Prevalence and Diagnosis of Obstructive Sleep Apnea in Atrial Fibrillation Patients: A Systematic Review
Abstract
1. Introduction
2. Methods
3. Results
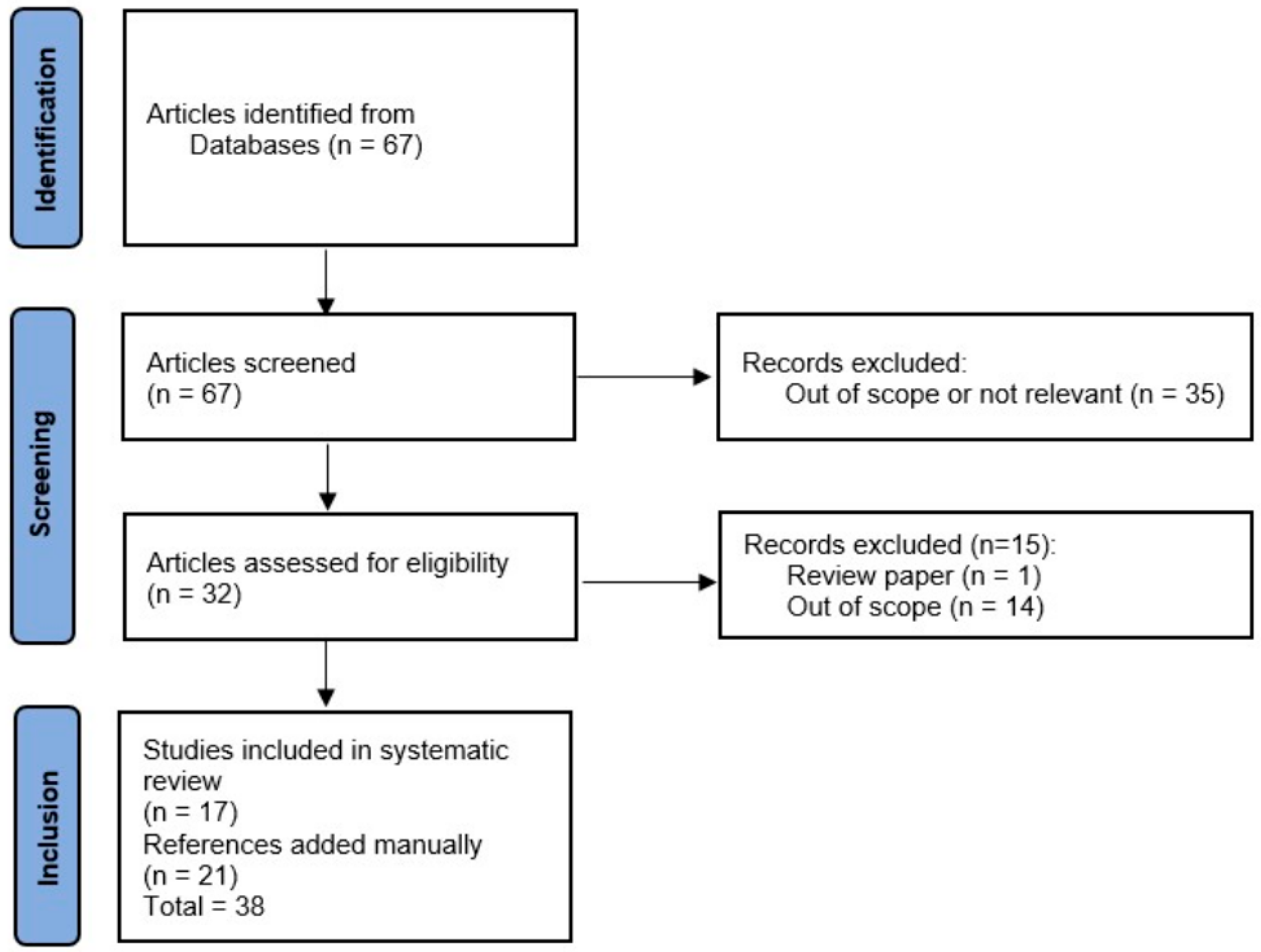
3.1. Search Results
3.2. Study Characteristics
3.3. Study Populations
3.4. Methods of Sleep-Disordered Breathing Assessment Across Studies
3.5. Prevalence of Sleep-Disordered Breathing in Atrial Fibrillation Populations
3.6. Severity Stratification of Sleep-Disordered Breathing in Atrial Fibrillation Populations
3.7. Prevalence of Atrial Fibrillation in Populations with Sleep-Related Breathing Disorders
3.8. Risk Factors for Sleep-Disordered Breathing in Patients with Atrial Fibrillation
3.9. Symptom Evaluation Scores for Sleep-Disordered Breathing
3.10. Self-Reported Symptoms of Sleep-Disordered Breathing
3.11. Laboratory, ECG, Echocardiographic and Polysomnographic Findings
4. Discussion
4.1. Overview
4.2. Heterogeneity in Study Populations and Methodological Designs
4.3. Diagnostic Approaches and Screening Tools
4.4. Underdiagnosis of OSA in AF Populations
4.5. Prevalence Estimates
4.6. Risk Factors and Clinical Correlates
4.7. Clinical and Research Implications
4.8. Study Limitations
4.9. Unexpected Findings and Research Gaps
4.10. Future Directions and Research Priorities
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Peker, Y.; Akdeniz, B.; Altay, S.; Balcan, B.; Başaran, Ö.; Baysal, E.; Çelik, A.; Dursunoğlu, D.; Dursunoğlu, N.; Fırat, S.; et al. Obstructive Sleep Apnea and Cardiovascular Disease: Where Do We Stand? Anatol. J. Cardiol. 2023, 27, 375–389. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA—J. Am. Med. Assoc. 2020, 323, 1380–1400. [Google Scholar] [CrossRef]
- Jun, J.; Polotsky, V.Y. Sleep disordered breathing and metabolic effects: Evidence from animal models. Sleep Med. Clin. 2007, 2, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [CrossRef]
- Lee, W.; Nagubadi, S.; Kryger, M.H.; Mokhlesi, B. Epidemiology of Obstructive Sleep Apnea: A Population-based Perspective. Expert Rev. Respir. Med. 2008, 2, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.S.; Agostinho, J.R.; Silva, G.; Guimarães, T.; Bernardes, A.; Santos, I.; Pinto, P.; Bárbara, C.; de Sousa, J.; Pinto, F.J.; et al. Accuracy and utility of a pacemaker respiratory monitoring algorithm for the detection of obstructive sleep apnea in patients with atrial fibrillation. Sleep Med. 2019, 61, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J. Am. Coll. Cardiol. 2008, 52, 686–717. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Young, T.; Skatrud, J.; Peppard, P.E. Risk factors for obstructive sleep apnea in adults. JAMA 2004, 291, 2013–2016. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Sreedharan, S.E.; Agrawal, P.; Rajith, R.S.; Nair, S.; Sarma, S.P.; Radhakrishnan, A. Clinical and polysomnographic predictors of severe obstructive sleep apnea in the South Indian population. Ann. Indian Acad. Neurol. 2016, 19, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Desteghe, L.; Hendriks, J.M.L.; McEvoy, R.D.; Chai-Coetzer, C.L.; Dendale, P.; Sanders, P.; Heidbuchel, H.; Linz, D. The why, when and how to test for obstructive sleep apnea in patients with atrial fibrillation. Clin. Res. Cardiol. 2018, 107, 617–631. [Google Scholar] [CrossRef] [PubMed]
- May, A.M.; Wang, L.; Kwon, D.H.; Van Wagoner, D.R.; Chung, M.K.; Dalton, J.E.; Mehra, R. Sleep apnea screening instrument evaluation and novel model development and validation in the paroxysmal atrial fibrillation population. Int. J. Cardiol. Heart Vasc. 2020, 31, 100624. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L.; Sahadevan, J.; Redline, S. Sleep Heart Health Study. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef]
- Young, T.; Finn, L.; Peppard, P.E.; Szklo-Coxe, M.; Austin, D.; Nieto, F.J.; Stubbs, R.; Hla, K.M. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008, 31, 1071–1078. [Google Scholar] [CrossRef]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Redline, S.; Yenokyan, G.; Gottlieb, D.J.; Shahar, E.; O’Connor, G.T.; Resnick, H.E.; Diener-West, M.; Sanders, M.H.; Wolf, P.A.; Geraghty, E.M.; et al. Obstructive sleep apnea-hypopnea and incident stroke: The sleep heart health study. Am. J. Respir. Crit. Care Med. 2010, 182, 269–277. [Google Scholar] [CrossRef]
- McEvoy, R.D. Obstructive. sleep apnoea and hypertension: The ESADA study. Eur. Respir. J. 2014, 44, 835–838. [Google Scholar] [CrossRef]
- Molnar, M.Z.; Mucsi, I.; Novak, M.; Szabo, Z.; Freire, A.X.; Huch, K.M.; Arah, O.A.; Ma, J.Z.; Lu, J.L.; Sim, J.J.; et al. Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax 2015, 70, 888–895. [Google Scholar] [CrossRef]
- Feng, T.R.; White, R.S.; Ma, X.; Askin, G.; Pryor, K.O. The effect of obstructive sleep apnea on readmissions and atrial fibrillation after cardiac surgery. J. Clin. Anesth. 2019, 56, 17–23. [Google Scholar] [CrossRef]
- Peker, Y.; Holtstrand-Hjälm, H.; Celik, Y.; Glantz, H.; Thunström, E. Postoperative Atrial Fibrillation in Adults with Obstructive Sleep Apnea Undergoing Coronary Artery Bypass Grafting in the RICCADSA Cohort. J. Clin. Med. 2022, 11, 2459. [Google Scholar] [CrossRef] [PubMed]
- Todd, K.; Mcintyre, W.F.; Baranchuk, A. Nature and Science of Sleep Dovepress Obstructive sleep apnea and atrial fibrillation. Nat. Sci. Sleep 2010, 2, 39–45. [Google Scholar] [PubMed][Green Version]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, 2071–2104. [Google Scholar] [CrossRef]
- Gahungu, N.; Judkins, C.; Gabbay, E.; Playford, D. Advances in screening for undiagnosed atrial fibrillation for stroke prevention and implications for patients with obstructive sleep apnoea: A literature review and research agenda. Sleep Med. 2019, 57, 107–114. [Google Scholar] [CrossRef]
- Selim, B.J.; Koo, B.B.; Qin, L.; Jeon, S.; Won, C.; Redeker, N.S.; Lampert, R.J.; Concato, J.P.; Bravata, D.M.; Ferguson, J.; et al. The Association between Nocturnal Cardiac Arrhythmias and Sleep-Disordered Breathing: The DREAM Study. J. Clin. Sleep Med. 2016, 12, 829–837. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- Hohl, M.; Linz, B.; Bohm, M.; Linz, D. Obstructive sleep apnea and atrial arrhythmogenesis. Curr. Cardiol. Rev. 2014, 10, 362–368. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Y.; Xu, J.; Yi, F. Association between obstructive sleep apnea (OSA) and atrial fibrillation (AF): A dose-response meta-analysis. Medicine 2022, 101, e29443. [Google Scholar] [CrossRef]
- Abumuamar, A.M.; Dorian, P.; Newman, D.; Shapiro, C.M. The prevalence of obstructive sleep apnea in patients with atrial fibrillation. Clin. Cardiol. 2018, 41, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Asirvatham, S.J.; Kapa, S. Sleep Apnea and Atrial Fibrillation. The Autonomic Link. J. Am. Coll. Cardiol. 2009, 54, 2084–2086. [Google Scholar] [CrossRef]
- Drager, L.F.; Lorenzi-Filho, G.; Cintra, F.D.; Pedrosa, R.P.; Bittencourt, L.R.A.; Poyares, D.; Carvalho, C.G.; Moura, S.M.G.P.T.; Santos-Silva, R.; Bruin, P.F.C.; et al. 1° Posicionamento Brasileiro sobre o Impacto dos Distúrbios de Sono nas Doenças Cardiovasculares da Sociedade Brasileira de Cardiologia. Arq. Bras. Cardiol. 2018, 111, 290–340. [Google Scholar] [CrossRef]
- Ben Halima, M.; Sammoud, K.; Ben Amar, J.; Boudiche, S.; Rekik, B.; Mghaieth, F.; Ouali, S.; Mourali, M.S. Prevalence and predictors of sleep apnea in atrial fibrillation patients. Tunis. Medicale 2020, 98, 1031–1037. [Google Scholar]
- Zhang, L.; Hou, Y.; Po, S.S. Obstructive sleep apnoea and atrial fibrillation. Arrhythmia Electrophysiol. Rev. 2015, 4, 14–18. [Google Scholar] [CrossRef]
- Monahan, K.; Brewster, J.; Wang, L.; Parvez, B.; Goyal, S.; Roden, D.M.; Darbar, D. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am. J. Cardiol. 2012, 110, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Liu, T.; Shehata, M.; Stevens, S.; Chugh, S.S.; Wang, X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am. J. Cardiol. 2011, 108, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Naruse, Y.; Tada, H.; Satoh, M.; Yanagihara, M.; Tsuneoka, H.; Hirata, Y.; Ito, Y.; Kuroki, K.; Machino, T.; Yamasaki, H.; et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: Clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013, 10, 331–337. [Google Scholar] [CrossRef]
- Kanagala, R.; Murali, N.S.; Friedman, P.A.; Ammash, N.M.; Gersh, B.J.; Ballman, K.V.; Shamsuzzaman, A.S.; Somers, V.K. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003, 107, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Congrete, S.; Bintvihok, M.; Thongprayoon, C.; Bathini, T.; Boonpheng, B.; Sharma, K.; Chokesuwattanaskul, R.; Srivali, N.; Tana-wuttiwat, T.; Cheungpasitporn, W. Effect of obstructive sleep apnea and its treatment of atrial fibrillation recurrence after radiofrequency catheter ablation: A meta-analysis. J. Evid. Based Med. 2018, 11, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Raza, A.; Guo, J. Treating obstructive sleep apnea with continuous positive airway pressure reduces risk of recurrent atrial fibrillation after catheter ablation: A meta-analysis. Sleep Med. 2018, 46, 5–11. [Google Scholar] [CrossRef]
- Li, F.; He, C.J.; Ding, C.H.; Wang, R.X.; Li, H. Continuous positive airway pressure therapy might be an effective strategy on reduction of atrial fibrillation recurrence after ablation in patients with obstructive sleep apnea: Insights from the pooled studies. Front. Neurol. 2023, 14, 1269945. [Google Scholar] [CrossRef]
- Traaen, G.M.; Aakerøy, L.; Hunt, T.E.; Øverland, B.; Bendz, C.; Sande, L.Ø.; Aakhus, S.; Fagerland, M.W.; Steinshamn, S.; Anfinsen, O.G.; et al. Effect of Continuous Positive Airway Pressure on Arrhythmia in Atrial Fibrillation and Sleep Apnea: A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 573–582. [Google Scholar] [CrossRef]
- Caples, S.M.; Mansukhani, M.P.; Friedman, P.A.; Somers, V.K. The impact of continuous positive airway pressure treatment on the recurrence of atrial fi brillation post cardioversion: A randomized controlled trial. Int. J. Cardiol. 2018, 278, 133–136. [Google Scholar] [CrossRef]
- Hojo, R.; Fukamizu, S.; Miyazawa, S.; Kawamura, I.; Sakurada, H.; Hiraoka, M. The relationship between obstructive sleep apnea and recurrence of atrial fibrillation after pulmonary vein isolation using a contact force-sensing catheter. J. Interv. Card. Electrophysiol. 2019, 54, 209–215. [Google Scholar] [CrossRef]
- Linz, D.; Brooks, A.G.; Elliott, A.D.; Nalliah, C.J.; Hendriks, J.M.L.; Middeldorp, M.E.; Gallagher, C.; Mahajan, R.; Kalman, J.M.; McEvoy, R.D.; et al. Variability of Sleep Apnea Severity and Risk of Atrial Fibrillation: The VARIOSA-AF Study. JACC Clin. Electrophysiol. 2019, 5, 692–701. [Google Scholar] [CrossRef]
- Providência, R.; Adragão, P.; de Asmundis, C.; Chun, J.; Chierchia, G.; Defaye, P.; Anselme, F.; Creta, A.; Lambiase, P.D.; Schmidt, B.; et al. Impact of Body Mass Index on the Outcomes of Catheter Ablation of Atrial Fibrillation: A European Observational Multicenter Study. J. Am. Heart Assoc. 2019, 8, e012253. [Google Scholar] [CrossRef]
- Bazan, V.; Vicente, I.; Lozano, L.; Villuendas, R.; González, M.; Adeliño, R.; Bisbal, F.; Sarrias, A.; Abad, J.; Sanz-Santos, J.; et al. Previously Undetected Obstructive Sleep Apnea in Patients With New-Onset Atrial Fibrillation. Am. J. Cardiol. 2021, 138, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Gellert, K.S.; Alonso, A.; Chen, L.Y.; Meyer, M.L.; Soliman, E.Z.; Suzuki, T.; Loehr, L.R. Association of Sleep Apnea, Diagnosed by Self-Reported Physician Diagnosis or Hospital Discharge Codes, With Atrial Fibrillation and Ectopy Using Ambulatory Electrocardiogram in the ARIC Study. Circ. Arrhythm. Electrophysiol. 2020, 13, E007574. [Google Scholar] [CrossRef]
- Marti-Almor, J.; Marques, P.; Jesel, L.; Garcia, R.; Di Girolamo, E.; Locati, F.; Defaye, P.; Venables, P.; Dompnier, A.; Barcelo, A.; et al. Incidence of sleep apnea and association with atrial fibrillation in an unselected pacemaker population: Results of the observational RESPIRE study. Heart Rhythm. 2020, 17, 195–202. [Google Scholar] [CrossRef]
- Mazza, A.; Bendini, M.G.; Leggio, M.; De Cristofaro, R.; Valsecchi, S.; Boriani, G. Continuous monitoring of sleep-disordered breathing with pacemakers: Indexes for risk stratification of atrial fibrillation and risk of stroke. Clin. Cardiol. 2020, 43, 1609–1615. [Google Scholar] [CrossRef]
- Shapira-Daniels, A.; Mohanty, S.; Contreras-Valdes, F.M.; Tieu, H.; Thomas, R.J.; Natale, A.; Anter, E. Prevalence of Undiagnosed Sleep Apnea in Patients With Atrial Fibrillation and its Impact on Therapy. JACC Clin. Electrophysiol. 2020, 6, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Starkey, S.Y.; Jonasson, D.R.; Alexis, S.; Su, S.; Johal, R.; Sweeney, P.; Brasher, P.M.A.; Fleetham, J.; Ayas, N.; Orenstein, T.; et al. Screening for Obstructive Sleep Apnea in an Atrial Fibrillation Population: What’s the Best Test? CJC Open 2020, 3, 442–449. [Google Scholar] [CrossRef]
- Szymanska, A.; Platek, A.E.; Sierdzinski, J.; Szymanski, F.M. Visfatin as a predictor of obstructive sleep apnea in atrial fibrillation patients. Sleep Breath. 2020, 24, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Traaen, G.M.; Øverland, B.; Aakerøy, L.; Hunt, T.E.; Bendz, C.; Sande, L.; Aakhus, S.; Zaré, H.; Steinshamn, S.; Anfinsen, O.G.; et al. Prevalence, risk factors, and type of sleep apnea in patients with paroxysmal atrial fibrillation. Int. J. Cardiol. Heart Vasc. 2019, 26, 100447. [Google Scholar] [CrossRef]
- Blanchard, M.; Gervès-Pinquié, C.; Feuilloy, M.; Le Vaillant, M.; Trzepizur, W.; Meslier, N.; Paris, A.; Pigeanne, T.; Racineux, J.L.; Balus-son, F.; et al. Association of nocturnal hypoxemia and pulse rate variability with incident atrial fibrillation in patients investigated for obstructive sleep apnea. Ann. Am. Thorac. Soc. 2021, 18, 1043–1051. [Google Scholar] [CrossRef]
- Delesie, M.; Knaepen, L.; Hendrickx, B.; Huygen, L.; Verbraecken, J.; Weytjens, K.; Dendale, P.; Heidbuchel, H.; Desteghe, L. The value of screening questionnaires/scoring scales for obstructive sleep apnoea in patients with atrial fibrillation. Arch. Cardiovasc. Dis. 2021, 114, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Mohammadieh, A.M.; Sutherland, K.; Kanagaratnam, L.B.; Whalley, D.W.; Gillett, M.J.; Cistulli, P.A. Clinical screening tools for obstructive sleep apnea in a population with atrial fibrillation: A diagnostic accuracy trial. J. Clin. Sleep Med. 2021, 17, 1015–1024. [Google Scholar] [CrossRef]
- Højager, A.; Schoos, M.M.; Tingsgaard, P.K.; Bock, T.G.; Homøe, P. Prevalence of silent atrial fibrillation and cardiovascular disease in patients with obstructive sleep apnea. Sleep Med. 2022, 100, 534–541. [Google Scholar] [CrossRef]
- Latif, Z.; Modest, A.M.; Ahn, A.; Thomas, R.; Tieu, H.; Tung, P. Effect of Widespread Sleep Apnea Screening on Progression of Atrial Fibrillation. Am. J. Cardiol. 2022, 182, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Mehawej, J.; Saczynski, J.S.; Kiefe, C.I.; Abu, H.O.; Tisminetzky, M.; Wang, W.; Bamgbade, B.A.; Ding, E.; Lessard, D.; Otabil, E.M.; et al. Association between risk of obstructive sleep apnea and cognitive performance, frailty, and quality of life among older adults with atrial fibrillation. J. Clin. Sleep Med. 2022, 18, 469–475. [Google Scholar] [CrossRef]
- Oster, M.; Thornsberry, J.D.; Howard, L.A.; Talley, M.H. Early detection of obstructive sleep apnea in patients with atrial fibrillation. J. Am. Assoc. Nurse Pract. 2022, 34, 1083–1089. [Google Scholar] [CrossRef]
- Verhaert, D.V.M.; Betz, K.; Gawałko, M.; Hermans, A.N.L.; Pluymaekers, N.A.H.A.; van der Velden, R.M.J.; Philippens, S.; Vorstermans, B.; Simons, S.O.; den Uijl, D.W.; et al. A VIRTUAL Sleep Apnoea management pathway For the work-up of Atrial fibrillation patients in a digital Remote Infrastructure: VIRTUAL-SAFARI. EP Europace. 2022, 24, 565–575. [Google Scholar] [CrossRef]
- Ahn, H.J.; Cha, M.J.; Lee, E.; Lee, S.R.; Choi, E.K.; Han, S.; Nam, G.B.; Choi, J.I.; Pak, H.N.; Oh, I.Y.; et al. The higher recurrence rate after catheter ablation in younger patients with atrial fibrillation suggesting different pathophysiology. J. Interv. Card. Electrophysiol. 2023, 66, 1609–1619. [Google Scholar] [CrossRef]
- Betz, K.; Verhaert, D.V.M.; Gawalko, M.; Hermans, A.N.L.; Habibi, Z.; Pluymaekers, N.A.H.A.; van der Velden, R.M.J.; Homberg, M.; Philippens, S.; Hereijgers, M.J.M.; et al. Atrial fibrillation-specific refinement of the STOP-Bang sleep apnoea screening questionnaire: Insights from the Virtual-SAFARI study. Clin. Res. Cardiol. 2023, 112, 834–845. [Google Scholar] [CrossRef]
- Holtstrand Hjälm, H.; Thunström, E.; Glantz, H.; Karlsson, M.; Celik, Y.; Peker, Y. Obstructive sleep apnea severity and prevalent atrial fibrillation in a sleep clinic cohort with versus without excessive daytime sleepiness. Sleep Med. 2023, 112, 63–69. [Google Scholar] [CrossRef]
- Jensen, M.H.; Dalgaard, F.; Rude Laub, R.; Gottlieb, V.; Nielsen, O.W.; Hansen, J.; Hansen, M.L.; Jennum, P.; Lamberts, M.; DAN-APNO Investigators. Prevalence of sleep apnea in unselected patients with atrial fibrillation by a home-monitoring device: The DAN-APNO study. Int. J. Cardiol. Heart Vasc. 2023, 47, 101219. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wei, H.; Jiang, F.; Zhu, P. Application of the STOP-BANG Questionnaire for Screening Obstructive Sleep Apnea Syndrome in Patients with Atrial Fibrillation. Panminerva Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.W.; Cassidy, M.; Sofer, T.; Tadros, T.; Zei, P.; Sauer, W.; Romero, J.; Martin, D.; Antman, E.M.; Javaheri, S. Evaluation of obstructive sleep apnea among consecutive patients with all patterns of atrial fibrillation using WatchPAT home sleep testing. Am. Heart J. 2023, 261, 95–103. [Google Scholar] [CrossRef]
- Ozkan, U.; Gurdogan, M. Novel Predictor of the AF Development in Patients with OSAS: Importance of Visceral Adipose Index. Medeni. Med. J. 2023, 38, 252–259. [Google Scholar] [CrossRef]
- Rivas, E.; Shehata, P.; Bravo, M.; Almonacid-Cardenas, F.; Shah, K.; Kopac, O.; Ruetzler, K.; Troianos, C.A.; Turan, A. Association between obstructive sleep apnea and atrial fibrillation and delirium after cardiac surgery. Sub-analysis of DECADE trial. J. Clin. Anesth. 2023, 87, 111109. [Google Scholar] [CrossRef]
- Tanaka, N.; Okada, M.; Tanaka, K.; Onishi, T.; Hirao, Y.; Harada, S.; Kawahira, M.; Koyama, Y.; Fujii, K.; Watanabe, H.; et al. Sleep apnea severity in patients undergoing atrial fibrillation ablation: Home sleep apnea-test and polysomnography comparison. J. Arrhythm. 2023, 39, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, J.; Vinck, T.; de Louw, B.; Slingerland, S.; van ’t Veer, M.; Regis, M.; Jansen, J.M.; van den Heuvel, E.; Dekker, L. Improving outcomes of AF ablation by integrated personalized lifestyle interventions: Rationale and design of the prevention to improve outcomes of PVI (POP) trial. Clin. Res. Cardiol. 2023, 112, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Hunt TE, F.; Traaen, G.M.; Aakerøy, L.; Massey, R.J.; Bendz, C.; Øverland, B.; Akre, H.; Steinshamn, S.; Loennechen, J.P.; Broch, K.; et al. Cardiac remodelling in patients with atrial fibrillation and obstructive sleep apnoea. Open Heart 2024, 11, e002718. [Google Scholar] [CrossRef]
- Kadhim, K.; Elliott, A.D.; Middeldorp, M.E.; Nalliah, C.J.; McEvoy, R.D.; Antic, N.A.; Pathak, R.K.; Emami, M.; Lau, D.H.; Kalman, J.M.; et al. Development of a Multivariable Prediction Model to Estimate Probability of Sleep-Disordered-Breathing in Patients With AF: MOODS-AF. JACC Clin. Electrophysiol. 2024, 11, 309–317. [Google Scholar] [CrossRef]
- Rogel, M.; Iverson, L.; Hall, A. Identifying Obstructive Sleep Apnea Risk Using the STOP-BANG Questionnaire in a Cardiology Clinic. J. Healthc. Qual. 2024, 46, 51–57. [Google Scholar] [CrossRef]
- Wei, H.; Luo, Y.; Wei, C.; Liao, H.; Nong, F. Cardiac structural and functional changes in OSAHS patients with heart failure with preserved ejection fraction and atrial fibrillation. BMC Cardiovasc. Disord. 2024, 24, 562. [Google Scholar] [CrossRef]
- Linz, D.; Dobrev, D. Sleep apnea and atrial fibrillation: Update 2020. IJC Heart Vasc. 2020, 31, 100681. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Baymukanov, A.M.; Weissman, Y.D.; Bulavina, I.A.; Ilyich, I.L.; Termosesov, S.A. Sleep Apnea and Atrial Fibrillation: Clinical Features and Screening Diagnostic Options. J. Pers. Med. 2024, 14, 618. [Google Scholar] [CrossRef]
- Linz, D.; McEvoy, R.D.; Cowie, M.R.; Somers, V.K.; Nattel, S.; Lévy, P.; Kalman, J.M.; Sanders, P. Associations of Obstructive Sleep Apnea With Atrial Fibrillation and Continuous Positive Airway Pressure Treatment: A Review. JAMA Cardiol. 2018, 3, 532–540. [Google Scholar] [CrossRef]
- O’Malley, K.J.; Cook, K.F.; Price, M.D.; Wildes, K.R.; Hurdle, J.F.; Ashton, C.M. Measuring diagnoses: ICD code accuracy. Health Serv. Res. 2005, 40, 1620–1639. [Google Scholar] [CrossRef]
- Oliveira, M.G.; Garbuio, S.; Treptow, E.C.; Polese, J.F.; Tufik, S.; Nery, L.E.; Bittencourt, L. The use of portable monitoring for sleep apnea diagnosis in adults. Expert Rev Respir. Med. 2014, 8, 123–132. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, Y. New Metrics from Polysomnography: Precision Medicine for OSA Interventions. Nat. Sci. Sleep 2023, 15, 69–77. [Google Scholar] [CrossRef]
- Sousa, S.R.; Caldeira, J.N.; Moita, J. Beyond Apnea-Hypopnea Index: How Clinical and Comorbidity Are Important in Obstructive Sleep Apnea. Adv. Respir. Med. 2022, 90, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Ayappa, I.; Ayas, N.; Collop, N.; Kirsch, D.; Mcardle, N.; Mehra, R.; Pack, A.I.; Punjabi, N.; White, D.; et al. Metrics of sleep apnea severity: Beyond the apnea-hypopnea index. Sleep 2021, 44, zsab030. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Thakur, S.; Rai, D.K.; Karmakar, S. Connecting the dots: Analysing the relationship between AHI and ODI in obstructive sleep apnea. Sleep Sci. Pract. 2024, 8, 1–8. [Google Scholar] [CrossRef]
- Sousa, S.; Silva Cunha, P.; Oliveira, M.M.; Drummond, M.; Bugalho, A. Sleep apnea and atrial fibrillation—A different kind of rhythm. IJC Heart Vasc. 2020, 29, 100548. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.L.S.S.; Cardoso, T.C.; Muniz, H.K.M.; Queiroz, R.C.; Souza, D.M.; Galli, L.C.P.; Cavalcante, R.L.C.; Junior, P.S.L.A. Síndrome da Apneia obstrutiva do sono: Risco para o desenvolvimento de fibrilação atrial. Braz. J. Health Rev. 2022, 5, 18092–18106. [Google Scholar] [CrossRef]
- Sousa, S.; Cunha, P.S.; Oliveira, M.; Bugalho, A.; Linz, D.; Drummond, M. Structured Pathway for Sleep-Disordered Breathing Screening in an Arrhythmia Unit. J. Atr. Fibrillation Electrophysiol. 2023, 16, 58–62. Available online: https://www.jafib-ep.com (accessed on 26 May 2025).
- Khan, A.; Patel, J.; Sharma, D.; Riaz, S.; Demissie, S.; Szerszen, A. Obstructive Sleep Apnea Screening in Patients With Atrial Fibrillation: Missed Opportunities for Early Diagnosis. J. Clin. Med. Res. 2018, 11, 21–25. [Google Scholar] [CrossRef]
- Szymanski, F.M.; Filipiak, K.J.; Platek, A.E.; Hrynkiewicz-Szymanska, A.; Kotkowski, M.; Kozluk, E.; Kiliszek, M.; Sierdzinski, J.; Opolski, G. Presence and severity of obstructive sleep apnea and remote outcomes of atrial fibrillation ablations-a long-term prospective, cross-sectional cohort study. Sleep Breath. 2015, 19, 849–856. [Google Scholar] [CrossRef]
- Wirth, D.; Nalliah, C.; Wong, G.; Voskoboinik, A.; Pathik, B.; Prabhu, S.; Morton, J.; Lee, G.; Joseph, S.; Linget, H.; et al. Obstructive Sleep Apnoea in Patients Having Atrial Fibrillation Ablation: Prevalence, Severity and Association with Arrhythmia Phenotype. Heart Lung Circ. 2017, 26, S187. [Google Scholar] [CrossRef]
- Latina, J.M.; Mark, N.A.; Iii, E.; Garlitski, A.C. The Relationship between Obstructive Sleep Apnea and Atrial Fibrillation: A Complex Interplay. Pulm. Med. 2013, 2013, 621736. [Google Scholar] [CrossRef]
- Saleeb-Mousa, J.; Nathanael, D.; Coney, A.M.; Kalla, M.; Brain, K.L.; Holmes, A.P. Mechanisms of Atrial Fibrillation in Obstructive Sleep Apnoea. Cells 2023, 12, 1661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lu, Q.; Li, S.; Pu, Z.; Gao, F.; Zhou, B. Risk factors associated with the severity of obstructive sleep apnea syndrome among adults. Sci. Rep. 2020, 10, 13508. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, Y.M.; Zhu, J.; Wu, S.; Wang, J.; Zhang, H.; Shao, X.H.; Mo, R.; Tan, J.S.; Wang, J.Y. Clinical characteristics and thrombotic risk of atrial fibrillation with obstructive sleep apnea: Results from a multi-center atrial fibrillation registry study. BMC Cardiovasc. Disord. 2022, 22, 59. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Bansal, A.; Yanamaladoddi, V.R.; Sarvepalli, S.S.; Vemula, S.L.; Aramadaka, S.; Mannam, R. Atrial Fibrillation in Obstructive Sleep Apnea Patients: Mechanisms, Risk Factors, and Management Strategies. Cureus 2023, 15, e36282. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kitey, P.; Kataria, V.; Nair, M. Polysomnography in AF patients without prior diagnosis of obstructive sleep apnea reveals significant sleep abnormality: A strong case for screening in all patients with atrial fibrillation? Indian Pacing Electrophysiol. J. 2022, 22, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, M.; Xue, P.; Lu, Y.; Tang, B. Prediction model and scoring system for the risk of atrial fibrillation recurrence in patients with atrial fibrillation and obstructive sleep apnoea syndrome: A retrospective case-control study. BMC Cardiovasc. Disord. 2025, 25, 308. [Google Scholar] [CrossRef]
- Dalgaard, F.; North, R.; Pieper, K.; Fonarow, G.C.; Kowey, P.R.; Gersh, B.J.; Mahaffey, K.W.; Pokorney, S.; Steinberg, B.A.; Naccarrelli, G. Risk of major cardiovascular and neurologic events with obstructive sleep apnea among patients with atrial fibrillation. Am. Heart J. 2020, 223, 65–71. [Google Scholar] [CrossRef]
- Szymanski, F.M.; Filipiak, K.J.; Platek, A.E.; Hrynkiewicz-Szymanska, A.; Karpinski, G.; Opolski, G. Assessment of CHADS2 and CHA2DS2-VASc scores in obstructive sleep apnea patients with atrial fibrillation. Sleep Breath. 2015, 19, 531–537. [Google Scholar] [CrossRef]
- Jean-Louis, G.; Zizi, F.; Clark, L.T.; Brown, C.D.; McFarlane, S.I. Obstructive Sleep Apnea and Cardiovascular Disease: Role of the Metabolic Syndrome and Its Components. J. Clin. Sleep Med. 2008, 4, 261–272. [Google Scholar] [CrossRef]
- Iacono, I.C.A.M.L.; Reali, E.; Perciballi, P.; Cacciafesta, M. Correlation between Obstructive Sleep Apnea Syndrome, Arrhythmias and Autonomic Cardiac Activity: A Retrospective Case-Control Study. J. Sleep Disord. Manag. 2020, 6. [Google Scholar] [CrossRef]
- Grandner, M.A.; Alfonso-Miller, P.; Fernandez-Mendoza, J.; Shetty, S.; Shenoy, S.; Combs, D. Sleep: Important considerations for the prevention of cardiovascular disease. Curr. Opin. Cardiol. 2016, 31, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Marulanda-Londoño, E.; Chaturvedi, S. The Interplay between Obstructive Sleep Apnea and Atrial Fibrillation. Front. Neurol. 2017, 8, 668. [Google Scholar] [CrossRef]
- Vazir, A.; Kapelios, C.J. Sleep-disordered breathing and cardiovascular disease: Who and why to test and how to intervene? Heart 2023, 109, 1864–1870. [Google Scholar] [CrossRef]
- Affas, Z.; Affas, S.; Tabbaa, K. Continuous positive airway pressure reduces the incidence of atrial fibrillation in patients with obstructive sleep apnea: A Meta-Analysis and Systematic Review. Spartan Med. Res. J. 2022, 7, 34521. [Google Scholar] [CrossRef]
- Lebkuchen, A.; Freitas, L.S.; Cardozo, K.H.M.; Drager, L.F. Advances and challenges in pursuing biomarkers for obstructive sleep apnea: Implications for the cardiovascular risk. Trends Cardiovasc. Med. 2021, 31, 242–249. [Google Scholar] [CrossRef]
- Yu, B.; Wei, J.; Zhao, J.; Fan, H.; Zhang, W.; Li, X.; Wang, L.; Zhang, Y.; Ren, Z.; Song, X.; et al. The neutrophil-to-lymphocyte ratio is a potential biomarker for the occurrence of atrial fibrillation in patients with obstructive sleep apnea. Sleep Med. 2023, 110, 259–267. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year Country | Objectives | Study Design | Patients Characteristics (N, % Male, Mean Age [Unless Otherwise Reported]) | Diagnostic and Monitoring Assessments | Reported Prevalence |
|---|---|---|---|---|---|
| Caples, 2019 [46] USA | To assess the impact of CPAP treatment on the recurrence of AF after direct current cardioversion in OSA pts | Randomized controlled trial | 25 pts with OSA and AF: PAP, 12 pts, 58% male, 63.5 ± 7.9 yrs Control, 13 pts, 54% male, 64.6 ± 10.1 yrs | OSA diagnosis: Attended split-night PSG | Mild OSA: PAP, 31% Control, 25% Moderate OSA: PAP, 31% Control, 42% Severe OSA: PAP, 38% Control, 33% |
| Feng, 2019 [23] USA | To understand the effect of OSA on hospital readmission rates and post-operative AF in the cardiac surgical population | Observational retrospective cohort study | 506,604 pts after CABG or valve surgery, 68.2% male, 66.2 ± 12.1 yrs | OSA diagnosis: Based on clinical documentation (hospital records and clinical databases) and ICD-9 coding | OSA, 6.4% Post-operative AF in OSA pts, 40.4% |
| Gonçalves, 2019 [7] Portugal | To evaluate the diagnostic utility and accuracy of a PM-incorporated respiratory monitoring algorithm and its interaction with AF | Single-centre, observational prospective diagnostic accuracy study | 81 pts with pacemakers, 58% male, 73 ± 11 yrs | OSA diagnosis: PSG Pacemaker respiratory monitoring algorithm tested as screening tool | OSAS, 62% OSAS severity: Mild, 40% Moderate, 30% Severe, 30% AF during follow-up in OSAS pts, 42% |
| Hojo, 2019 [47] Japan | To evaluate the impact of OSA on AF recurrence after multiple pulmonary vein isolation (PVI) sessions using a contact force-sensing catheter (CF-C) | Non-randomized, observational prospective cohort study | 100 AF pts Non-OSA, 66 pts, 66.7% male, 60.4 ± 12.2 yrs Treated OSA (CPAP), 11 pts, 72.7% male, 66.3 ± 9.6 yrs Untreated OSA, 23 pts, 82.6% male, 68.3 ± 8.8 yrs | OSA Diagnosis: AHI ≥ 15 (Type 3 sleep study or PSG in selected cases) | OSA, 34% AF recurrence, 1st session: Non-OSA, 18.2% OSA, 14.7% AF recurrence, 2nd session: Non-OSA, 12.1% Treated OSA, 9.1% Untreated OSA, 8.7% |
| Linz, 2019 [48] Australia | To investigate night-to-night variability in SDB severity and its relationship with the daily risk of incident AF using simultaneous long-term day-by-day SDB and AF monitoring | Observational prospective cohort study (VARIOSA-AF Study) | 72 pts with dual-chamber pacemakers capable of monitoring nightly SDB and daily AF burden, sex and age not reported | SDB Monitoring: Pacemaker-based SAM algorithm (RDI via transthoracic impedance) | Severe SDB, 32% |
| Providência, 2019 [49] Europe (7 countries) | To assess the impact of BMI on AF ablation outcomes, with emphasis on OSA as an independent predictor | Non-randomized, multicentre, international, observational prospective cohort study | 2497 consecutive AF pts undergoing CA for AF, 70.6% male, 61.1 ± 10.2 yrs | OSA Diagnosis: Clinical history and sleep specialist referral | OSA, 7.0% |
| Bazan, 2020 [50] Spain | To investigate the prevalence of previously undetected OSA in pts with new-onset AF and provide a practical screening strategy for CPAP indication | Observational prospective diagnostic study | 73 pts with new-onset AF, 67% male, 61 ± 10 yrs | OSA Diagnosis: Ambulatory RP (ApneaLink, ResMed); AHI ≥ 5 | OSA, 82% OSA severity: Mild, 26% Moderate, 26% Severe, 30% |
| Ben Halima, 2020 [36] Tunisia | To determine the prevalence and severity of OSAS in pts with NVAF and identify OSAS’s predictive factors in this population | Cross-sectional study | 100 NVAF, 45% male, 66.4 ± 9.7 yrs | OSAS Diagnosis: PSG confirmation Screening Tools: Berlin Questionnaire and ESS | OSAS, 90% OSAS severity: Mild, 32% Moderate, 27% Severe, 31% |
| Gellert, 2020 [51] USA | To investigate the association between SA and AF, premature atrial contractions, and premature ventricular contractions using 48 h ambulatory ECG | Observational, retrospective population-based cohort study from the Atherosclerosis Risk in Communities (ARIC) study | 1154 pts, middle-aged to older adults SA, 45% male Non-SA, 34% male | SA Diagnosis: ICD-9 codes or self-reported physician diagnosis | SA, 18.8% AF, 6% |
| Martí-Almor, 2020 [52] Europe | To evaluate the incidence and severity of SA and its association with AF in an unselected population after implantation with an SA monitoring–enabled dual-chamber pacemaker | Multicentre, international, open-label, observational prospective cohort study (RESPIRE study) | 553 pts, 61.5% male, 72.2% aged 65–85 yrs | SA Severity: Pacemaker SAM algorithm (transthoracic impedance for respiratory disturbances) | Severe SA, 31.1% Significant AF at 12 months: 25.0% (severe SA) vs. 13.9% (non-severe SA), p = 0.002 Persistent AF at 12 months: 16.9% (severe SA) vs. 7.3% (non-severe SA), CI 2.5–16.6% (p not explicitly stated but significant) |
| May, 2020 [15] USA | To evaluate and optimize SA screening tools in AF pts and to develop a novel screening model | Case–control study with matched controls (sex, race, age, BMI) within the SAFEBEAT trial | 300 pts, 63.3% male, 61.4 ± 11.9 yrs PAF, 150 pts Control, 150 pts | SA Diagnosis: Full overnight PSG with manual scoring by certified polysomnologists; AHI ≥ 5 and ≥15 used to define SA presence and severity Screening Tools: STOP, STOP-BANG, Berlin, NoSAS, and ESS; a novel model (NABS: neck circumference, age, BMI, snoring) was developed and validated | SA PAF group AHI ≥ 5, 68.0% AHI ≥ 15, 43.3% SA Control group AHI ≥ 5, 69.3% AHI ≥ 15, 46.0% |
| Mazza, 2020 [53] Italy | To investigate pacemaker-detected SA variability, assess its association with AF and evaluate the associated long-term risk of stroke and death | Observational prospective cohort study, using pacemaker algorithms to detect SDB | 439 pacemaker recipients, 57% male, 78 ± 8 yrs | SA Monitoring: Pacemakers with ApneaScan algorithm (Boston Scientific) to detect SA via thoracic impedance changes | Severe SA, RDI ≥ 30 episodes/h, 80% AF ≥ 6 h, 29% |
| Shapira-Daniels, 2020 [54] USA | To assess the prevalence of undiagnosed SA in AF pts undergoing ablation and its impact on SA therapy adherence | Observational prospective study at two tertiary referral centres | 188 AF pts scheduled for CA, without a prior diagnosis of SA, 65.4% male, 62 ± 11.3 yrs | OSA Diagnosis: HSAT (WatchPAT), single-night session | SA, 82.4% SA severity: Mild, 43.8% Moderate, 32.9% Severe, 23.2% |
| Starkey, 2020 [55] Canada | To compare the accuracy of different OSA screening tools (NoSAS, STOP-BANG, AP) with HSAT in an AF population | Observational prospective diagnostic accuracy study | 188 pts with NVAF, 61.2% male, 69.0 ± 9.5 yrs | OSA Diagnosis: HSAT (ApneaLink, ResMed); manually scored by respiratory therapists and interpreted by a sleep physician | OSA, 86% OSA severity: Mild, 36.7% Moderate, 32.4% Severe, 17.0% |
| Szymańska, 2020 [56] Poland | To assess the association between visfatin concentrations and OSA in AF pts | Observational study | 266 hospitalized pts with AF, 65%, 57.6 ± 10.1 yrs | OSA Diagnosis: Overnight respiratory polygraphy (Embletta MPR); manually scored per AASM guidelines by certified sleep specialist | OSA, 45.5% |
| Traaen, 2020 [57] Norway | To study the prevalence, characteristics, risk factors, and type of SA in ablation candidates with PAF | Observational prospective cohort study | 579 pts with PAF, 72.9% male, 59.9 ± 9.6 yrs | SA Diagnosis: Home Type 3 polygraphy over two nights (NOX Medical device) | SA, 82.7% SA severity: Moderate–severe (AHI ≥ 15), 42.1% Severe (AHI ≥ 30), 12.1% |
| Blanchard, 2021 [58] France | To evaluate whether oxygen desaturation and PRV indices derived from nocturnal oximetry are associated with incident AF in pts investigated for OSA | Multicentre, observational prospective cohort study (Pays de la Loire Sleep Cohort) | 7205 AF-free pts, 62.3% male, median age 60 (IQR 50–70) yrs | OSA Diagnosis: PSG (52%) and RP (48%) Hypoxemia Assessment: Pulse oximetry (ODI, T90, nadir SpO2) and PRV metrics | OSA, 86.7% OSA severity: Mild, 22.8% Moderate, 22.4% Severe 41.5% AF, 2.5% |
| Delesie, 2021 [59] Belgium | To test the performance of OSA screening questionnaires/scoring scales in AF pts referred for PSG | Multicentre, observational prospective diagnostic validation study | 100 pts with known previous AF, referred for PSG, 73% male, 64.0 ± 8.7 yrs | OSA Diagnosis: PSG | OSA (AHI ≥ 15), 69% Severe OSA (AHI ≥ 30), 33% |
| Mohammadieh, 2021 [60] Australia | To evaluate the diagnostic accuracy of commonly used OSA screening tools in AF pts and assess the prevalence of undiagnosed OSA in a hospital-based AF cohort | Prospective diagnostic accuracy study comparing multiple screening tools with gold standard PSG | 107 pts with ECG-documented AF, 65.4% male, 61.3 ± 11.7 yrs | OSA Diagnosis: In-laboratory PSG (gold standard) and Level 3 HSAT (ApneaLink Air, ResMed) Screening Tools: Berlin Questionnaire, STOP-BANG, ESS, Mallampati score, BMI, snoring | OSA, 62.6% OSA severity: Mild, 31.8% Moderate, 18.7% Severe, 12.1% |
| Højager, 2022 [61] Denmark | To investigate the prevalence of silent AF and associated risk factors in pts with OSA | Multicentre, prospective cross-sectional study, conducted in two centres (hospital and private ENT clinic) | 303 pts investigated for OSA, 68.6% male, 56.4 ± 12.4 yrs No/mild, 65 pts Moderate/severe, 238 pts | OSA Diagnosis: One-night home Type 3 sleep study (NoxT3™, ResMed); AHI used to classify severity | OSA, 78.5% OSA severity: Moderate, 30.7% Severe, 47.9% AF overall, 7.3% AF per OSA severity Mild, 1.5% Moderate/severe, 8.8% Severe, 10.7% |
| Latif, 2022 [62] USA | To analyze the prevalence of SA in AF pts and assess the impact of widespread SA screening on AF progression | Observational retrospective cohort study | 185 AF pts, 72% male, median age 64 (IQR 58–71) yrs | OSA Diagnosis: HST and PSG | OSA, 49% OSA severity: Mild, 75% Moderate, 18% Severe, 7% |
| Mehawej, 2022 [63] USA | To examine the associations of OSA with frailty, cognitive performance, and AF-related QoL among older adults with AF | Observational, cross-sectional study based on prospective cohort data (the SAGE-AF study) | 970 AF pts, 51% male, 75 yrs, 680 pts without OSA Low risk, 179 pt Intermediate, 360 pts High risk, 141 pts | OSA Diagnosis: Risk assessed using STOP-BANG questionnaire (not confirmed by PSG or HSAT) OSA Classification: STOP-BANG scores | OSA, 29.9% |
| Oster, 2022 [64] USA | To implement and evaluate the effectiveness of an evidence-based OSA bundle (including screening, education, and referral) in a hospitalized high-risk AF population | Pre-Post Quality Improvement project, with retrospective comparison group, evaluating the impact of a 3-month OSA intervention bundle on OSA screening, sleep clinic referral and follow-up | 101 hospitalized AF pts Comparison, 68 pts, 48.5% male, 73.0 ± 11.6 yrs Intervention, 33 pts, 51.6% male, 69.0 ± 10.4 yrs | OSA Diagnosis: Screening using the STOP-Bang questionnaire; follow-up diagnosis via PSG or HSAT in referred cases STOP-Bang ≥ 3 triggered education and referral to a sleep clinic; OSA confirmed through formal sleep testing | Moderate to high-risk for OSA (modified STOP-Bang score ≥ 3), 52.9% |
| Verhaert, 2022 [65] Netherlands | To implement and evaluate a virtual sleep-SDB management pathway for AF pts scheduled for ablation | Observational prospective, remote SA screening study (VIRTUAL-SAFARI) | 119 pts,55% male, 65 ± 9.5 yrs | OSA Diagnosis: One-night home sleep apnea test using WatchPAT-ONE or WatchPAT 300; data reviewed and interpreted by sleep physicians | Moderate-to-severe SDB, 55% SDB severity: Mild (AHI 5 < 15), 30% Moderate (AHI 15 < 30), 38% Severe (AHI ≥ 30), 17% |
| Ahn, 2023 [66] South Korea | To investigate age-related differences in clinical features, recurrence of atrial tachyarrhythmia, and predictors after radiofrequency CA for AF | Multicentre, observational prospective cohort study (Korean Heart Rhythm Society Ablation Registry for AF KARA) | 2799 AF pts, 73.5% male, 59.9 ± 9.6 yrs Group A (<60 yrs), 1269 pts, 79.2% male, 51.6 ± 6.7 yrs Group B (≥60 yrs), 1530 pts, 68.7% male, 66.8 ± 5.2 yrs | OSA Diagnosis: Clinical history; no formal sleep study protocol described | OSA, 4.6% OSA prevalence: Group A, 6.5% Group B, 3.0% |
| Betz, 2023 [67] Netherlands | To assess the accuracy of STOP-BANG in detecting SDB in AF pts and to develop an AF-specific screening tool (BOSS-GAP score) to improve pre-selection for SDB testing | Observational prospective diagnostic accuracy study (sub-study of ISOLATION cohort and registry) | 206 symptomatic AF pts scheduled for CA, without known SDB, 58% male, median age 65 (IQR 58–70) yrs | SDB Diagnosis: Home sleep apnea test using WatchPAT-ONE or WatchPAT 300; results reviewed by a sleep physician; AHI ≥ 15 defined moderate-to-severe SDB Screening Tools: STOP-Bang questionnaire assessed; refined into AF-specific BOSS-GAP score for improved pre-selection | Moderate-to-severe SDB, 51% SDB severity: Mild, 34% Moderate, 34% Severe, 17% |
| Holtstrand, 2023 [68] Sweden | To explore the association of OSA severity with AF in a sleep clinic cohort stratified by EDS | Cross-sectional study | 3814 pts, 65.9% male, 53.8 ± 12.8 yrs | OSA Diagnosis: Home Sleep Apnea Test (HSAT) using Embletta® device; manually scored by two sleep physicians; AHI used to define OSA severity (≥5, ≥15, ≥30 events/h for mild, moderate, and severe OSA) Sleepiness Assessment: ESS; EDS defined as ESS ≥ 11 | OSA, 90.3% OSA severity: Mild, 23.7% Moderate, 35% Severe, 31.6% Baseline AF, 5.3% AF prevalence: Non-OSA, 1.6% Mild OSA, 3.9% Moderate OSA, 5.2% Severe OSA, 7.6% |
| Jensen, 2023 [69] Denmark | To assess SA prevalence and severity in AF pts using a home-monitoring device (NightOwl™) and evaluate its predictive value against CRM | Cross-sectional study | 126 AF pts without known SA, 67% male, median age 68 yrs (IQR 60–75) | SA Diagnosis: Four-night home monitoring using NightOwl™ device; AHI ≥ 15 used to define moderate-to-severe SA; validation in a subgroup with CRM Screening Tool: NightOwl™ device (photoplethysmography -based sensor with smartphone integration); positive predictive value validated against CRM | Moderate to severe SA (AHI > 15), 56% SA severity: Mild (AHI 5–15), 36% Moderate (AHI 15–30), 28% Severe (AHI > 30), 29% |
| Lin, 2023 [70] China | To evaluate the application of STOP-BANG in screening OSAHS in AF pts | Cross-sectional study | 63 AF pts, 50.8% male, 62.5 ± 10.7 yrs | OSAHS Diagnosis: STOP-BANG questionnaire and full overnight PSG (Philips Alice 5/6 system); AHI ≥ 5 events/h used to confirm OSAHS, with severity classification Screening Tool: STOP-BANG questionnaire | OSAHS, 82.5% OSAHS severity: Mild, 32.7% Moderate, 34.6% Severe, 32.7% |
| Mills, 2023 [71] USA | To define the prevalence of undiagnosed OSA in consecutive ambulatory AF pts and evaluate its impact on AF-related QoL | Observational prospective, cohort study (single-centre phase IV registry) | 38 consecutive ambulatory AF pts investigated for OSA with WatchPAT, 68.4% male, median age 58.3 (IQR 52.0–69.0) yrs | OSA Diagnosis: One-night home sleep test using WatchPAT-One or WatchPAT-300; interpreted by board-certified sleep medicine specialists; AHI ≥ 5 defined OSA, AHI ≥ 15 defined moderate-to-severe OSA Screening Tools: STOP-Bang questionnaire and ESS used alongside HST | OSA, 79% OSA severity: Mild, 42,1% Moderate/Severe, 36.8% |
| Ozkan, 2023 [72] Turkey | To investigate the relationship between the visceral adiposity index and AF development in OSAS pts | Observational retrospective study | 207 pts with OSAS, 80.1% male, 49.9 ± 4.06 yrs AF group, 44 pts NSR group, 163 pts | OSAS Diagnosis: Based on AHI; severity defined as mild (5–15), moderate (15–30), or severe (>30) | AF, 21.3% |
| Rivas, 2023 [73] USA | To evaluate the association between OSA and postoperative AF and delirium after cardiac surgery in DECADE trial pts | Sub-analysis of a multicentre, randomized, double-blind, placebo-controlled clinical trial (DECADE trial) | 590 pts, sex and age not re-ported | OSA Diagnosis: STOP-BANG questionnaire score >5 and/or preoperative diagnosis of OSA (ICD-9 codes) | OSA, 23% AF incidence, 37% |
| Tanaka, 2023 [74] Japan | To compare HSAT using Watch-PAT and PSG in AF pts undergoing CA | Single-centre, observational retrospective study | 464 consecutive AF pts, 76.5% male, 64.9 ± 10.6 yrs | SA Diagnosis: Both WatchPAT (home sleep apnea test) and in-lab PSG; AHI ≥ 5 used for SA classification; PSG used to determine CPAP indication | SA, 88.6% SA severity: Mild, 18.3% Moderate, 50.9% Severe, 30.0% |
| Vermeer, 2023 [75] Netherlands | To evaluate the impact of integrated, nurse-led lifestyle intervention program including OSA screening on AF ablation outcomes | Prospective, 1:1 randomized, controlled, single-centre, open-label, investigator-initiated clinical trial (POP Trial) | 150 pts with paroxysmal or persistent AF referred for 1st PVI, sex not yet reported, age range 18–75 yrs | OSA Diagnosis: HSAT using WatchPAT™ 300; OSA suspected if AHI ≥ 5/h | Prevalence data not yet reported (ongoing study) |
| Hunt, 2024 [76] Norway | To evaluate the impact of CPAP treatment on LA and LV remodelling in pts with OSA and PAF before and after CA | Randomized controlled trial (A3 trial) | 108 pts with PAF and moderate-to-severe OSA, 76% male, 63 ± 7 yrs CPAP, 55 pts Standard care, 54 pts | OSA Diagnosis: Respiratory polygraphy over two nights; OSA defined as AHI ≥ 15 events/hour | Moderate/severe OSA, 42% |
| Kadhim, 2024 [77] Australia | To develop and validate a prediction model (MOODS-AF) to estimate the probability of moderate-to-severe SDB (AHI ≥ 15/h) in AF pts | Multicentre, prospective cohort study with external validation cohort | 851 total pts Derivation cohort, 442 ambulatory AF pts undergoing PSG, 69.2% male, 60 ± 11 yrs Validation cohort, 409 pts, 75.8% male, 59 ± 10 yrs | SDB Diagnosis: In-lab PSG; moderate-to-severe SDB defined as AHI ≥ 15 events/h Screening Tool: MOODS score developed and validated as a simplified clinical prediction tool based on sex, BMI, diabetes, and prior stroke/TIA | Any SDB: Derivation cohort, 66.1% Validation cohort, 86% Significant SDB: Derivation cohort, 34% Validation cohort, 54% |
| Rogel, 2024 [78] USA | To screen and stratify OSA risk in adult pts using the STOP-BANG during clinic visits over 6 weeks. To increase provider referrals for sleep studies in high-risk identified pts (STOP-Bang ≥ 5) following questionnaire’s implementation | Quality Improvement Project (prospective, observational, comparative study focused on evaluating OSA systematic screening impact in a cardiology patient population) | 963 pts Pre implementation, 535 pts, 48% male, 68.2 ± 13.3 yrs Implementation, 428 pts, 279 pts screened with STOP-Bang, 49% male, 68.8 ± 11.1 yrs | OSA Risk Assessment: STOP-BANG questionnaire administered in outpatient cardiology clinic | High risk for OSA, 25% AF prevalence: Pre implementation, 19% Implementation, 16% |
| Wei, 2024 [79] China | To explore cardiac structural and functional changes in OSAHS pts with HFpEF and AF | Observational retrospective study | 336 OSAHS pts with HFpEF Group A (non-AF), 187 pts, 50.3% male, 74.9 ± 9.2 yrs Group B (AF history, no episodes), 56 pts, 55.4% male, 71.3 ± 11.7 yrs Group C (AF history and episodes), 93 pts, 57.0% male, 73.9 ± 10.4 yrs | OSAHS Diagnosis: In-lab PSG using Embletta X20; severity classified based on AHI: mild (5–15), moderate (15–30), severe (>30) | Mild OSA: Group A, 63.1% Group B, 42.9% Group C, 11.8% Moderate OSA: Group A, 27.3% Group B, 37.5% Group C, 45.2% Severe OSA: Group A, 9.6% Group B, 19.6% Group C, 41.7% AF prevalence: Mild OSA, 22.9% Moderate OSA, 55.3% Severe OSA, 74.0%. |
| Very High Prevalence (≥80%) | High Prevalence (60–79%) | Moderate Prevalence (30–59%) | Low Prevalence (<30%) |
|---|---|---|---|
| OSA Prevalence | |||
| Bazan 2020 [50], ~82% | Gonçalves 2019 [7], ~62% | Mehawej 2022 [63], ~30% | Ahn 2023 [66], ~5% |
| Lin 2023 [70], ~83% | Mohammadieh 2021 [60], ~63% | Hojo 2019 [47], ~34% | Feng 2019 [23], ~6% |
| Starkey 2020 [55], ~86% | Delesie 2021 [59], AHI ≥ 15: ~69% | Hunt 2024 [76], 42% | Providência 2019 [49], ~7% |
| Blanchard 2021 [58], ~87% | Højager, 2022 [61], ~79% | Szymanska, 2020 [56], ~46% | Gellert 2020 [51], ~19% |
| Ben Halima 2020 [36], ~90% | Mills 2023 [71], ~79% | Latif 2022 [62], ~49% | Rivas 2023 [73], 23% |
| Holtstrand 2023 [68], ~90% | |||
| SA prevalence | |||
| Mazza 2020 [53], ~80% severe SA | Marti-Almor 2020 [52], ~31% severe SA | ||
| Shapira-Daniels 2020 [54], ~82% | May 2020 [15], AHI ≥ 15: ~45% | ||
| Traaen 2020 [57], ~83% | Jensen, 2023 [69], 56% moderate to severe SA | ||
| Tanaka 2023 [74], ~89% | |||
| SDB prevalence | |||
| Linz 2019 [80], ~32% severe SDB | |||
| Kadhim 2024 [77], ~76% any SDB, 44% significant SDB | |||
| Betz 2023 [67], ~51% moderate to severe SDB | |||
| Verhaert 2022 [65], ~55%, moderate to severe SDB | |||
| First Author, Year | Risk Factors | Symptoms of OSA/SA/SDB in AF |
|---|---|---|
| Caples, 2019 [46] | Control vs. PAP (all pts had OSA): No significant differences between groups in age (64.6 vs. 63.5 yrs), sex (54% vs. 58% male), BMI (35.8 vs. 36.0 kg/m2), LVEF (57.3% vs. 58.1%), or LAVI (35.6 vs. 40.6 mL/m2), all p > 0.05 | Control vs. PAP (all pts had OSA): Baseline ESS: 7.3 ± 3.3 vs. 4.8 ± 2.1, p = 0.04; follow-up ESS: 5.7 vs. 5.8, p = 0.17 Baseline FOSQ: 17.6 ± 1.6 vs. 19.1 ± 0.7, p = 0.01; follow-up FOSQ: 17.5 vs. 18.3, p = 0.26 |
| Feng, 2019 [23] | Risk factors for OSA vs. non-OSA: Younger age, male sex, white race, hypertension (uncomplicated and complicated), chronic pulmonary disease, diabetes (uncomplicated and complicated), renal failure, obesity, and depression were more prevalent among OSA pts | OSA diagnosis based on ICD-9 codes; symptoms not assessed |
| Gonçalves, 2019 [7] | No significant differences between pts with and without OSAS | Snoring, witnessed apneas, restless sleep, and EDS were more frequent in OSAS, but differences were not statistically significant Snoring: 68% (OSAS) vs. 42% (non-OSAS), p = 0.14. Witnessed SA: 20% (OSAS) vs. 19% (non-OSAS), p = 0.45. Restless sleep: 28% (OSAS) vs. 32% (non-OSAS), p = 0.74 EDS (ESS > 10): 14% (OSAS) vs. 6.5% (non-OSAS), p = 0.31. |
| Hojo, 2019 [47] | Risk factors for OSA vs. non-OSA: Older age, higher BMI, higher AHI, hypertension, and diabetes were significantly associated with OSA | Symptoms were not assessed; OSA diagnosed via sleep study based on AHI ≥ 15 |
| Linz, 2019 [48] | Not reported | Not reported |
| Providência, 2019 [49] | Risk factors for OSA: Higher BMI | Not reported |
| Bazan, 2020 [50] | Risk factors for OSA vs. non-OSA: Male sex, higher BMI, hypertension, higher CHA2DS2-VASc score | Snoring, observed apneas, fatigue, daytime sleepiness: Not individually reported STOP-BANG score: 4.1 ± 1.6 (OSA) vs. 2.6 ± 1.9 (non-OSA), p = 0.004 ESS: 8.4 ± 4 (OSA) vs. 9.3 ± 3 (non-OSA), p = 0.47 (NS) Berlin Questionnaire: 1.6 ± 0.9 (OSA) vs. 1.2 ± 1.1 (non-OSA), p = 0.12 (NS) |
| Ben Halima, 2020 [36] | Risk factors for OSAS vs. non-OSAS: Age > 61 yrs and AF duration > 2 yrs | Snoring: 90% (OSAS) vs. 60% (non-OSAS), p = 0.024 [Independent predictor: OR = 18.9 (95% CI 1.62–221.1), p = 0.019] ESS: 10.5 ± 4.1 (OSAS) vs. 9 ± 4.5 (non-OSAS), p = 0.2 (NS) ESS > 10: 56% (OSAS) vs. 30% (non-OSAS), p = 0.1 (NS) Berlin Questionnaire: Positive in 34% (OSAS) vs. 50% (non-OSAS), p = NS Other symptoms (e.g., sleep disorders, nocturia, daytime sleepiness, fatigue, memory, and cognitive impairment): Not significantly different |
| Gellert, 2020 [51] | Risk factors for SA vs. non-SA: Male sex, white race, current smoking, diabetes, CHD | SA was identified via self-reported physician diagnosis or ICD-9 codes; symptoms not assessed or compared |
| Martí-Almor, 2020 [52] | Risk factors for severe SA (RDI ≥ 20) and non-severe SA: Older age, male sex, AF history, CAD, cardiomyopathy, MI, HF, cardiac surgery history, smoking history, more frequent use of beta-blockers | Not reported |
| May, 2020 [15] | Risk factors for AF vs. non-AF (controls): Lower HR, larger LAV, and more frequent use of beta-blockers and calcium channel blockers | STOP-BANG Score: 3.5 ± 1.4 (PAF) vs. 3.4 ± 1.5 (controls), p = 0.91 Berlin Questionnaire: 1.6 ± 0.8 (PAF) vs. 1.6 ± 0.9 (controls), p > 0.99 NoSAS Score: 10.8 ± 4.1 (PAF) vs. 11.0 ± 4.0 (controls), p = 0.64 ESS: 7.5 ± 4.3 (PAF) vs. 8.3 ± 4.3 (controls), p = 0.12 |
| Mazza, 2020 [53] | Predictors of AF among pts with SA: RDI max ≥ 63 ep/h and RDI mean ≥ 46 ep/h RDI burden ≥ 7% not significantly associated with AF Death/stroke risk higher with AF ≥ 6 h In pts without AF history, RDI max ≥ 63 predicted death/stroke | Not reported, SA diagnosed via pacemaker-derived RDI; symptoms not assessed |
| Shapira-Daniels, 2020 [54] | Risk factors for SA vs. non-SA: Older age, higher BMI, and hypertension | Sleep symptoms (snoring, daytime sleepiness, fatigue, apneas): 69.0% (SA) vs. 69.7% (non-SA), p = 1.00 (NS) STOP-BANG score: median 4 [IQR 3–5] (SA) vs. 3 [IQR 2–4.5] (non-SA), p = 0.003 STOP-BANG positive: 81.2% (SA) vs. 57.6% (non-SA), p = 0.01 |
| Starkey, 2020 [55] | Risk factors for OSA: Male sex, higher BMI, larger neck circumference, smoking, hypertension, and more frequent use of beta-blockers | Average ESS score: 5.9 ± 3.9, range 0–19; ESS > 11: 9% OSA screening-related parameters (non-OSA vs. mild OSA vs. moderate OSA vs. severe OSA, no p-values reported): Snoring: 46.2% vs. 73.9% vs. 63.9% vs. 75.0% ESS: 5.2 ± 4.1 vs. 6.2 ± 4.0 vs. 6.1 ± 3.8 vs. 5.2 ± 3.4 NC: 35.2 ± 3.7 vs. 38.1 ± 3.7 vs. 39.3 ± 3.7 vs. 40.7 ± 4.4 cm MCA: 2.4 ± 0.7 vs. 2.4 ± 0.9 vs. 2.5 ± 1.1 vs. 2.2 ± 0.6 cm2 NoSAS score: 8.1 ± 3.4 vs. 9.6 ± 3.7 vs. 11.2 ± 3.6 vs. 12.6 ± 4.1 STOP-BANG score: 2.9 ± 1.4 vs. 3.4 ± 1.4 vs. 3.9 ± 1.3 vs. 4.3 ± 1.6 |
| Szymańska, 2020 [56] | Risk factors for OSA: Male sex, older age, higher BMI, more often arterial hypertension, history of stroke/peripheral thromboembolism and vascular disease, visfatin concentration > 1.25 ng/mL, permanent AF | Not reported |
| Traaen, 2020 [57] | Risk factors for SA Male sex, older age, higher BMI, habitual snoring, and AF duration | Values per SA severity (non-SA vs. mild SA vs. moderate SA vs. severe SA): Snoring: 55.6% vs. 66.5% vs. 85.9% vs. 81.2%, p = 0.056 Observed Apneas: 12.1% vs. 17.6% vs. 37.3% vs. 43.8%, p < 0.001 ESS score: 6.9 ± 4.3 vs. 7.1 ± 4.0 vs. 7.5 ± 3.6 vs. 7.1 ± 4.2, p = 0.367 Berlin Questionnaire (score ≥ 2): 25.3% vs. 36.4% vs. 54.1% vs. 58.8%, p < 0.001 STOP-BANG (score ≥ 3): 35.9% vs. 62.8% vs. 83.1% vs. 84.6%, p < 0.001 |
| Blanchard, 2021 [58] | Risk factors for AF vs. non-AF: Older age, male sex, higher BMI, diabetes, cardiac diseases, hypertension, more frequent use of beta-blockers and calcium channel blockers, greater PAP adherence, more severe nocturnal hypoxemia (higher T90), and altered autonomic regulation (increased RMSSD and SDNN; lower LF/HF ratio) | ESS: median 10 [IQR 6–13] (AF) vs. 10 [IQR 6–14] (non-AF), p = 0.2096 Fatigue and EDS not significantly different; no significant difference in classic symptoms |
| Delesie, 2021 [59] | Not reported | Not reported |
| Mohammadieh, 2021 [60] | Risk factors for OSA (AHI ≥ 15) vs. non-OSA (AHI < 15): Higher BMI, larger neck circumference, higher Modified Mallampati score, hypertension, congestive cardiac failure, higher CHA2DS2-VASc score, persistent/permanent AF, and larger left atrial area | Self-reported snoring: 78.8% (AHI ≥ 15) vs. 58.1% (AHI < 15), p = 0.039 ESS score: 6.9 ± 3.4 (AHI ≥ 15) vs. 5.7 ± 3.3 (AHI < 15), p = 0.079 (NS) STOP-BANG score: 4.7 ± 1.7 (AHI ≥ 15) vs. 3.0 ± 1.3 (AHI < 15), p < 0.001 Berlin questionnaire (high risk): 75.8% (AHI ≥ 15) vs. 25.7% (AHI < 15), p < 0.001 |
| Højager, 2022 [61] | Risk factors for OSA severity (moderate/severe vs. mild OSA): Older age (moderate and severe OSA), male sex (severe OSA), higher BMI (moderate and severe OSA), hypertension (moderate and severe OSA), dysregulated hypertension (moderate and severe OSA), type 2 diabetes (severe OSA), prediabetes (severe OSA), higher HbA1c (severe OSA), higher waist/hip ratio (severe OSA), higher systolic blood pressure (moderate and severe OSA), higher diastolic blood pressure (severe OSA), higher heart rate (severe OSA), metabolic syndrome (moderate and severe OSA), higher central apneas (severe OSA) | ESS mean score: 9.9 ± 4.8 No significant differences in ESS across OSA severity (p = 0.73): AHI < 15 (mild OSA): 9.5 ± 4.1 AHI 15–30 (moderate OSA): 9.9 ± 4.9 AHI > 30 (severe OSA): 10.1 ± 5.1 |
| Latif, 2022 [62] | Risk factors for OSA vs. non-SA: Older age, male sex, higher BMI, higher diabetes prevalence, lower CAD prevalence, lower stroke prevalence, more paroxysmal AF and less persistent AF at diagnosis, smaller left atrial diameter, and lower ejection fraction | Not reported |
| Mehawej, 2022 [63] | Risk factors for high-risk OSA vs. low-risk OSA: Male sex, higher BMI, hypertension, diabetes, renal disease, and frailty | STOP-BANG used for risk stratification; no symptoms reported separately Cognitive impairment (MoCA ≤ 23): 45.6% (intermediate-risk OSA) vs. 35.2% (low-risk) vs. 32.6% (high-risk), p < 0.01 |
| Oster, 2022 [64] | Not reported | Intervention group: Snoring: 80.6% Daytime tiredness: 71.0% Observed apnea: 32.3% Neck (>40 cm): 45.2% STOP-Bang score: Modified STOP-Bang (comparison): 2.6 ± 0.8 Complete STOP-Bang (intervention): 4.7 ± 1.3 |
| Verhaert, 2022 [65] | Risk factors for moderate-to-severe SDB vs. none/mild SDB: Higher BMI, higher CHA2DS2-VASc score, congestive heart failure, hypertension, higher number of cardiovascular drugs (≥4), and more frequent use of beta-blockers and RAS inhibitors | Not reported |
| Ahn, 2023 [66] | Risk factors for OSA: Age < 60 yrs | Not reported |
| Betz, 2023 [67] | Risk factors for moderate-to-severe SDB vs. none/mild SDB: Age > 50 yrs, higher BMI, higher CHA2DS2-VASc score, dyslipidemia, hypertension, previous thromboembolic events (stroke or TIA), vascular disease, vitamin K antagonists use | Snoring: 40% in moderate-to-severe SDB vs. 0–28% in mild or non-SDB (p = 0.012) Observed apneas: 34% in moderate-to-severe SDB vs. 10–17% in mild or non-SDB (p = 0.003) |
| Holtstrand, 2023 [68] | Risk factors for OSA: Higher age, male sex, obesity, current smoking, hypertension, CAD, and stroke Risk for prevalent AF: increased in severe OSA without EDS, OR 2.54, 95% CI 1.05–6.16, p = 0.039, independent of confounding factors | Mean ESS score: 10.6 ± 5.0 ESS score per OSA severity (non-OSA, mild, moderate, severe): 10.0 (6.0–14.0) vs. 10.0 (6.0–13.0) vs. 11.0 (7.0–15.0) vs. 11.0 (7.0–15.0), p < 0.001 EDS (ESS ≥ 11): 50.8% EDS per OSA severity (non-OSA, mild, moderate, severe): 172 (46.5%) vs. 421 (46.6%) vs. 707 (52.9%) vs. 566 (53.0%), p-value not reported |
| Jensen, 2023 [69] | Risk factors for moderate-to-severe SA vs. none/mild SA: Older age, BMI, and neck circumference; higher resting HR; permanent AF; higher CHA2DS2-VASc score; hypertension; history of thromboembolic events; higher LV ejection fraction; and more frequent use of benzodiazepines and steroids | Not reported |
| Lin, 2023 [70] | Risk factors for OSAHS vs. non-OSAHS: Older age, higher BMI, postmenopausal women, and more frequent use of statins | STOP-Bang questionnaire (all NS): Snoring: 64.7% (OSAHS) vs. 36.4% (non-OSAHS), p = 0.101 Daytime fatigue: 72.6% (OSAHS) vs. 45.5% (non-OSAHS), p = 0.151 Observed apnea: 21.6% (OSAHS) vs. 9.1% (non-OSAHS), p = 0.675 |
| Mills, 2023 [71] | Risk factors for moderate–severe OSA vs. mild and non-OSA: Older age, male sex, white race, higher BMI and CHA2DS2-VASc score, persistent AF | Non-OSA, mild, and moderate-to-severe OSA, no p-values reported: ESS: 6.0 (4.5–9.0), 6.0 (4.5–8.5), 5.0 (3.0–7.0) STOP-BANG: 3.5 (2.3–4.0) vs. 5.0 (3.0–5.0) vs. 4.5 (4.0–5.0) |
| Ozkan, 2023 [72] | Risk factors for AF vs. non-AF (NSR): Higher VAI, PAP, and SII | Not reported |
| Rivas, 2023 [73] | OSA was not associated with AF | Not reported |
| Tanaka, 2023 [74] | Risk factors for OSA vs. non-OSA: Male sex, WP-AHI ≥ 18.1 and ESS ≥ 11 | ESS score: 6.8 ± 4.5 (overall, no group comparison provided) ESS ≥ 11: independent predictor of CPAP indication |
| Vermeer, 2023 [75] | Not reported | Not reported. STOP-BANG or symptom scores (ESS, fatigue, etc.) not included; OSA suspected if AHI ≥ 5 on WatchPAT |
| Hunt, 2024 [76] | No significant differences in cardiac structure and function changes between pts with or without OSA | ESS score: 7.4 ± 3.2 |
| Kadhim, 2024 [77] | Risk factors for moderate-to-severe SDB vs. no/mild SDB: Male sex, higher body weight and BMI, diabetes, history of stroke/TIA, higher CHA2DS2-VASc score, nonparoxysmal AF, increased LV internal diameter end-diastole and LA diameter | Derivation cohort: ESS score: 6.1 ± 4.0 (moderate–severe SDB) vs. 5.5 ± 4.1 (no/mild SDB), p = 0.099 (NS) |
| Rogel, 2024 [78] | Not reported | Not reported |
| Wei, 2024 [79] | Risk factors for AF (group B and C) vs. non-AF (group A): Higher BMI, severe OSA, lower SBP, higher DBP, higher HR, larger LA diameter and volume, larger RV and atrial diameter (end-diastole and systole), larger RV outflow tract, and larger right atrial area | Not reported |
| First Author, Year | Laboratory Findings | ECG and Echocardiographic Findings | Polysomnographic Findings |
|---|---|---|---|
| Caples, 2019 [46] | Not reported | No significant differences between groups (p > 0.05) LVEF: 57.3 ± 7.1% (control) vs. 58.1 ± 7.5% (PAP) LAVI: 35.6 ± 6.5 vs. 40.6 ± 6.8 mL/m2 | Control vs. PAP: AHI: 29.8 ± 21 (control) vs. 30.3 ± 19.5 (PAP) events/h Obstructive Apnea Index: 13.8 ± 16.0 vs. 13.7 ± 16.1 Central Apnea Index: 0.6 ± 2.3 vs. 0.8 ± 2.3 Min SpO2: 80.1 ± 8.2% vs. 80.2 ± 8.1% Time with SpO2 > 90%: 90.2 ± 14.1% vs. 90.1 ± 14.2% (p > 0.9) |
| Feng, 2019 [23] | Not reported | Not reported | Not reported |
| Gonçalves, 2019 [7] | Not reported | Not reported | RDI-PM correlated with PSG (R = 0.34, p = 0.004); cut-off 13.3 had 78% sensitivity/specificity for OSAS, 90% sensitivity for moderate-to-severe OSAS; AF reduced specificity to 57% |
| Hojo, 2019 [47] | Non-OSA vs. treated OSA vs. untreated OSA: BNP: 80.4 ± 157.6 vs. 40.9 ± 24.3 vs. 154.9 ± 210.6 Cre: 0.89 ± 0.22 vs. 0.80 ± 0.10 vs. 1.3 ± 1.8 BNP (pg/mL) and Cre (g/dL) levels did not differ between groups. | No statistically significant differences in other parameters such as LA diameter and volume, EF, and E/e’, were found between groups Non-OSA vs. treated OSA vs. untreated OSA (NS): LA diameter: 37.4 ± 9.7, 41.5 ± 6.2, 41.7 ± 7.4 mm; EF: 62.7 ± 7.1%, 63.6 ± 10.3%, 62.4 ± 14.1%; E/e′: 12.8 ± 10.5, 11.4 ± 3.2, 17.6 ± 14.2 LAV: 103.7 ± 25.1 mL, 107.5 ± 30.0 mL, 120.3 ± 40.1 mL | AHI (type 3 study), events/h: 5.8 ± 3.8 non-OSA; 26.0 ± 15.8 treated OSA; 28.1 ± 10.9 untreated OSA, p = 0.0001 CPAP initiated after 2nd session in severe OSA cases |
| Linz, 2019 [48] | Not reported | Not reported | Mean RDI: 17.9 ± 11.5 events/h RDI ≥ 20/h in ≥1 night: 85% of pts; Severe SDB (mean RDI ≥ 20/h): 32% of pts; High night-to-night RDI variability (SD ≈ 6.3 events/h); RDI quartile 4 vs. quartile 1 associated with 1.7×, 2.3×, and 10.2× increased risk of AF >5 min, >1 h, and >12 h/day, respectively (p < 0.001 for all) |
| Providência, 2019 [49] | eGFR: 75.1 ± 18.4 mL/min (declined with BMI); no other laboratory values reported | Indexed LA volume: 48.6 ± 18.6 mL/m2 LVEF: 62 ± 9% LVEF < 35% in 2% overall | Not reported |
| Bazan, 2020 [50] | CHA2DS2-VASc: 1.9 ± 1.7 (higher in OSA: 2.1 ± 1.7 vs. 0.8 ± 1.4, p = 0.012); no other labs reported | OSA vs. non-OSA: LA diameter: 44.8 ± 5 mm vs. 43.5 ± 7 mm, p = NS LVEF: 57.7 ± 12% vs. 56.7 ± 10%, p = NS PR: 189 ± 35 ms vs. 167 ± 30 ms, p = 0.056 p-wave duration: 133 ± 25 ms vs. 121 ± 20 ms, p = 0.11 | AHI ≥ 5 in 82% of pts: 26% 5 ≤ AHI < 15, 26% 15 ≤ AHI < 30, 30% AHI ≥ 30 AHI: 28 ± 22 (men) vs. 17 ± 15 (women), p = 0.048 STOP-BANG ≥ 4 predicted CPAP indication (OR 4.5 [1.9–10.6]) AHI correlated with BMI (r = 0.36, p = 0.003) and STOP-BANG (r = 0.45, p < 0.001) |
| Ben Halima, 2020 [36] | Not reported | Not reported | Mean AHI (ambulatory study): 21.6 ± 13.6 e/h |
| Gellert, 2020 [51] | Total cholesterol, glucose levels collected but no groupwise data reported | 48 h aECG: AF prevalence 9.3%; SA associated with AF (OR 7.3, 95% CI: 3.7–14.5); increased PACs (OR 1.2–1.5) and PVCs in SA (non-significant) | SA defined by ICD-9 codes and/or self-reported diagnosis; 217/1154 had SA; no PSG or AHI data |
| Martí-Almor, 2020 [52] | Not reported | Not reported | SA severity (RDI ≥ 20 via pacemaker) associated with increased AF burden over 12–18 months; persistent AF: 16.9% vs. 7.3%; 15.3% treated pts; fallback mode switching > 7 d: Δ 13.4%, 95% CI: 4.1–22.7% |
| May, 2020 [15] | Not reported | PAF vs. controls: LAV: 64.2 [IQR 48.9, 77.4] vs. 56.2 [IQR 45.1, 70.0], p = 0.006 HR: 64.99 ± 10.2 bpm vs. 68.76 ± 10.4, p = 0.002 | AHI (median [IQR]): 10.6 [3.6–23.4] (AF) vs. 12.7 [3.9–24.5] (controls), p = 0.33 STOP-BANG AUC = 0.75, new NABS model (neck, age, BMI, snoring) outperformed STOP-BANG (AUC = 0.88 for AHI ≥ 15) |
| Mazza, 2020 [53] | Not reported | EF: 58 ± 7%; LA diameter: 42 ± 5 mm | AF ≥ 6 h vs. non-AF or AF < 6 h RDI max: 58 ± 25 episodes/h vs. 49 ± 29 episodes/h, p = 0.001 RDI mean: 32 ± 18 vs. 30 ± 19, p = 0.156 RDI burden: 14% [2–33%] vs. 14% [0–36%], p = 0.774 |
| Shapira-Daniels, 2020 [54] | Not reported | Not reported | Not reported (sleep study via WatchPAT) |
| Starkey, 2020 [55] | Not reported | Minimal cross-sectional area: 2.4 ± 0.7 cm2 (non-OSA) vs. 2.4 ± 0.9 cm2 (mild OSA) vs. 2.5 ± 1.1 cm2 (moderate OSA) vs. 2.2 ± 0.6 cm2 (severe OSA), no p-values reported | Not reported |
| Szymańska, 2020 [56] | Mean visfatin: 1.9 ± 2.1 ng/mL Visfatin (ng/mL) levels correlated with OSA severity: 1.77 ± 0.17 (mild OSA) vs. 2.38 ± 0.18 (moderate OSA) vs. 3.55± 0.61 (severe OSA), p for trend = 0.017 | Not reported | Portable RP study (Embletta MPR) Mean AHI (all patients): 8.1 ± 10.7 events/hour |
| Traaen, 2020 [57] | Not reported | Not reported | ODI, SPO2, SaO2 < 90% assessed and presented by sex Values per SA severity (non-SA vs. mild SA vs. moderate SA vs. severe SA): Central Apnea Index: 0.1 (IQR 0–0.3) vs. 0.2 (IQR 0.1–0.6) vs. 0.4 (IQR 0.1–0.9) vs. 0.8 (IQR 0.2–2.8), p < 0.001 Central AHI > Obstructive AHI: 14% vs. 3.5% vs. 1.2% vs. 2.9%, p < 0.001 AHI associated with higher STOP-BANG and neck circumference |
| Blanchard, 2021 [58] | Not reported | Pts with incident AF vs. without incident AF: RMSSD (ms): 82 (55–121) vs. 60 (45–85), p < 0.0001 SDNN (ms): 71 (51–103) vs. 63 (48–84), p = 0.0183 pNN50 (%): 18 (11–32) vs. 18 (9–33), p = 0.4691 LF/HF ratio: 1 (1–1) vs. 1 (1–2), p < 0.0001 | Pts with incident AF vs. without incident AF: AHI: 32 (18–49) vs. 22 (39–39) events/h, p < 0.0001 ODI 3%: 23 (11–43) vs. 14 (5–30) events/h, p < 0.0001 T90 (SaO2 < 90%): 4% (1–22) vs. 1% (0–6), p < 0.0001 Nadir SaO2: 81% (73–85) vs. 84% (78–88), p < 0.0001 Hypoxic burden: 52 (18–108) vs. 25 (8–62) % min/h, p < 0.0001 |
| Delesie, 2021 [59] | Not reported | Not reported | Not reported |
| Mohammadieh, 2021 [60] | Not reported | OSA (AHI ≥ 15) vs. non-OSA (AHI < 15): LVEF: 54.6 ± 9.8 vs. 58.8 ± 7.7, p = 0.075 LA area: 26.6 ± 4.7 vs. 23.4 ± 5.2 cm2, p = 0.048 LA diameter: 4.3 ± 0.6 vs. 4.0 ± 0.6 cm, p = 0.211 | OSA (AHI ≥ 15) vs. non-OSA (AHI < 15): AHI: 31.9 ± 15.5 vs. 5.2 ± 4.3, p < 0.001 ODI: 18.4 ± 13.1 vs. 2.1 ± 2.3, p < 0.001 CAI: 1.4 ± 2.5 vs. 0.2 ± 0.5, p < 0.001 |
| Højager, 2022 [61] | AHI < 15 vs. AHI 15–30 vs. AHI > 30: HbA1c (mmol/mol): 36.8 ± 11.4 vs. 38.7 ± 7.5 vs. 42.3 ± 10.6, p < 0.001 Triglycerides (mmol/L): 1.8 ± 1.4 vs. 2.1 ± 1.2 vs. 2.4 ± 1.7, p = 0.019 Total cholesterol (mmol/L): 5.9 ± 5.9 vs. 5.2 ± 1.1 vs. 5.1 ± 1.0, p = 0.14 (NS) LDL-C (mmol/L): 3.1 ± 1.0 vs. 2.9 ± 0.9 vs. 2.8 ± 1.0, p = 0.07 (NS) | AHI < 15 vs. AHI 15–30 vs. AHI > 30: HR: 70.8 ± 11.5 vs. 68.8 ± 12.6 vs. 75.6 ± 14.3 bpm, p < 0.001 | AHI: 34.2 (0.2–115.8) Central apneas (events/hour): 2.1 ± 3.3 (AHI < 15) vs. 6.0 ± 8.1 (AHI 15–30) vs. 16.8 ± 36.4 (AHI > 30), p < 0.001 |
| Latif, 2022 [62] | Not reported | OSA vs. non-SA, no p-values reported: LA diameter: 5.0 (5–6) cm vs. 6.0 (5–6) cm EF: 57% (55–65) vs. 60% (55–65) | OSA vs. non-SA, no p-values reported: AHI: 4%: 9 (5–14) events/h vs. 1 (1–2) Minimum oxygen saturation (%): 89 (85–90) vs. 91 (91–92) |
| Mehawej, 2022 [63] | Hb: 14 ± 2 g/dL (high-risk OSA) vs. 13 ± 2 g/dL (low-risk OSA), p < 0.01 | LVEF: 54 ± 11.4% (high-risk OSA) vs. 55 ± 11.3% (low-risk OSA), NS Heart rate: 72 ± 15.5 bpm vs. 71 ± 13.5 bpm, NS | Not reported (OSA assessed via STOP-BANG questionnaire only; no PSG or polygraphy performed) |
| Oster, 2022 [64] | Not reported | Not reported | OSA was screened using STOP-BANG only; no PSG or oximetry data reported |
| Verhaert, 2022 [65] | Not reported | Not reported | WatchPAT-based sleep parameters across SDB severity (none vs. severe): Awakenings: 7.0 [5,6,7,8,9,10] (none) vs. 12 [9,10,11,12,13,14,15,16,17] (severe), p = 0.01 pAHI: 3.1 [1.5–3.8] vs. 43 [33,37,38,39,40,41,42,43,44,45,46,59,60,70], p < 0.01 Central pAHI: 0 [0–0.2] vs. 3.0 [1.1–7.2], p < 0.01 ODI: 0.6 [0.3–1.0] vs. 22 [16,17,18,19,20,21,22,23,24,25,26], p < 0.01 SpO2 nadir: 92% vs. 91%, p < 0.01 Time SpO2 < 90%: 0 vs. 7.0 [0.9–16] min, p < 0.01 SpO2 < 90% (% sleep time): 0% vs. 1.2% [0.2–2.8], p < 0.01 |
| Ahn, 2023 [66] | Not reported | Not reported | Not reported |
| Betz, 2023 [67] | Not reported | Not reported | Not reported |
| Holtstrand, 2023 [68] | Not reported | Not reported | Mean AHI: 25.4 ± 19.7 events/h AHI (events/h) per OSA severity (non-OSA, mild, moderate, severe): 3.0 (2.0–4.0) vs. 9.4 (7.0–12.0) vs. 20.6 (17.0–24.9) vs. 44.0 (35.1–58.0), p = 0.000 ODI (events/h): 1.0 (0.5–2.0) vs. 4.0 (2.0–6.0) vs. 11.0 (7.0–16.0) vs. 32.0 (21.5–47.8) p = 0.000 Mean SpO2 (%): 95.9 (94.9–96.6) vs. 95.0 (94.0–96.0) vs. 94.0 (93.0–95.0) vs. 93.0 (91.8–94.0), p < 0.001 Nadir SpO2 (%): 91.0 (88.0–92.0) vs. 88.0 (85.0–90.0) vs. 85.0 (81.0–87.0) vs. 79.0 (73.0–83.0), p < 0.001 |
| Jensen, 2023 [69] | Not reported | Moderate/severe SA vs. none/mild SA: LVEF: 55.0 (50.0–60.0) vs. 55.0 (55.0–60.0) p = 0.016 LA dilatation: 46.5% vs. 32.7%, NS | Moderate-to-severe SA vs. none/mild SA: AHI: 30.0 (20.0–38.0) vs. 7.0 (5.0–11.4), p < 0.001 ODI < 3% (events/hour): 27.0 (17.0–35.0) vs. 8.0 (6.0–11.0), p < 0.001 ODI < 4% (events/hour): 18.0 (10.6–26.5) vs. 4.0 (3.0–6.0), p < 0.001 SpO2 minimum (%): 79 (74–84) vs. 85 (78–87), p < 0.001 |
| Lin, 2023 [70] | Total cholesterol: 3.7 ± 1.1 mmol/L (OSAHS) vs. 4.9 ± 1.2 mmol/L (non-OSAHS), p = 0.003 LDL-C: 2.5 (2.0–2.9) mmol/L (OSAHS) vs. 3.2 (2.4–4.2) mmol/L (non-OSAHS), p = 0.022 Triglycerides, creatinine, uric acid, and glucose also assessed, not significant | Not reported | AHI: 20.3 (12.5–31.9) events/h (OSAHS) vs. 2.9 (1.4–3.5) events/h (non-OSAHS), p < 0.001 |
| Mills, 2023 [71] | Not reported | Not reported | Non-OSA, mild, and moderate-to-severe OSA, no p-values reported: pAHI 3%: 3.15 (2.8–4.0) vs. 10.75 (7.9–11.7) vs. 32.6 (19.6–45.2) pAHI 4%: 0.85 (0.8–1.1) vs. 2.75 (2.2–4.8) vs. 14.8 (10.1–26.8) ODI 3%: 3.05 (2.5–3.4) vs. 10.4 (7.35–11.8) vs. 31.4 (18.6–45.0) Time SpO2 < 90% (min): 0.03 (0.0–0.10) vs. 3.40 (0.0–44.9) vs. 8.16 (0.0–40.8) Central Sleep Apnea Index: 0.1 (0.0–0.23) vs. 0.65 (0.0–1.0) vs. 1.75 (1.15–3.5) |
| Ozkan, 2023 [72] | AF vs. non-AF (NSR): HDL-C: 39 (30–45) vs. 41 (28–53), p = 0.003 Triglycerides: 213 (174–267) vs. 197 (144–255), p = 0.001 Lower LDL-C: 143.5 ± 17.8 vs. 152.3 ± 14.9, p < 0.001 Cre levels: 1.1 ± 0.2 vs. 1 ± 0.2, p = 0.02 Platelet count: 232.7 ± 32.3 vs. 211.2 ± 31.4, p < 0.001 Lymphocyte count: 1.8 (1.3–2.3) vs. 2.2 (1.3–3.1), p < 0.001 Neutrophil count: 5 ± 1 vs. 5.4 ± 1.5, p = 0.03 VAI: 8 (5.3–15.5) vs. 6.7 (3.8–14.6), p < 0.001 SII: 624.4 (284.5–950.6) vs. 539.4 (238.7–1131.0), p < 0.001 | AF vs. non-AF (NSR): EF: 58 (51–69) vs. 57 (50+), p = 0.013 PAP: 38 (27–46) vs. 35 (4–46), p < 0.001 | AF vs. non-AF (NSR): AHI: median 27 (21–33) vs. 23 (8–36), p < 0.001 |
| Rivas, 2023 [73] | Not reported | Not reported | Not reported |
| Tanaka, 2023 [74] | Not reported | LVDd: 47.7 ± 5.0 mm; LAD: 40.8 ± 6.1 mm; LVEF: 63.5 ± 10.5%; LAD ≥ 40 mm: not an independent predictor of CPAP indication (multivariate OR 1.37, p = 0.23) | WP-AHI: 25.9 ± 12.7; PSG-AHI: 31.4 ± 18.9; correlation r = 0.48, p < 0.001; 35.7% of patients with WP-AHI < 30 had PSG-AHI ≥ 30; WP-AHI ≥ 18.1 best cutoff to predict PSG-AHI ≥ 20 (AUC 0.72) |
| Vermeer, 2023 [75] | Total and LDL-cholesterol, HbA1c, fasting glucose assessed at baseline and follow-up; no results yet reported (protocol paper) | ECG and echocardiographic parameters part of standard care, but not specified; to be assessed pre- and post-ablation | OSA screening by WatchPAT™ 300; OSA suspected if AHI ≥ 5; no AHI, ODI or SpO2 values reported |
| Hunt, 2024 [76] | Baseline: Mean Hb: 14.7 ± 1.1 g/L Mean Cre: 79 ± 15 µmol/L NT-proBNP: 140 ng/L (IQR 205) | Not reported | AHI: 27 (IQR 15–86) ODI: 27 (IQR 13–90) |
| Kadhim, 2024 [77] | Not reported | Moderate-to-severe SDB (AHI ≥ 15) vs. no/mild SDB (AHI < 15) (derivation cohort): LVEF (%): 60.5 ± 10.4 vs. 61.0 ± 8.0, p = 0.056 LVIDd (cm): 5.1 ± 0.8 vs. 4.9 ± 0.6, p = 0.001 LA diameter (cm): 4.2 ± 0.6 vs. 3.9 ± 0.6, p < 0.001 LA volume index (mL/m2): 34.3 ± 11.3 vs. 32.0 ± 11.0, p = 0.053 (borderline) | Validation cohort: AHI: 35.9 ± 20.1 (moderate–severe SDB) vs. 7.2 ± 3.9 (no/mild SDB), p < 0.001 |
| Rogel, 2024 [78] | Not reported | Not reported | Not reported |
| Wei, 2024 [79] | BNP (pg/mL): Group A, 674 ± 132 Group B, 752 ± 145 (p < 0.05 vs. A) Group C, 1053 ± 156 (p < 0.05 vs. A & B) (p < 0.001). | AF (group B and C) vs. non-AF (group A): Left heart: LAD: 52.5 ± 6.0 vs. 38.7 ± 5.7 mm, p < 0.001; LAV: 105 ± 39 vs. 87 ± 34 mL, p = 0.001; LVEF: 63 ± 15 vs. 69 ± 16%, p = 0.006; FS: 31 ± 15 vs. 38 ± 14%, p = 0.001; E/A: 1.29 ± 0.48 vs. 0.98 ± 0.29, p < 0.001; e′: 5.3 ± 1.8 vs. 6.6 ± 1.8 cm/s, p < 0.001; E/e′: 10.9 ± 3.9 vs. 14.3 ± 3.5, p < 0.001; PV-D: 58 ± 20 vs. 45 ± 20 cm/s, p < 0.001; PV-S: 45 ± 20 vs. 53 ± 20 cm/s, p < 0.001; FPV: 53 ± 27 vs. 42 ± 24 cm/s, p = 0.003. Right heart: RV D1: 38.1 ± 6.3 vs. 34.2 ± 5.7 mm, p < 0.001; RV D2: 30.7 ± 5.4 vs. 27.6 ± 4.4 mm, p < 0.001; RVOT2: 30.3 ± 3.4 vs. 23.2 ± 3.2 mm, p < 0.001; RA D1: 46.9 ± 7.1 vs. 39.5 ± 5.9 mm, p < 0.001; RA D2: 40.4 ± 6.1 vs. 34.6 ± 5.2 mm, p < 0.001; RA area: 26.7 ± 7.1 vs. 17.3 ± 5.9 cm2, p < 0.001. | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, S.; Drummond, M.; Bugalho, A. Prevalence and Diagnosis of Obstructive Sleep Apnea in Atrial Fibrillation Patients: A Systematic Review. J. Clin. Med. 2025, 14, 5708. https://doi.org/10.3390/jcm14165708
Sousa S, Drummond M, Bugalho A. Prevalence and Diagnosis of Obstructive Sleep Apnea in Atrial Fibrillation Patients: A Systematic Review. Journal of Clinical Medicine. 2025; 14(16):5708. https://doi.org/10.3390/jcm14165708
Chicago/Turabian StyleSousa, Susana, Marta Drummond, and António Bugalho. 2025. "Prevalence and Diagnosis of Obstructive Sleep Apnea in Atrial Fibrillation Patients: A Systematic Review" Journal of Clinical Medicine 14, no. 16: 5708. https://doi.org/10.3390/jcm14165708
APA StyleSousa, S., Drummond, M., & Bugalho, A. (2025). Prevalence and Diagnosis of Obstructive Sleep Apnea in Atrial Fibrillation Patients: A Systematic Review. Journal of Clinical Medicine, 14(16), 5708. https://doi.org/10.3390/jcm14165708





