106 (97.2) | 153 (96.8) | 0.85 |
| LM equivalent disease * | 8 (3.0) | 3 (2.8) | 5 (3.2) | 0.85 |
| Bifurcation or trifurcation disease of distal LM; n (%) | 227 (85.0) | 91 (83.5) | 136 (86.1) | 0.56 |
| LM considered as culprit lesion in ACS, n (%) | 29/174 (16.7) | 12/66 (18.2) | 17/108 (15.7) | 0.68 |
| Affected non-LM arteries, n (%) | ||||
| 0 | 48 (18.0) | 19 (17.4) | 29 (18.4) | 0.0971 |
| 1 | 69 (25.8) | 29 (26.6) | 40 (25.3) | |
| 2 | 88 (33.0) | 37 (33.9) | 51 (32.3) | |
| 3 | 62 (23.2) | 24 (22.0) | 38 (24.1) | |
| Severe calcification; n (%) | 86 (32.2) | 34 (31.2) | 52 (32.9) | 0.77 |
| Total occlusion; n (%) | 70 (26.2) | 25 (22.9) | 45 (28.5) | 0.31 |
| Number of stents implanted per patient; median (Q1; Q3) | NA | NA | 2 (1;3) | NA |
| Number of conduits (arterial and venous) per patient; median (Q1; Q3) | NA | 3 (2;3) | NA | NA |
| IVUS utilization; n (%) | NA | NA | 154 (97.5) | NA |
| FFR utilization; n (%) | NA | NA | 48 (30.4) | NA |
| OCT utilization; n (%) | NA | NA | 23 (14.6) | NA |
| Rotational atherectomy; n (%) | NA | NA | 11 (7.0) | NA |
| SYNTAX score; median (Q1;Q3) | 34.0 (28.0; 38.8) | 34.0 (29.0; 41.0) | 33.8 (23.8; 37.5) | 0.03 |
| Medications on Admission | Overall (267) | CABG (109) | PCI (158) | p Value |
| Statin; n (%) | 231 (86.5) | 81 (74.3) | 150 (94.9) | <0.001 |
| ACE inhibitor; n (%) | 196 (63.3) | 62 (56.9) | 107 (67.7) | 0.07 |
| ARB; n (%) | 65 (24.3) | 31 (28.4) | 34 (21.5) | 0.20 |
| Beta-blocker; n (%) | 208 (77.9) | 85 (78.0) | 123 (77.8) | 0.98 |
| Medications at discharge | Overall (267) | CABG (109) | PCI (158) | p value |
| Aspirin; n (%) | 234 (87.6) | 101 (92.7) | 133 (84.2) | 0.04 |
| P2Y12 inhibitors; n (%) | 145 (54.3) | 21 (19.3) | 124 (78.5) | <0.001 |
| Clopidogrel, n (%) | 88 (60.7) | 19 (90.5) | 69 (55.6) | <0.001 |
| Ticagrelol, n (%) | 37 (25.5) | 1 (4.8) | 36 (29.0) | <0.001 |
| Prasugrel, n (%) | 20 (13.8) | 1 (4.8) | 19 (15.3) | <0.001 |
| Statin; n (%) | 243 (91.0) | 91 (83.5) | 152 (96.2) | <0.001 |
| ACE inhibitor; n (%) | 191 (71.5) | 74 (67.9) | 117 (74.1) | 0.27 |
| ARB; n (%) | 70 (26.2) | 29 (26.6) | 41 (25.9) | 0.91 |
| Beta-blocker; n (%) | 213 (79.8) | 78 (71.6) | 135 (85.4) | 0.006 |
| Loop diuretic; n (%) | 144 (53.9) | 80 (73.4) | 64 (40.5) | <0.001 |
| Aldosterone antagonist; n (%) | 57 (21.3) | 13 (11.9) | 44 (27.8) | 0.002 |
| Endpoints | Overall (267) | CABG (109) | PCI (158) | RR PCI vs. CABG (95% CI) | p Value |
|---|---|---|---|---|---|
| All-cause death, n (%) | 64 (24.0) | 25 (22.9) | 39 (24.7) | 1.08 (0.69–1.67) | 0.74 |
| MACCE, n (%) | 182 (68.1) | 60 (55.0) | 122 (77.2) | 1.40 (1.16–1.70) | <0.001 |
| MI, n (%) | 33 (12.4) | 8 (7.3) | 25 (15.8) | 2.16 (1.01–4.60) | 0.04 |
| Stroke, n (%) | 18 (6.7) | 12 (11.0) | 6 (3.8) | 0.34 (0.13–0.89) | 0.02 |
| Periprocedural, n (%) | 4 (1.5) | 3 (2.8) | 1 (0.6) | 0.27 (0.10–0.75) | 0.01 |
| Repeat revascularization, n (%) | 67 (25.1) | 15 (13.8) | 52 (32.9) | 2.40 (1.45–4.10) | <0.001 |
| In-hospital mortality, n (%) | 11 (4.1) | 5 (4.6) | 6 (3.8) | 0.83 (0.26–2.65) | 0.75 |
| Postprocedural hospital stay, days; median (Q1; Q3) | 4 (1;7) | 6 (4;11) | 1 (1;3) | - | <0.001 |
| Outcome | Number of Patients with Event | Event Rate (%/yr) | Hazard Ratio (95% CI) | p Value |
|---|---|---|---|---|
| All-cause death, n (%) | ||||
| PCI | 12/53 (22.6) | 4.5 | 1.09 (0.43–2.75) | 0.82 |
| CABG | 11/53 (20.8) | 4.2 | Reference | |
| MACCE, n (%) | ||||
| PCI | 36/53 (67.9) | 13.6 | 1.33 (0.60–2.94) | 0.08 |
| CABG | 27/53 (50.9) | 11.0 | Reference | |
| MI, n (%) | ||||
| PCI | 7/53 (13.2) | 2.6 | 1.17 (0.48–6.37) | 0.34 |
| CABG | 4/53 (7.5) | 1.5 | Reference | |
| Stroke, n (%) | ||||
| PCI | 2/53 (3.8) | 0.75 | 0.33 (0.06–1.73) | 0.14 |
| CABG | 6/53 (11.3) | 2.3 | Reference | |
| Repeat revascularization, n (%) | ||||
| PCI | 15/53 (28.3) | 5.7 | 2.5 (0.88–7.06) | 0.03 |
| CABG | 6/53 (11.3) | 2.3 | Reference | |
| In-hospital mortality, n (%) | ||||
| PCI | 1/53 (1.9) | 0.38 | 0.33 (0.03–3.31) | 0.31 |
| CABG | 3/53 (5.7) | 1.1 | Reference | |
| Postprocedural hospital stay, days; mean (SD) | ||||
| PCI | 1.6 (1.1) | - | - | <0.001 |
| CABG | 8.5 (4.4) | - | Reference | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonik, S.; Gumiężna, K.; Baruś, P.; Wilimski, R.; Kuśmierczyk, M.; Opolski, G.; Grabowski, M.; Kochman, J.; Huczek, Z.; Mazurek, T. Coronary Artery Bypass Grafting Versus Percutaneous Coronary Intervention for Left Main Coronary Artery Disease—Long-Term Outcomes. J. Clin. Med. 2025, 14, 5747. https://doi.org/10.3390/jcm14165747
Jonik S, Gumiężna K, Baruś P, Wilimski R, Kuśmierczyk M, Opolski G, Grabowski M, Kochman J, Huczek Z, Mazurek T. Coronary Artery Bypass Grafting Versus Percutaneous Coronary Intervention for Left Main Coronary Artery Disease—Long-Term Outcomes. Journal of Clinical Medicine. 2025; 14(16):5747. https://doi.org/10.3390/jcm14165747
Chicago/Turabian StyleJonik, Szymon, Karolina Gumiężna, Piotr Baruś, Radosław Wilimski, Mariusz Kuśmierczyk, Grzegorz Opolski, Marcin Grabowski, Janusz Kochman, Zenon Huczek, and Tomasz Mazurek. 2025. "Coronary Artery Bypass Grafting Versus Percutaneous Coronary Intervention for Left Main Coronary Artery Disease—Long-Term Outcomes" Journal of Clinical Medicine 14, no. 16: 5747. https://doi.org/10.3390/jcm14165747
APA StyleJonik, S., Gumiężna, K., Baruś, P., Wilimski, R., Kuśmierczyk, M., Opolski, G., Grabowski, M., Kochman, J., Huczek, Z., & Mazurek, T. (2025). Coronary Artery Bypass Grafting Versus Percutaneous Coronary Intervention for Left Main Coronary Artery Disease—Long-Term Outcomes. Journal of Clinical Medicine, 14(16), 5747. https://doi.org/10.3390/jcm14165747






