Long-Term Effectiveness of Acetylsalicylic Acid in Primary Prevention of Cardiovascular Diseases and Mortality in Patients at High Risk, a Retrospective Cohort Study—The JOANA Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design
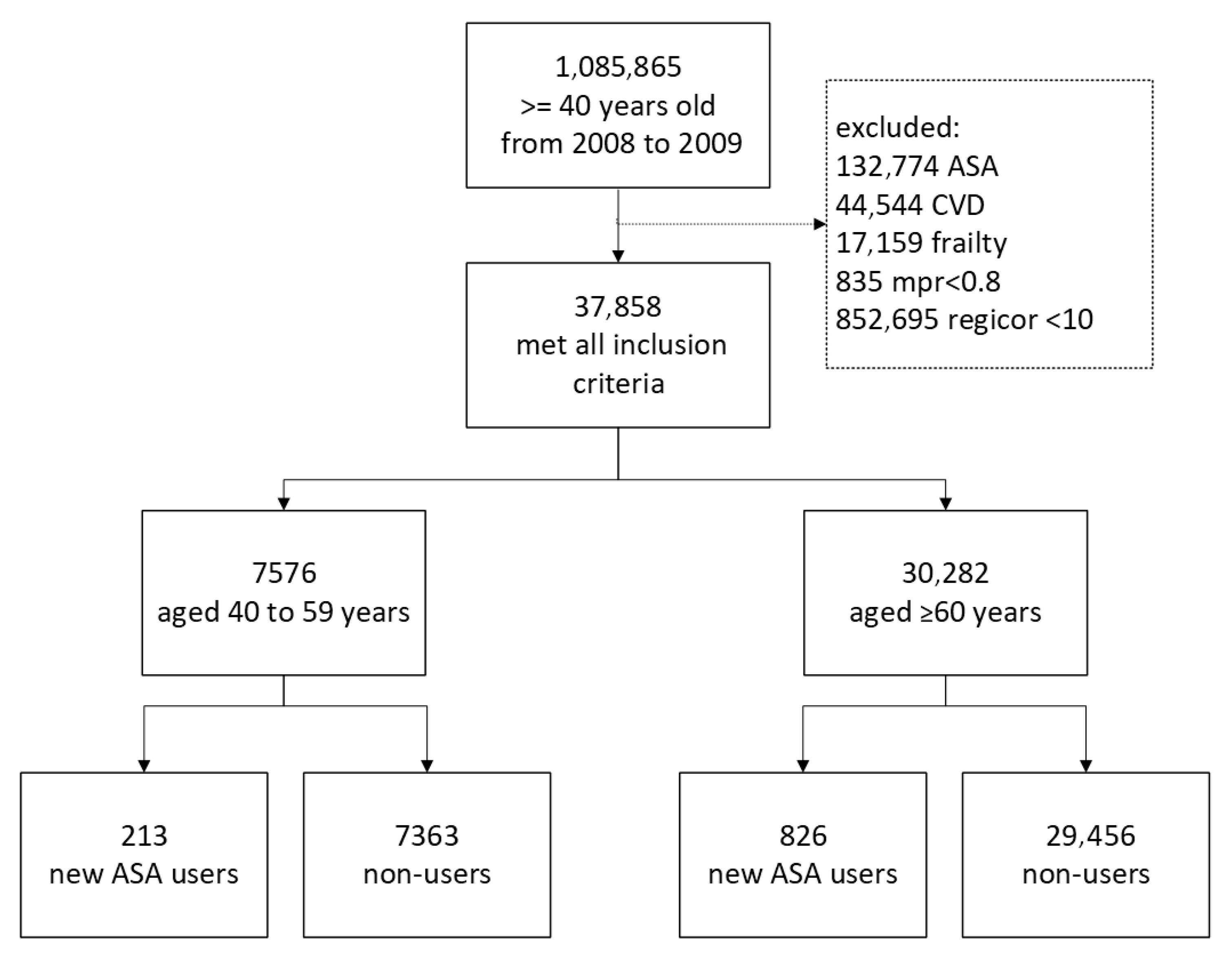
2.3. Study Population
2.4. ASA Exposure, Outcomes, and Covariates
2.5. Statistical Analyses
3. Results
4. Discussion
Study Characteristics That Merit Consideration
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | acetylsalicylic acid |
| ASCVD | atherosclerotic cardiovascular disease |
| ATT | antithrombotic trialists |
| BMI | body mass index |
| CHD | coronary heart disease |
| CVD | cardiovascular diseases |
| HR | hazard ratios |
| IQR | interquartile range |
| MPR | medication possession ratio |
| NNH | number needed to harm |
| NNT | number needed to treat |
| PS | propensity score |
| SIDIAP | Information System for the Development of Research in Primary Care |
| USPSTF | United States Preventive Services Task Force |
Appendix A
Appendix A.1. Methods
Appendix A.1.1. Propensity Score Building
Appendix A.1.2. Validation of the Imputation Process [39,40]
Appendix A.2. Tables
| Variables | Missing Values | Observed Values | Imputed Values |
|---|---|---|---|
| Participants < 60 years | |||
| Systolic blood pressure | 5022 (66.29%) | 145.95 (145.3–146.6) | 146.23 (146.1–146.35) |
| Diastolic blood pressure | 5022 (66.29%) | 85.6 (85.19–86.02) | 86.75 (86.66–86.84) |
| BMI | 5883 (77.65%) | 30.54 (30.33–30.74) | 29.99 (29.95–30.03) |
| Total cholesterol | 5339 (70.47%) | 234.44 (232.77–236.11) | 243.92 (243.56–244.27) |
| HDL cholesterol | 6013 (79.37%) | 40.37 (40.04–40.71) | 38.01 (37.94–38.08) |
| Triglycerides | 5659 (74.70%) | 204.84 (201.3–208.38) | 228.67 (227.5–229.84) |
| Glucose | 5360 (70.75%) | 143.46 (141.06–145.87) | 114.24 (113.87–114.61) |
| Glomerular filtration rate | 5421 (71.55%) | 86.79 (86.11–87.48) | 85.12 (84.97–85.26) |
| Participants ≥ 60 years | |||
| Systolic blood pressure | 17,558 (57.98%) | 143.5 (143.19–143.8) | 144(143.92–144.09) |
| Diastolic blood pressure | 17,558 (57.98%) | 80.69 (80.5–80.88) | 82.71 (82.66–82.77) |
| BMI | 21,579 (71.26%) | 29.54 (29.45–29.63) | 29.27 (29.25–29.3) |
| Total cholesterol | 19,054 (62.92%) | 220.28 (219.54–221.02) | 229.15 (228.93–229.36) |
| HDL cholesterol | 22,088 (72.94%) | 44.66 (44.47–44.85) | 42.23 (42.18–42.28) |
| Triglycerides | 20,825 (68.77%) | 165.18 (163.76–166.6) | 179.5 (178.96–180.05) |
| Glucose | 19,105 (63.09%) | 127.88 (126.99–128.77) | 109.79 (109.59–109.99) |
| Glomerular filtration rate | 19,360 (63.93%) | 78.09 (77.78–78.4) | 78.45 (78.37–78.53) |
| 40–59 Years | ≥60 Years | |||||||
|---|---|---|---|---|---|---|---|---|
| ASA Non-Users n = 1340 | ASA Users n = 80 | Standardised Difference | Adjusted Standardised Difference | ASA Non-Users n = 6246 | ASA Users n = 344 | Standardised Difference | Adjusted Standardised Difference | |
| Age | 55.73 (3.4) | 55.36 (3.8) | 0.1066 | 0.3809 | 68.25 (4.3) | 68.07(4.2) | 0.0461 | 0.0462 |
| Men | 67.39% | 67.5% | −0.0024 | 0.2603 | 85.29% | 82.85% | 0.0648 | 0.0981 |
| Systolic blood pressure | 145.16 (15.2) | 145.78 (15.6) | −0.0405 | 0.1917 | 143.57 (14.8) | 145.78 (16.7) | 0.1093 | 0.1097 |
| Diastolic blood pressure | 84.71 (9.4) | 84.44 (11.3) | 0.0287 | 0.0859 | 80.55 (9.3) | 79.26 (9.2) | 0.1183 | 0.1189 |
| BMI | 31.05 (4.9) | 31.42 (5.5) | −0.0765 | 0.0959 | 29.71 (4.2) | 29.82(4.1) | 0.0074 | 0.0074 |
| Vascular risk factors | ||||||||
| DM | 73.36% | 93.75% | −0.7040 | 0.5941 | 58.63% | 82.56% | −0.5835 | 0.0000 |
| Hypertension | 58.51% | 56.25% | 0.0455 | 0.0557 | 67.26% | 71.51% | −0.0938 | 0.0276 |
| Smoking | 56.34% | 50% | 0.1265 | 0.0890 | 39.16% | 37.5% | 0.0343 | 0.0865 |
| High alcohol consumption | 10.37% | 16.25% | −0.1589 | 0.2671 | 6.96% | 9.3% | −0.0806 | 0.1757 |
| Other comorbidities | ||||||||
| Arthritis | 0.6% | 0 | 1.1450 | 1.1603 | 0.66% | 0.58% | 0.0098 | 0.0698 |
| Asthma | 2.91% | 0 | 2.6018 | 11.2420 | 2.05% | 2.03% | 0.0010 | 0.0263 |
| Hypothyroidism | 2.84% | 5% | −0.0990 | 0.0717 | 2.74% | 2.62% | 0.0076 | 0.1183 |
| Other medications | ||||||||
| Statins | 31.64% | 57.5% | −0.5001 | 0.1441 | 35.27% | 57.27% | −0.4307 | 0.0000 |
| Other lipid-lowering drugs | 37.69% | 68.75% | −0.6237 | 0.0701 | 39.88% | 61.63% | −0.4327 | 0.0000 |
| Diuretics | 28.81% | 33.75% | −0.1045 | 0.0828 | 37.43% | 45.93% | −0.1699 | 0.0035 |
| Beta-blockers | 8.73% | 12.5% | −0.1140 | 0.0342 | 10.63% | 13.37% | −0.0807 | 0.0684 |
| Calcium channel blockers | 11.72% | 10% | 0.0569 | 0.0831 | 13.4% | 19.19% | −0.1468 | 0.0021 |
| ACEI | 44.1% | 52.5% | −0.1673 | 0.2799 | 53.59% | 68.9% | −0.3235 | 0.0015 |
| Anti-diabetics | 50.82% | 76.25% | −0.5604 | 0.1865 | 41.93% | 72.09% | −0.6255 | 0.0000 |
| Anti-inflammatory drugs | 27.09% | 30% | −0.0635 | 0.1412 | 31.06% | 32.27% | −0.0258 | 0.0655 |
| Laboratory tests | ||||||||
| Total cholesterol | 231.93 (39.8) | 230.41 (38.7) | 0.0380 | 0.4128 | 218.14 (36.5) | 214.47(37) | 0.1004 | 0.1057 |
| HDL cholesterol | 40.49 (7.6) | 41.2 (8.1) | −0.0929 | 0.1359 | 44.83 (9.3) | 44.43(9.1) | 0.0433 | 0.0615 |
| Triglycerides | 204.63 (81.5) | 207.29 (78.6) | −0.0327 | 0.0781 | 164.15 (72.3) | 169.61(72) | −0.0755 | 0.0807 |
| Glucose | 145.99 (55.1) | 185.6 (67.1) | −0.7089 | 0.5374 | 130.13 (43.1) | 152.86 (55.4) | −0.5189 | 0.4490 |
| Glomerular filtration rate | 87.62 (15.9) | 85.3 (15.5) | 0.1459 | 0.2590 | 77.84 (15.1) | 76.74 (15.5) | 0.0732 | 0.0439 |
| ASA Non-Users n = 1340 | ASA New Users n = 80 | ||||
|---|---|---|---|---|---|
| Number of Events | Incidence Rate/1000 Person-Years (95% CI) | Number of Events | Incidence Rate/1000 Person-Years (95% CI) | Hazard Ratio (95% CI) | |
| 40–59 years | |||||
| Outcomes of interest | |||||
| Total mortality | 145 | 10.21 (8.67–12.01) | 5 | 5.74 (2.39–13.79) | 0.42 (0.17–1.08) |
| ASCV | 199 | 15.17 (13.2–17.43) | 11 | 13.65 (7.56–24.66) | 0.61 (0.32–1.17) |
| CHD | 120 | 8.88 (7.42–10.62) | 7 | 8.48 (4.04–17.78) | 0.63 (0.28–1.4) |
| Ischemic stroke | 93 | 6.76 (5.52–8.29) | 5 | 5.89 (2.45–14.15) | 0.66 (0.26–1.68) |
| Adverse effects | |||||
| Gastric ulcer | 15 | 1.07 (0.65–1.78) | 2 | 2.34 (0.59–9.35) | – |
| Gastrointestinal bleeding | 22 | 1.56 (1.03–2.37) | 6 | 7.21 (3.24–16.06) | 5.2 (1.89–14.31) |
| Haemorrhagic stroke | 14 | 0.99 (0.59–1.67) | 0 | NA | NA |
| ≥60 years | n = 6246 | n = 344 | |||
| Outcomes of interest | |||||
| Total mortality | 1516 | 24.03 22.85–25.27) | 97 | 28.48 (23.34–34.75) | 1.17 (0.94–1.44) |
| ASCVD | 1004 | 17.25 (16.22–18.35) | 78 | 26.06 (20.87–32.53) | 1.24 (0.98–1.58) |
| CHD | 491 | 8.1 (7.42–8.85) | 34 | 10.45 (7.47–14.63) | 1.11 (0.78–1.6) |
| Ischemic stroke | 585 | 9.68 (8.93–10.5) | 52 | 16.66 (12.7–21.87) | 1.39 (1.04–1.86) |
| Adverse effects | |||||
| Gastric ulcer | 73 | 1.17 (0.93–1.48) | 5 | 1.5 (0.62–3.6) | 2.21 (1.13–4.32) |
| Gastrointestinal bleeding | 136 | 2.17 (1.84–2.57) | 13 | 3.85 (2.24–6.64) | 1.79 (1–3.22) |
| Haemorrhagic stroke | 92 | 1.46 (1.19–1.8) | 7 | 2.07 (0.99–4.34) | 1.31 (0.75–2.28) |
References
- GBD 2021 Causes of Death Collaborators. Global Burden of 288 Causes of Death and Life Expectancy Decomposition in 204 Countries and Territories and 811 Subnational Locations, 1990-2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Estadística de Defunciones Según la Causa De Muerte. Available online: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176780&menu=ultiDatos&idp=1254735573175 (accessed on 4 December 2024).
- Ministerio de Sanidad. Estrategia en Salud Cardiovascular del Sistema Nacional de Salud (ESCAV); Ministerio de Sanidad: Madrid, Spain, 2022.
- Visseren, F.L.J.; MacH, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Jaén, C.R.; Krist, A.H.; et al. Aspirin Use to Prevent Cardiovascular Disease. JAMA 2022, 327, 1577. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification; NICE Guideline 181; NICE: London, UK, 2023; pp. 1–44. [Google Scholar]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Gaziano, J.M.; Brotons, C.; Coppolecchia, R.; Cricelli, C.; Darius, H.; Gorelick, P.B.; Howard, G.; Pearson, T.A.; Rothwell, P.M.; Ruilope, L.M.; et al. Use of Aspirin to Reduce Risk of Initial Vascular Events in Patients at Moderate Risk of Cardiovascular Disease (ARRIVE): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2018, 392, 1036–1046. [Google Scholar] [CrossRef]
- The ASCEND Study Collaborative Group. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Wolfe, R.; Woods, R.L.; Tonkin, A.M.; Donnan, G.A.; Nelson, M.R.; Reid, C.M.; Lockery, J.E.; Kirpach, B.; Storey, E.; et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, D.; Alves, M.; Gonçalves, N.; Costa, J.; Ferreira, J.J.; Pinto, F.J. Association of Aspirin Use in Primary Prevention and Cardiovascular Events: A Retrospective Analysis of the VITAL Cohort. J. Pers. Med. 2025, 15, 89. [Google Scholar] [CrossRef]
- Dehmer, S.P.; Maciosek, M.V.; La France, A.B.; Flottemesch, T.J. Health Benefits and Cost-Effectiveness of Asymptomatic Screening for Hypertension and High Cholesterol and Aspirin Counseling for Primary Prevention. Ann. Fam. Med. 2017, 15, 23–36. [Google Scholar] [CrossRef]
- Slawson, D.C. USPSTF Recommends Against Initiating Aspirin for Primary Prevention of CVD in Adults 60 Years or Older. Am. Fam. Physician 2022, 106, 586. [Google Scholar] [PubMed]
- Bolíbar, B.; Fina Avilés, F.; Morros, R.; Garcia-Gil, M.d.M.; Hermosilla, E.; Ramos, R.; Rosell, M.; Rodríguez, J.; Medina, M.; Calero, S.; et al. SIDIAP Database: Electronic Clinical Records in Primary Care as a Source of Information for Epidemiologic Research. Med. Clin. 2012, 138, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Balló, E.; Marrugat, J.; Elosua, R.; Sala, J.; Grau, M.; Vila, J.; Bolíbar, B.; García-Gil, M.; Martí, R.; et al. Validity for Use in Research on Vascular Diseases of the SIDIAP (Information System for the Development of Research in Primary Care): The EMMA Study. Rev. Esp. Cardiol. (Engl. Ed.) 2012, 65, 29–37. [Google Scholar] [CrossRef]
- Ramos, R.; García-Gil, M.; Comas-Cufí, M.; Quesada, M.; Marrugat, J.; Elosua, R.; Sala, J.; Grau, M.; Martí, R.; Ponjoan, A.; et al. Statins for Prevention of Cardiovascular Events in a Low-Risk Population with Low Ankle Brachial Index. J. Am. Coll. Cardiol. 2016, 67, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gil, M.; Alves-Cabratosa, L.; Cunillera, O.; Blanch, J.; Martí-Lluch, R.; Ponjoan, A.; Ribas-Aulinas, F.; Tornabell-Noguera, È.; Zacarías-Pons, L.; Domínguez-Armengol, G.; et al. Effectiveness of the Low-density Lipoprotein Cholesterol Goals in Secondary Cardiovascular Prevention. Eur. J. Clin. Investig. 2024, 54, 14258. [Google Scholar] [CrossRef]
- Marrugat, J.; Subirana, I.; Comin, E.; Cabezas, C.; Vila, J.; Elosua, R.; Nam, B.-H.; Ramos, R.; Sala, J.; Solanas, P.; et al. Validity of an Adaptation of the Framingham Cardiovascular Risk Function: The VERIFICA Study. J. Epidemiol. Community Health (1978) 2007, 61, 40–47. [Google Scholar] [CrossRef]
- Ray, W.A. Evaluating Medication Effects Outside of Clinical Trials: New-User Designs. Am. J. Epidemiol. 2003, 158, 915–920. [Google Scholar] [CrossRef]
- White, I.R.; Royston, P.; Wood, A.M. Multiple Imputation Using Chained Equations: Issues and Guidance for Practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef]
- Austin, P.C. Goodness-of-fit Diagnostics for the Propensity Score Model When Estimating Treatment Effects Using Covariate Adjustment with the Propensity Score. Pharmacoepidemiol. Drug Saf. 2008, 17, 1202–1217. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Team, R. Development Core R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 15 April 2025).
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Kim, K.; Hennekens, C.H.; Martinez, L.; Gaziano, J.M.; Pfeffer, M.A.; Biglione, B.; Gitin, A.; McCabe, J.B.; Cook, T.D.; Demets, D.L.; et al. Primary Care Providers Should Prescribe Aspirin to Prevent Cardiovascular Disease Based on Benefit-Risk Ratio, Not Age. Fam. Med. Community Health 2021, 9, e001475. [Google Scholar] [CrossRef]
- Barnett, H.; Burrill, P.; Iheanacho, I. Don’t Use Aspirin for Primary Prevention of Cardiovascular Disease. BMJ 2010, 340, c1805. [Google Scholar] [CrossRef] [PubMed]
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; et al. Aspirin in the Primary and Secondary Prevention of Vascular Disease: Collaborative Meta-Analysis of Individual Participant Data from Randomised Trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Marquis-Gravel, G.; Roe, M.T.; Harrington, R.A.; Muñoz, D.; Hernandez, A.F.; Schuyler Jones, W. Revisiting the Role of Aspirin for the Primary Prevention of Cardiovascular Disease. Circulation 2019, 140, 1115–1124. [Google Scholar] [CrossRef]
- Smith, C.L.; Kasza, J.; Woods, R.L.; Lockery, J.E.; Kirpach, B.; Reid, C.M.; Storey, E.; Nelson, M.R.; Shah, R.C.; Orchard, S.G.; et al. Compliance-Adjusted Estimates of Aspirin Effects Among Older Persons in the ASPREE Randomized Trial. Am. J. Epidemiol. 2023, 192, 2063–2074. [Google Scholar] [CrossRef]
- Chowdhury, E.K.; Ernst, M.E.; Nelson, M.R.; Beilin, L.J.; Neumann, J.T.; Tonkin, A.; Woods, R.L.; Stocks, N.; Lacaze, P.; Orchard, S.G.; et al. Stratification by CVD Risk Equations Does Not Inform the Use of Aspirin for Primary Prevention in Older Adults. Eur. J. Prev. Cardiol. 2025, 2025, zwaf329. [Google Scholar] [CrossRef] [PubMed]
- Wittes, J.; DeMets, D.L.; Kim, K.; Maki, D.G.; Pfeffer, M.A.; Gaziano, J.M.; Kitsantas, P.; Hennekens, C.H.; Wood, S.K. Aspirin in Primary Prevention: Undue Reliance on an Uninformative Trial Led to Misinformed Clinical Guidelines. Clin. Trials 2025, 22, 17407745251324866. [Google Scholar] [CrossRef]
- Califf, R.M.; Sanderson, I.; Miranda, M.L. The Future of Cardiovascular Clinical Research: Informatics, Clinical Investigators, and Community Engagement. JAMA 2012, 308, 1747–1748. [Google Scholar] [CrossRef]
- Lauer, M. Time for a Creative Transformation of Epidemiology in the United States. JAMA 2012, 308, 1804–1805. [Google Scholar] [CrossRef] [PubMed]
- Tannen, R.L.; Weiner, M.G.; Xie, D. Use of Primary Care Electronic Medical Record Database in Drug Efficacy Research on Cardiovascular Outcomes: Comparison of Database and Randomised Controlled Trial Findings. BMJ 2009, 338, b81. [Google Scholar] [CrossRef] [PubMed]
- Dahabreh, I.J.; Sheldrick, R.C.; Paulus, J.K.; Chung, M.; Varvarigou, V.; Jafri, H.; Rassen, J.A.; Trikalinos, T.A.; Kitsios, G.D. Do Observational Studies Using Propensity Score Methods Agree with Randomized Trials? A Systematic Comparison of Studies on Acute Coronary Syndromes. Eur. Heart J. 2012, 33, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Rahme, E.; Abrahamowicz, M.; Pilote, L. Survival Bias Associated with Time-to-Treatment Initiation in Drug Effectiveness Evaluation: A Comparison of Methods. Am. J. Epidemiol. 2005, 162, 1016–1023. [Google Scholar] [CrossRef]
- Janssen, K.J.M.; Donders, A.R.T.; Harrell, F.E.; Vergouwe, Y.; Chen, Q.; Grobbee, D.E.; Moons, K.G.M. Missing Covariate Data in Medical Research: To Impute Is Better than to Ignore. J. Clin. Epidemiol. 2010, 63, 721–727. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple Imputation for Missing Data in Epidemiological and Clinical Research: Potential and Pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]

| 40–59 Years | ≥60 Years | |||||||
|---|---|---|---|---|---|---|---|---|
| ASA Non-Users n = 7363 | ASA Users n = 213 | Standardised Difference | Adjusted Standardised Difference | ASA Non-Users n = 29,456 | ASA Users n = 826 | Standardised Difference | Adjusted Standardised Difference | |
| Age | 55.72 (3.5) | 55.34 (3.9) | 0.1081 | 0.1010 | 68.18 (4.3) | 67.69 (4.2) | 0.1136 | 0.1355 |
| Men | 76.8% | 71.96% | 0.1076 | 0.1892 | 89.58% | 82.97% | 0.1743 | 0.0069 |
| Systolic blood pressure | 145.67 (14.5) | 147.07 (15.7) | −0.0966 | 0.1018 | 142.99 (15) | 146.06 (17) | −0.2043 | 0.0613 |
| Diastolic blood pressure | 86.01 (9.9) | 84.7 (10.6) | 0.1316 | 0.0882 | 81.64 (9.7) | 80.77 (9.8) | 0.0890 | 0.0747 |
| BMI | 30.42 (4.6) | 30.83 (5) | −0.0872 | 0.0089 | 29.28 (4.4) | 29.87 (4.5) | −0.1355 | 0.0775 |
| Vascular risk factors | ||||||||
| DM | 45% | 85.89% | −0.9840 | 0.0000 | 32.29% | 72.99% | −0.8097 | 0.0000 |
| Hypertension | 41.53% | 55.91% | −0.2855 | 0.0228 | 44.59% | 68.43% | −0.4914 | 0.0002 |
| Smoking | 58.97% | 52.67% | 0.1259 | 0.0880 | 42.72% | 36.05% | 0.1383 | 0.0132 |
| High alcohol consumption | 11.32% | 11.74% | −0.0132 | 0.0151 | 7.47% | 8.24% | −0.0282 | 0.0267 |
| Other comorbidities | ||||||||
| Arthritis | 0.56% | 0.02% | 0.1851 | 0.0988 | 0.6% | 0.61% | −0.0017 | 0.0365 |
| Asthma | 2.04% | 0.52% | 0.1895 | 0.0786 | 2% | 1.77% | 0.0174 | 0.0146 |
| Hypothyroidism | 2.08% | 3.59% | -0.0806 | 0.0133 | 1.96% | 2.25% | −0.0195 | 0.0854 |
| Other medications | ||||||||
| Statins | 18.79% | 53.56% | −0.6310 | 0.0000 | 20.6% | 51.3% | −0.5693 | 0.0000 |
| Other lipid-lowering drugs | 24.83% | 66.81% | −0.7824 | 0.0000 | 24.03% | 57.74% | −0.6275 | 0.0000 |
| Diuretics | 17.63% | 32.96% | −0.3185 | 0.0001 | 22.71% | 44.86% | −0.4283 | 0.0001 |
| Beta-blockers | 6.49% | 14.37% | −0.2189 | 0.0041 | 6.98% | 12.8% | −0.1719 | 0.0126 |
| Calcium channel blockers | 6.37% | 12.35% | −0.1790 | 0.0000 | 8.16% | 20.41% | −0.2924 | 0.0002 |
| ACEI | 27.84% | 57.36% | −0.5611 | 0.0000 | 32.3% | 64.12% | −0.6179 | 0.0000 |
| Anti-diabetics | 27.18% | 74.24% | −0.9088 | 0.0000 | 20.82% | 65.54% | −0.8064 | 0.0000 |
| Anti-inflammatory drugs | 22.59% | 27.89% | −0.1182 | 0.1311 | 24.52% | 28.93% | −0.0972 | 0.1486 |
| Laboratory tests | ||||||||
| Total cholesterol | 240.36 (40.8) | 236.39 (45.1) | 0.0973 | 0.2283 | 223.98 (38.1) | 219.94 (38.3) | 0.1060 | 0.1388 |
| HDL cholesterol | 39.35 (8.1) | 39.55 (8.4) | −0.0249 | 0.0143 | 44.24 (10) | 43.6 (9.2) | 0.0651 | 0.0753 |
| Triglycerides | 242.17 (162.3) | 277.3 (198.7) | −0.2150 | 0.0646 | 175.23 (103.6) | 188.67 (111.9) | −0.1294 | 0.1033 |
| Glucose | 129.52 (51.5) | 183.83 (71.6) | −1.0417 | 0.2948 | 116.61 (38.7) | 152.95 (57.2) | −0.9231 | 0.3315 |
| Glomerular filtration rate | 86.12 (16) | 85.86 (16.2) | 0.0166 | 0.0259 | 78.43 (14.8) | 76.85 (16.9) | 0.1066 | 0.0212 |
| 40–59 Years | ASA Non-Users n = 7363 | ASA New Users n = 213 | ||
|---|---|---|---|---|
| Number of Events | Incidence Rate/1000 Person-Years (95% CI) | Number of Events | Incidence Rate/1000 Person-Years (95% CI) | |
| Outcomes of interest | ||||
| Total mortality | 3559 | 8.88 (8.59–9.18) | 31 | 6.58 (4.63–9.35) |
| ASCVD | 4309 | 11.38 (11.04–11.72) | 58 | 13.2 (10.2–17.07) |
| CHD | 2439 | 6.29 (6.04–6.54) | 33 | 7.3 (5.19–10.27) |
| Ischemic stroke | 2094 | 5.36 (5.13–5.59) | 31 | 6.8 (4.78–9.67) |
| Adverse effects | ||||
| Gastric ulcer | 437 | 1.11 (1.01–1.21) | 5 | 1.07 (0.45–2.57) |
| Gastrointestinal bleeding | 583 | 1.46 (1.35–1.59) | 16 | 3.46 (2.12–5.65) |
| Haemorrhagic stroke | 304 | 0.76 (0.68–0.85) | 1 | 0.21 (0.03–1.51) |
| ≥60 Years | ASA Non-Users n= 29,456 | ASA New Users n= 826 | ||
| Number of Events | Incidence Rate/1000 Person-Years (95% CI) | Number of Events | Incidence Rate/1000 Person-Years (95% CI) | |
| Outcomes of interest | ||||
| Total mortality | 19,084 | 22.32 (22.01–22.64) | 340 | 26.42 (23.76–29.39) |
| ASCVD | 9284 | 11.46 (11.23–11.7) | 244 | 21.02 (18.54–23.83) |
| CHD | 4490 | 5.40 (5.24–5.56) | 118 | 9.63 (8.04–11.53) |
| Ischemic stroke | 5310 | 6.39 (6.22–6.56) | 146 | 12.02 (10.22–14.14) |
| Adverse effects | ||||
| Gastric ulcer | 892 | 1.06 (0.99–1.13) | 22 | 1.74 (1.15–2.64) |
| Gastrointestinal bleeding | 1375 | 1.62 (1.53–1.71) | 41 | 3.22 (2.37–4.37) |
| Haemorrhagic stroke | 875 | 1.03 (0.96–1.1) | 20 | 1.56 (1.01–2.42) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Cabratosa, L.; López, C.; Garcia-Gil, M.; Tornabell-Noguera, È.; Comas-Cufí, M.; Blanch, J.; Martí-Lluch, R.; Ponjoan, A.; Domínguez-Armengol, G.; Zacarías-Pons, L.; et al. Long-Term Effectiveness of Acetylsalicylic Acid in Primary Prevention of Cardiovascular Diseases and Mortality in Patients at High Risk, a Retrospective Cohort Study—The JOANA Study. J. Clin. Med. 2025, 14, 5710. https://doi.org/10.3390/jcm14165710
Alves-Cabratosa L, López C, Garcia-Gil M, Tornabell-Noguera È, Comas-Cufí M, Blanch J, Martí-Lluch R, Ponjoan A, Domínguez-Armengol G, Zacarías-Pons L, et al. Long-Term Effectiveness of Acetylsalicylic Acid in Primary Prevention of Cardiovascular Diseases and Mortality in Patients at High Risk, a Retrospective Cohort Study—The JOANA Study. Journal of Clinical Medicine. 2025; 14(16):5710. https://doi.org/10.3390/jcm14165710
Chicago/Turabian StyleAlves-Cabratosa, Lia, Carles López, Maria Garcia-Gil, Èric Tornabell-Noguera, Marc Comas-Cufí, Jordi Blanch, Ruth Martí-Lluch, Anna Ponjoan, Gina Domínguez-Armengol, Lluís Zacarías-Pons, and et al. 2025. "Long-Term Effectiveness of Acetylsalicylic Acid in Primary Prevention of Cardiovascular Diseases and Mortality in Patients at High Risk, a Retrospective Cohort Study—The JOANA Study" Journal of Clinical Medicine 14, no. 16: 5710. https://doi.org/10.3390/jcm14165710
APA StyleAlves-Cabratosa, L., López, C., Garcia-Gil, M., Tornabell-Noguera, È., Comas-Cufí, M., Blanch, J., Martí-Lluch, R., Ponjoan, A., Domínguez-Armengol, G., Zacarías-Pons, L., Ribas-Aulinas, F., Balló, E., & Ramos, R. (2025). Long-Term Effectiveness of Acetylsalicylic Acid in Primary Prevention of Cardiovascular Diseases and Mortality in Patients at High Risk, a Retrospective Cohort Study—The JOANA Study. Journal of Clinical Medicine, 14(16), 5710. https://doi.org/10.3390/jcm14165710






