Endoscopic Ultrasound-Directed Transgastric Endoscopic Retrograde Cholangiopancreatography (EDGE): Techniques, Outcomes and Safety Profiles
Abstract
1. Introduction
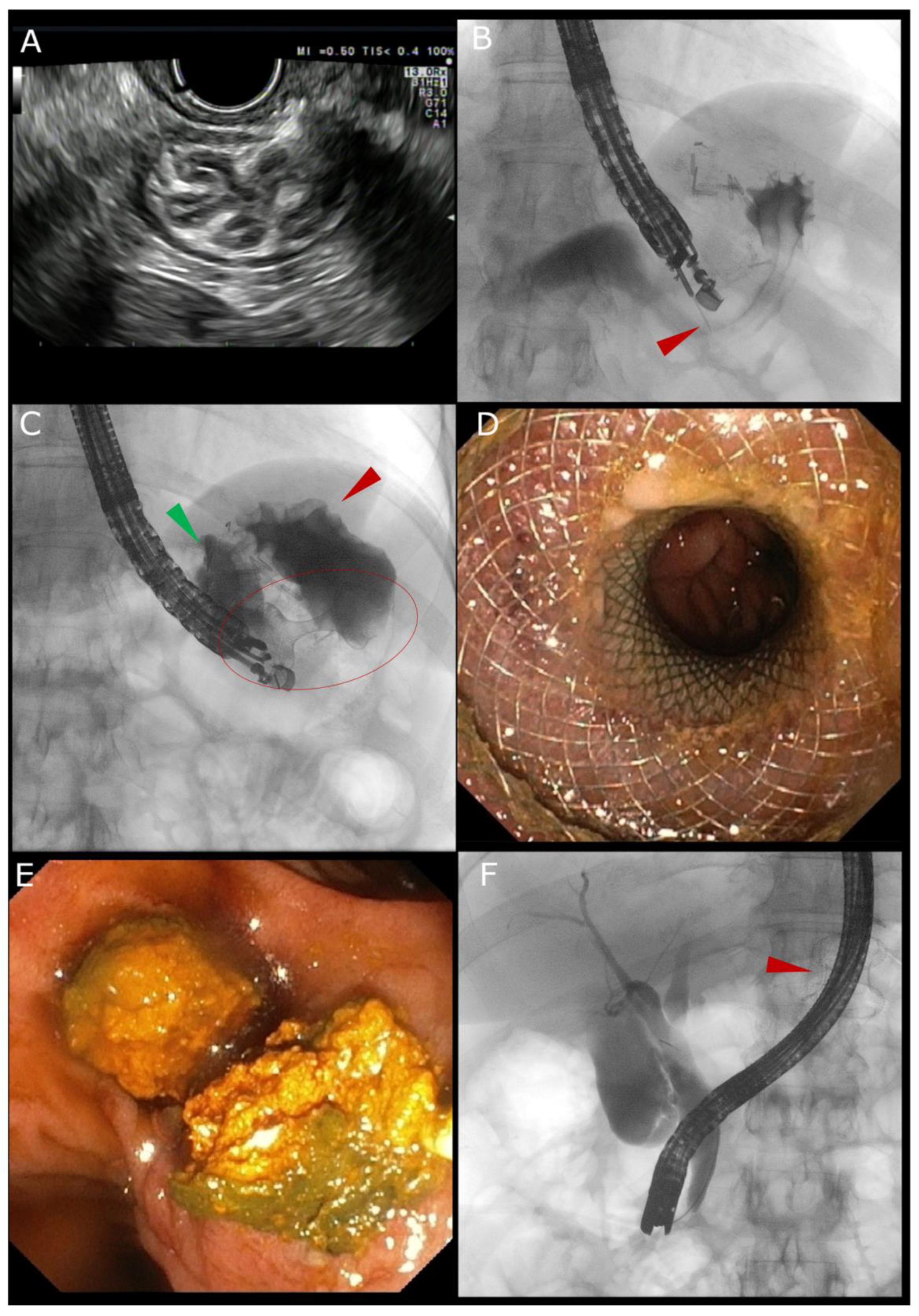
2. Technical Overview of the EDGE Procedure
2.1. EUS-Guided LAMS Placement
2.2. ERCP via the Created Access
2.3. Other Procedures Through the LAMS
2.4. LAMS Removal
3. Technical and Clinical Success
4. Safety
4.1. Stent Migration/Dislodgement
4.2. Fistula Persistence
4.3. Weight Regain
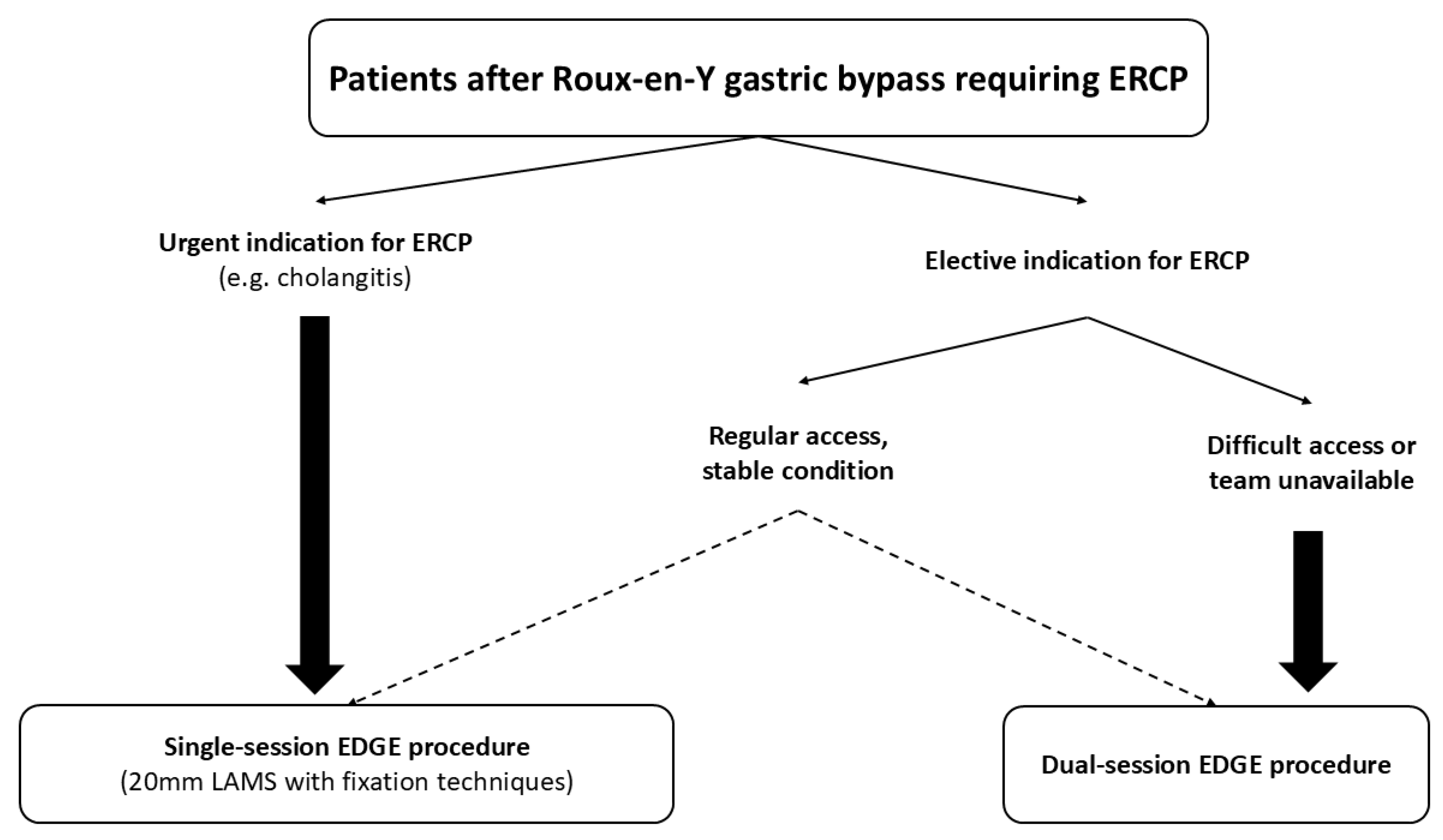
5. Training Implications for EDGE
6. Economic Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef]
- Seidell, J.C.; Halberstadt, J. The global burden of obesity and the challenges of prevention. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 7–12. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.E.; Thornley, C.J.; Blackstone, R.P. Outcomes in Bariatric and Metabolic Surgery: An Updated 5-Year Review. Curr. Obes. Rep. 2020, 9, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Nagem, R.G.; Lázaro-da-Silva, A.; de Oliveira, R.M.; Morato, V.G. Gallstone-related complications after Roux-en-Y gastric bypass: A prospective study. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Tustumi, F.; Pinheiro Filho, J.E.L.; Stolzemburg, L.C.P.; Serigiolle, L.C.; Costa, T.N.; Pajecki, D.; Santo, M.A.; Nahas, S.C. Management of biliary stones in bariatric surgery. Ther. Adv. Gastrointest. Endosc. 2022, 15, 26317745221105090. [Google Scholar] [CrossRef]
- Chang, J.; Corcelles, R.; Boules, M.; Jamal, M.H.; Schauer, P.R.; Kroh, M.D. Predictive factors of biliary complications after bariatric surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2016, 12, 1706–1710. [Google Scholar] [CrossRef]
- Dhindsa, B.S.; Dhaliwal, A.; Mohan, B.P.; Mashiana, H.S.; Girotra, M.; Singh, S.; Ohning, G.; Bhat, I.; Adler, D.G. EDGE in Roux-en-Y gastric bypass: How does it compare to laparoscopy-assisted and balloon enteroscopy ERCP: A systematic review and meta-analysis. Endosc. Int. Open 2020, 8, E163–E171. [Google Scholar] [CrossRef]
- Gangwani, M.K.; Aziz, M.; Haghbin, H.; Iqbal, A.; Dillard, J.; Dahiya, D.S.; Ali, H.; Hayat, U.; Khuder, S.; Lee-Smith, W.; et al. Comparing EUS-Directed Transgastric ERCP (EDGE) Versus Laparoscopic-Assisted ERCP Versus Enteroscopic ERCP: A Network Meta-Analysis. J. Clin. Gastroenterol. 2024, 58, 110–119. [Google Scholar] [CrossRef]
- Tarantino, I.; Rizzo, G.E.M. Biliopancreatic Endoscopy in Altered Anatomy. Medicina 2021, 57, 1014. [Google Scholar] [CrossRef]
- Mauro, A.; Vanella, G.; Mirante, V.G.; Fugazza, A.; Binda, C.; Elli, L.; Forti, E.; Di Mitri, R.; Berretti, D.; Bertani, H.; et al. Endoscopic biliary drainage in patients with surgically-altered anatomy: The street multicenter study. Gastrointest. Endosc. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- de Oliveira, V.L.; de Moura, D.T.H.; do Monte, E.S., Jr.; Proença, I.M.; Ribeiro, I.B.; Sánchez-Luna, S.A.; Ribas, P.H.B.V.; Hemerly, M.C.; Bernardo, W.M.; de Moura, E.G.H. Laparoscopic-Assisted Endoscopic Retrograde Cholangiopancreatography (ERCP) Versus Endoscopic Ultrasound-Directed Transgastric ERCP in Patients with Roux-en-Y Gastric Bypass: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e30196. [Google Scholar] [CrossRef]
- Kedia, P.; Sharaiha, R.Z.; Kumta, N.A.; Kahaleh, M. Internal EUS-directed transgastric ERCP (EDGE): Game over. Gastroenterology 2014, 147, 566–568. [Google Scholar] [CrossRef]
- Thompson, C.C.; Ryou, M.K.; Kumar, N.; Slattery, J.; Aihara, H.; Ryan, M.B. Single-session EUS-guided transgastric ERCP in the gastric bypass patient. Gastrointest. Endosc. 2014, 80, 517. [Google Scholar] [CrossRef] [PubMed]
- Kedia, P.; Tyberg, A.; Kumta, N.A.; Gaidhane, M.; Karia, K.; Sharaiha, R.Z.; Kahaleh, M. EUS-directed transgastric ERCP for Roux-en-Y gastric bypass anatomy: A minimally invasive approach. Gastrointest. Endosc. 2015, 82, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, M.; Kowalski, T.; Nieto, J.; Kunda, R.; Ahuja, N.; Irani, S.; Shah, A.; Loren, D.; Brewer, O.; Sanaei, O.; et al. An international, multicenter, comparative trial of EUS-guided gastrogastrostomy-assisted ERCP versus enteroscopy-assisted ERCP in patients with Roux-en-Y gastric bypass anatomy. Gastrointest. Endosc. 2017, 88, 486–494. [Google Scholar] [CrossRef]
- Tyberg, A.; Nieto, J.; Salgado, S.; Weaver, K.; Kedia, P.; Sharaiha, R.Z.; Gaidhane, M.; Kahaleh, M. Endoscopic Ultrasound (EUS)-Directed Transgastric Endoscopic Retrograde Cholangiopancreatography or EUS: Mid-Term Analysis of an Emerging Procedure. Clin. Endosc. 2017, 50, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Antonini, F.; Merlini, I.; Di Saverio, S. Endoscopic ultrasound-guided biliary drainage after failed endoscopic retrograde cholangiopancreatography: The road is open for almighty biliopancreatic endoscopists! World J. Gastrointest. Surg. 2024, 16, 2765–2768. [Google Scholar] [CrossRef]
- Ghandour, B.; Bejjani, M.; Irani, S.S.; Sharaiha, R.Z.; Kowalski, T.E.; Pleskow, D.K.; Do-Cong Pham, K.; Anderloni, A.A.; Martinez-Moreno, B.; Khara, H.S.; et al. Classification, outcomes, and management of misdeployed stents during EUS-guided gastroenterostomy. Gastrointest. Endosc. 2022, 95, 80–89. [Google Scholar] [CrossRef]
- Rizzo, G.E.M.; Carrozza, L.; Tammaro, S.; Ligresti, D.; Traina, M.; Tarantino, I. Complete intraperitoneal maldeployment of a lumen-apposing metal stent during EUS-guided gastroenteroanastomosis for malignant gastric outlet obstruction: Rescue retrieval with peritoneoscopy through natural orifice transluminal endoscopic surgery. VideoGIE 2023, 8, 310–312. [Google Scholar] [CrossRef]
- Adler, D.G.; Taylor, L.J.; Hasan, R.; Siddiqui, A.A. A retrospective study evaluating endoscopic ultrasound-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery enhanced delivery system. Endosc. Ultrasound 2017, 6, 389–393. [Google Scholar] [CrossRef]
- ASGE Technology Committee; Lo, S.K.; Fujii-Lau, L.L.; Enestvedt, B.K.; Hwang, J.H.; Konda, V.; Manfredi, M.A.; Maple, J.T.; Murad, F.M.; Pannala, R.; et al. The use of carbon dioxide in gastrointestinal endoscopy. Gastrointest. Endosc. 2016, 83, 857–865. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Khashab, M.A.; Chithadi, K.V.; Acosta, R.D.; Bruining, D.H.; Chandrasekhara, V.; Eloubeidi, M.A.; Fanelli, R.D.; Faulx, A.L.; Fonkalsrud, L.; et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest. Endosc. 2015, 81, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Mosko, J.D.; Kobayashi, R.; Fecso, A.; Kim, B.S.; Scott, S.; May, G.R. Endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography for patients with Roux-en-Y gastric bypass anatomy: Technical overview. Clin. Endosc. 2022, 55, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Bahdi, F.; George, R.; Paneerselvam, K.; Nguyen, D.; Abidi, W.M.; Othman, M.O.; Raijman, I. Comparison of endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography outcomes using various technical approaches. Endosc. Int. Open 2022, 10, E459–E467. [Google Scholar] [CrossRef] [PubMed]
- Khara, H.S.; Parvataneni, S.; Park, S.; Choi, J.; Kothari, T.H.; Kothari, S.T. Review of ERCP Techniques in Roux-en-Y Gastric Bypass Patients: Highlight on the Novel EUS-Directed Transgastric ERCP (EGDE) Technique. Curr. Gastroenterol. Rep. 2021, 23, 10. [Google Scholar] [CrossRef]
- Binmoeller, K.F.; Smith, I.; Gaidhane, M.; Kahaleh, M. A kit for eus-guided access and drainage of pancreatic pseudocysts: Efficacy in a porcine model. Endosc. Ultrasound 2012, 1, 137–142. [Google Scholar] [CrossRef]
- Pérez-Cuadrado-Robles, E.; Alric, H.; Quénéhervé, L.; Monino, L.; Poghosyan, T.; Benosman, H.; Vienne, A.; Perrod, G.; Rebibo, L.; Aidibi, A.; et al. Risk factors of anastomosis-related difficult endoscopic retrograde cholangiopancreatography following endoscopic ultrasound-guided gastro-gastrostomy using a standardized protocol (with video). Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2023, 35, 909–917. [Google Scholar] [CrossRef]
- Runge, T.M.; Chiang, A.L.; Kowalski, T.E.; James, T.W.; Baron, T.H.; Nieto, J.; Diehl, D.L.; Krafft, M.R.; Nasr, J.Y.; Kumar, V.; et al. Endoscopic ultrasound-directed transgastric ERCP (EDGE): A retrospective multicenter study. Endoscopy 2021, 53, 611–618. [Google Scholar] [CrossRef]
- Ayoub, F.; Brar, T.S.; Banerjee, D.; Abbas, A.M.; Wang, Y.; Yang, D.; Draganov, P.V. Laparoscopy-assisted versus enteroscopy-assisted endoscopic retrograde cholangiopancreatography (ERCP) in Roux-en-Y gastric bypass: A meta-analysis. Endosc. Int. Open 2020, 8, E423–E436. [Google Scholar] [CrossRef]
- van der Merwe, S.W.; van Wanrooij, R.L.J.; Bronswijk, M.; Everett, S.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Kunda, R.; Badaoui, A.; Law, R.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 185–205. [Google Scholar] [CrossRef]
- Mangiavillano, B.; Ramai, D.; Kahaleh, M.; Tyberg, A.; Shahid, H.; Sarkar, A.; Samanta, J.; Dhar, J.; Bronswijk, M.; Van der Merwe, S.; et al. Outcomes of lumen apposing metal stent placement in patients with surgically altered anatomy: Multicenter international experience. Endosc. Int. Open 2024, 12, E1143–E1149. [Google Scholar] [CrossRef]
- Khan, H.M.A.; Hussain, A.; Kumar, V.C.S.; Yang, D.; Hasan, M.K. Fixation of Proximal flange of Lumen Apposing Metal Stent using Through-the-Scope Endoscopic Suturing System to Prevent Stent Migration in Single-Session EUS-directed Transgastric ERCP: A Pilot Study. Gastrointest. Endosc. 2024, 100, 132–135. [Google Scholar] [CrossRef]
- Binda, C.; Giuffrida, P.; Fabbri, S.; Coluccio, C.; Petraroli, C.; Perini, B.; Fabbri, C. Same-session EUS-directed transgastric interventions: From tissue acquisition to choledochoduodenostomy. VideoGIE Off. Video J. Am. Soc. Gastrointest. Endosc. 2024, 9, 182–184. [Google Scholar] [CrossRef]
- Krafft, M.R.; Hsueh, W.; James, T.W.; Runge, T.M.; Baron, T.H.; Khashab, M.A.; Irani, S.S.; Nasr, J.Y. The EDGI new take on EDGE: EUS-directed transgastric intervention (EDGI), other than ERCP, for Roux-en-Y gastric bypass anatomy: A multicenter study. Endosc. Int. Open 2019, 7, E1231–E1240. [Google Scholar] [CrossRef]
- Ghandour, B.; Shinn, B.; Dawod, Q.M.; Fansa, S.; El Chafic, A.H.; Irani, S.S.; Pawa, R.; Gutta, A.; Ichkhanian, Y.; Paranandi, B.; et al. EUS-directed transgastric interventions in Roux-en-Y gastric bypass anatomy: A multicenter experience. Gastrointest. Endosc. 2022, 96, 630–638. [Google Scholar] [CrossRef]
- Shah, R.M.; Tarnasky, P.; Kedia, P. A review of endoscopic ultrasound guided endoscopic retrograde cholangiopancreatography techniques in patients with surgically altered anatomy. Transl. Gastroenterol. Hepatol. 2018, 3, 90. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Skinner, M.J. Cardiac septal occluder for closure of persistent gastrogastric fistula. VideoGIE 2021, 6, 294–296. [Google Scholar] [CrossRef]
- Aghaie Meybodi, M.; Johal, A.S.; Abdulsamad, M. Persistent Fistula Closure After Endoscopic Ultrasound-Directed Transgastric Endoscopic Retrograde Cholangiopancreatography by Postinfarct Ventricular Septal Defect Occluder. ACG Case Rep. J. 2024, 11, e01519. [Google Scholar] [CrossRef]
- Wang, T.J.; Thompson, C.C.; Ryou, M. Gastric access temporary for endoscopy (GATE): A proposed algorithm for EUS-directed transgastric ERCP in gastric bypass patients. Surg. Endosc. 2019, 33, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.E.M.; Coluccio, C.; Forti, E.; Fugazza, A.; Binda, C.; Vanella, G.; Di Matteo, F.M.; Crinò, S.F.; Lisotti, A.; Maida, M.F.; et al. Endoscopic Ultrasound-Guided Anastomoses of the Gastrointestinal Tract: A Multicentric Experience. Cancers 2025, 17, 910. [Google Scholar] [CrossRef] [PubMed]
- Marzioni, M.; Crinò, S.F.; Lisotti, A.; Fuccio, L.; Vanella, G.; Amato, A.; Bertani, H.; Binda, C.; Coluccio, C.; Forti, E.; et al. Biliary drainage in patients with malignant distal biliary obstruction: Results of an Italian consensus conference. Surg. Endosc. 2024, 38, 6207–6226. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.E.M.; Carrozza, L.; Rancatore, G.; Binda, C.; Fabbri, C.; Anderloni, A.; Tarantino, I. The Role of Endoscopy in the Palliation of Pancreatico-Biliary Cancers: Biliary Drainage, Management of Gastrointestinal Obstruction, and Role in Relief of Oncologic Pain. Cancers 2023, 15, 5367. [Google Scholar] [CrossRef]
- Kedia, P.; Shah-Khan, S.; Tyberg, A.; Gaidhane, M.; Sarkar, A.; Shahid, H.; Zhao, E.; Thakkar, S.; Winkie, M.; Krafft, M.; et al. Endoscopic ultrasound-directed transgastric ERCP (EDGE): A multicenter US study on long-term follow-up and fistula closure. Endosc. Int. Open 2023, 11, E529–E537. [Google Scholar] [CrossRef]
- Bronswijk, M.; Gökce, E.; Hindryckx, P.; Van der Merwe, S. Single-session endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography with a dedicated over-the-scope fixation device: Feasibility study (with video). Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2025, 37, 176–182. [Google Scholar] [CrossRef]
- Keane, M.G.; Higa, J.T.; La Selva, D.; Khashab, M.A.; Irani, S.S. Suturing a 20-mm lumen-apposing metal stent allows for safe same-session EUS-directed transgastric intervention in patients with Roux-en-Y gastric bypass anatomy: A multicenter study (with video). Gastrointest. Endosc. 2023, 97, 291–299. [Google Scholar] [CrossRef]
- Chhabra, P.; On, W.; Paranandi, B.; Huggett, M.T.; Robson, N.; Wright, M.; Maher, B.; Tehami, N. Initial United Kingdom experience of endoscopic ultrasound-directed transgastric endoscopic retrograde cholangiopancreatography. Ann. Hepato-Biliary-Pancreat. Surg. 2022, 26, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Barclay, R.L.; Jain, A.; Buteau Ferland, A.-S.; Chen, Y.-I.; Donnellan, F. Living on the EDGE: Canadian Experience With EUS-directed Transgastric ERCP (EDGE) in Patients with Roux-en-Y Gastric Bypass Anatomy. J. Can. Assoc. Gastroenterol. 2022, 5, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Tyberg, A.; Kedia, P.; Tawadros, A.; Tarnasky, P.R.; Gaidhane, M.; Nieto, J.; Kahaleh, M. EUS-Directed Transgastric Endoscopic Retrograde Cholangiopancreatography (EDGE): The First Learning Curve. J. Clin. Gastroenterol. 2020, 54, 569–572. [Google Scholar] [CrossRef]
- De Benito Sanz, M.; Carbajo López, A.Y.; Sánchez-Ocaña Hernández, R.; Chavarría Herbozo, C.; Bagaza Pérez De Rozas, S.; García-Alonso, J.; De La Serna Higuera, C.; Pérez-Miranda Castillo, M. Endoscopic ultrasound-directed transgastric ERCP in patients with Roux-en-Y gastric bypass using lumen-apposing metal stents or duodenal self-expandable metal stents. A European single-center experience. Rev. Esp. Enfermedades Dig. 2020, 112, 211–215. [Google Scholar] [CrossRef] [PubMed]
- James, T.W.; Baron, T.H. Endoscopic Ultrasound-Directed Transgastric ERCP (EDGE): A Single-Center US Experience with Follow-up Data on Fistula Closure. Obes. Surg. 2019, 29, 451–456. [Google Scholar] [CrossRef]
- Ngamruengphong, S.; Nieto, J.; Kunda, R.; Kumbhari, V.; Chen, Y.-I.; Bukhari, M.; El Zein, M.; Bueno, R.; Hajiyeva, G.; Ismail, A.; et al. Endoscopic ultrasound-guided creation of a transgastric fistula for the management of hepatobiliary disease in patients with Roux-en-Y gastric bypass. Endoscopy 2017, 49, 549–552. [Google Scholar] [CrossRef]
- Attam, R.; Leslie, D.; Arain, M.; Freeman, M.; Ikramuddin, S. EUS-guided sutured gastropexy for transgastric ERCP (ESTER) in patients with Roux-en-Y gastric bypass: A novel, single-session, minimally invasive approach. Endoscopy 2015, 47, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, R.; Razzak, F.A.; Kerbage, A.; Brunaldi, V.; Storm, A.C.; Vargas, E.J.; Bofill-Garcia, A.; Chandrasekhara, V.; Law, R.J.; Martin, J.A.; et al. Endoscopic retrograde cholangiopancreatography (ERCP) approach for patients with Roux-en-Y gastric bypass: A comparative study between four ERCP techniques with proposed management algorithm. Surg. Obes. Relat. Dis. 2024, 20, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Cortes, P.; Jirapinyo, P.; Thompson, C.C.; Ryou, M. A comparison of clinical outcomes and cost utility among laparoscopy, enteroscopy, and temporary gastric access-assisted ERCP in patients with Roux-en-Y gastric bypass anatomy. Surg. Endosc. 2021, 35, 4469–4477. [Google Scholar] [CrossRef] [PubMed]
- Kröll, D.; Müller, A.C.; Nett, P.C.; Wiest, R.; Maubach, J.; Stirnimann, G.; Candinas, D.; Borbély, Y.M. Tailored access to the hepatobiliary system in post-bariatric patients: A tertiary care bariatric center experience. Surg. Endosc. 2020, 34, 5469–5476. [Google Scholar] [CrossRef]
- Kochhar, G.S.; Mohy-Ud-Din, N.; Grover, A.; Carleton, N.; Kulkarni, A.; Farah, K.; Dhawan, M.; Thakkar, S. EUS-directed transgastric endoscopic retrograde cholangiopancreatography versus laparoscopic-assisted ERCP versus deep enteroscopy-assisted ERCP for patients with RYGB. Endosc. Int. Open 2020, 8, E877–E882. [Google Scholar] [CrossRef]
- Kedia, P.; Tarnasky, P.R.; Nieto, J.; Steele, S.L.; Siddiqui, A.; Xu, M.; Tyberg, A.; Gaidhane, M.; Kahaleh, M. EUS-directed Transgastric ERCP (EDGE) Versus Laparoscopy-Assisted ERCP (LA-ERCP) for Roux-en-Y Gastric Bypass (RYGB) Anatomy: A Multicenter Early Comparative Experience of Clinical Outcomes. J. Clin. Gastroenterol. 2019, 53, 304–308. [Google Scholar] [CrossRef]
- Klair, J.S.; Jayaraj, M.; Chandrasekar, V.T.; Priyan, H.; Law, J.; Murali, A.R.; Singh, D.; Larsen, M.; Irani, S.; Kozarek, R.; et al. ERCP with overtube-assisted enteroscopy in patients with Roux-en-Y gastric bypass anatomy: A systematic review and meta-analysis. Endoscopy 2020, 52, 824–832. [Google Scholar] [CrossRef]
- Bronswijk, M.; Vanella, G.; van Wanrooij, R.L.J.; Van der Merwe, S. Through-the-scope proximal flange fixation: “Edging” toward single-session procedures for all? Gastrointest. Endosc. 2025, 101, 486–487. [Google Scholar] [CrossRef]
- Gkolfakis, P.; Papaefthymiou, A.; Facciorusso, A.; Tziatzios, G.; Ramai, D.; Dritsas, S.; Florou, T.; Papanikolaou, I.S.; Hassan, C.; Repici, A.; et al. Comparison between Enteroscopy-, Laparoscopy- and Endoscopic Ultrasound-Assisted Endoscopic Retrograde Cholangio-Pancreatography in Patients with Surgically Altered Anatomy: A Systematic Review and Meta-Analysis. Life 2022, 12, 1646. [Google Scholar] [CrossRef]
- Gellért, B.; Rancz, A.; Hoferica, J.; Teutsch, B.; Sipos, Z.; Veres, D.S.; Hegyi, P.J.; Ábrahám, S.; Hegyi, P.; Hritz, I. Understanding the Role of Different ERCP Techniques in Post-Roux-en-Y Gastric Bypass Patients: A Systematic Review and Meta-analysis. Obes. Surg. 2025, 35, 285–304. [Google Scholar] [CrossRef]
- Gangwani, M.K.; Haghbin, H.; Priyanka, F.; Hadi, Y.; Dahiya, D.S.; Kamal, F.; Lee-Smith, W.; Nawras, A.; Aziz, M.; Adler, D.G. Efficacy and safety of EUS-directed transgastric ERCP (EDGE) versus laparoscopic-assisted ERCP: A systematic review and meta-analysis. Endosc. Ultrasound 2024, 13, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Chen, T.; Wang, J.; Feng, Y.; Wang, R.; Zhao, S. Endoscopic-Directed Trans-Gastric Retrograde Cholangiopancreatography in Patients with Roux-en-Y gastric Bypasses: A Meta-Analysis. J. Clin. Gastroenterol. 2023, 57, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Deliwala, S.S.; Mohan, B.P.; Yarra, P.; Khan, S.R.; Chandan, S.; Ramai, D.; Kassab, L.L.; Facciorusso, A.; Dhawan, M.; Adler, D.G.; et al. Efficacy & safety of EUS-directed transgastric endoscopic retrograde cholangiopancreatography (EDGE) in Roux-en-Y gastric bypass anatomy: A systematic review & meta-analysis. Surg. Endosc. 2023, 37, 4144–4158. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W. Clinical and economic value of bispectral index monitoring for adequate endoscopic sedation. Clin. Endosc. 2022, 55, 518–519. [Google Scholar] [CrossRef]
- Esckilsen, S.; Sagatelian, M.; Nietert, P.J.; Baig, M.U.; Sharaiha, R.Z.; Elmunzer, B.J. Systematic review of technical factors associated with persistent fistula after EUS-directed transgastric ERCP in patients with Roux-en-Y gastric bypass. Gastrointest. Endosc. 2025. [Google Scholar] [CrossRef]
- Ghandour, B.; Keane, M.G.; Shinn, B.; Dawod, Q.M.; Fansa, S.; El Chafic, A.H.; Irani, S.S.; Pawa, R.; Gutta, A.; Ichkhanian, Y.; et al. Factors predictive of persistent fistulas in EUS-directed transgastric ERCP: A multicenter matched case-control study. Gastrointest. Endosc. 2023, 97, 260–267. [Google Scholar] [CrossRef]
- Ichkhanian, Y.; Runge, T.; Jovani, M.; Vosoughi, K.; Brewer Gutierrez, O.I.; Khashab, M.A. Management of adverse events of EUS-directed transgastric ERCP procedure. VideoGIE Off. Video J. Am. Soc. Gastrointest. Endosc. 2020, 5, 260–263. [Google Scholar] [CrossRef]
- Baldwin, D.; Ali, A.M.; Altieri, M.S.; DeMaria, E.J. Marginal ulceration after Roux-en-Y gastric bypass-literature review and management algorithm. Metab. Target Organ Damage 2024, 4, 6. [Google Scholar] [CrossRef]
- Krafft, M.R.; Lorenze, A.; Croglio, M.P.; Fang, W.; Baron, T.H.; Nasr, J.Y. “Innocent as a LAMS”: Does Spontaneous Fistula Closure (Secondary Intention), After EUS-Directed Transgastric ERCP (EDGE) via 20-mm Lumen-Apposing Metal Stent, Confer an Increased Risk of Persistent Fistula and Unintentional Weight Gain? Dig. Dis. Sci. 2022, 67, 2337–2346. [Google Scholar] [CrossRef]
- Jovani, M.; Ichkhanian, Y.; Parsa, N.; Singh, S.; Brewer Gutierrez, O.I.; Keane, M.G.; Al Ghamdi, S.S.; Ngamruengphong, S.; Kumbhari, V.; Khashab, M.A. Assessment of the learning curve for EUS-guided gastroenterostomy for a single operator. Gastrointest. Endosc. 2021, 93, 1088–1093. [Google Scholar] [CrossRef]
- Tyberg, A.; Kats, D.; Choi, A.; Gaidhane, M.; Nieto, J.; Kahaleh, M. Endoscopic Ultrasound Guided Gastroenterostomy: What Is the Learning Curve? J. Clin. Gastroenterol. 2021, 55, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Montanelli, J.; Hernandez Lara, A.H.; Uribe Rivera, A.K.; Verde, J.M.; Burmester, E.; Al-Haddad, M.A.; Hollerbach, S.; Vilmann, P.; Abu-Suboh Abadia, M.; Badaoui, A.; et al. An optimal curriculum for training in endoscopic ultrasound: A summarized evidence-based literature systematic review. Surg. Endosc. 2025, 39, 4076–4093. [Google Scholar] [CrossRef]
- Sosa-Valencia, L.; Huppertz, J.; Wanert, F.; Haberzetser, F.; Swanström, L.; Mangiavillano, B.; Eisendrath, P.; Deprez, P.; Robles-Medranda, C.; Carrara, S.; et al. Design and validation of a therapeutic EUS training program using a live animal model: Taking training to the next level. Endosc. Ultrasound 2022, 11, 112–121. [Google Scholar] [CrossRef]
- James, H.J.; James, T.W.; Wheeler, S.B.; Spencer, J.C.; Baron, T.H. Cost-effectiveness of endoscopic ultrasound-directed transgastric ERCP compared with device-assisted and laparoscopic-assisted ERCP in patients with Roux-en-Y anatomy. Endoscopy 2019, 51, 1051–1058. [Google Scholar] [CrossRef]
| Phase | Aspect | Recommendation |
|---|---|---|
| Pre-procedure | Imaging | Contrast-enhanced CT and/or MRCP to identify remnant stomach anatomy and landmarks |
| Antibiotic prophylaxis | Not routinely indicated; advised if cholangitis or complex drainage expected | |
| Diet | Fast for 8 h pre-procedure | |
| Intra-procedure | LAMS selection | Preferably 20 mm LAMS, especially in case of single-session procedure |
| LAMS fixation | Consider fixation using OTSC or suturing, especially in case of single-session procedure | |
| Post-procedure | PPI | Consider treatment until fistula closure |
| Diet | Liquid diet for 24 h, followed by gradual refeeding based on clinical tolerance | |
| Fistula closure | Remove LAMS at 4 weeks; close fistula if needed via clips or suturing; consider use of APC on the fistula edges |
| First Author | Year | Study Design | Total N/EDGE | Technical Success, n (%) | Clinical Success, n (%) | AEs, n (%) | Severe AEs, n (%) | Comparison with Other Approaches | Sessions |
|---|---|---|---|---|---|---|---|---|---|
| Bronswijk [59] | 2025 | Retrospective | 20/20 | 19 (95) | NA | 2 (10) | 0 | No | Single session (95%); Dual session (5%) |
| Rizzo [40] | 2025 | Retrospective | 216/9 | 9 (100) | 8 (88.9) | 1 (11.1) | 0 | No | Single session (66%); Dual session (34%) |
| Mangiavillano [31] | 2024 | Retrospective | 270/79 | 77 (97.5) | 77 (100) | 4 (5.1) | NA | No | NA |
| Ghazi [53] | 2024 | Retrospective | 132/25 | 22 (89.5) | NA | 4 (15.8) | NA | Yes (LA-ERCP, EA-ERCP) | Single session (46.2%) Dual session (53.8%) |
| Pérez-Cuadrado-Robles [27] | 2023 | Retrospective | 33/31 | 30 (96.8) | 30 (100) | 1 (3.2) | 1 (3.2) | No | Dual session (100%) |
| Kedia [43] | 2023 | Retrospective | 172/172 | 171 (99.4) | 163 (95.3) | 48 (27.9) | 1 (0.6) | No | Single session (44%) Dual session (56%) |
| Keane [45] | 2023 | Retrospective | 37/37 | 37 (100) | 37 (100) | 4 (10.8) | 2 (5.4) | No | Single session (100%) |
| Chhabra [46] | 2022 | Retrospective | 14/14 | 13 (92.8) | 13 (100) | 3 (21.4) | NA | No | Single session (67%) Dual session (43%) |
| Bahdi [24] | 2022 | Retrospective | 32/32 | 31 (96.9) | 27 (87.1) | 11 (34.4) | 3 (9.4) | No | Single session (53.1%) Dual session (46.9%) |
| Runge [28] | 2021 | Retrospective | 178/178 | 175 (98.3) | 172 (98.3) | 28 (15.7) | 4 (2.2) | No | Single session (49%) Dual session (51%) |
| Khara [25] | 2021 | Retrospective | 76/17 | 17 (100) | 17 (100) | 1(6) | 0 (0) | Yes (LA-ERCP) | Single session (51%) Dual session (49%) |
| Wang [54] | 2021 | Retrospective | 130/18 | 18 (100) | NA | 1 | NA | Yes (LA-ERCP, EA-ERCP) | NA |
| Barclay [47] | 2022 | Retrospective | 7/7 | 7 (100) | 7 (100) | 0 | 0 | No | Dual session (100%) |
| Tyberg [48] | 2020 | Retrospective | 19/19 | 19 (100) | 18 (94.7) | 4 (21.1) | NA | No | Single session (26%) Dual session (74%) |
| Kröll [55] | 2020 | Retrospective | 19/2 | 2 (100) | NA | 0 | 0 | Yes (LA-ERCP, HGS, EDGE) | Dual session (100%) |
| Kochhar [56] | 2020 | Retrospective | 56/26 | 26 (100) | NA | 3 (11.5) | NA | Yes (LA-ERCP, EA-ERCP) | Single session (50%) Dual session (50%) |
| de Benito Sanz [49] | 2020 | Retrospective | 14/14 | 14 (100) | 13 (92.9) | 5 (35.7) | NA | No | NA |
| James [50] | 2019 | Retrospective | 19/19 | 19 (100) | 19 (100) | 6 (31.6) | 0 | No | Single session (21%) Dual session (79%) |
| Wang [39] | 2019 | Prospective | 10/10 | 10 (100) | 10 (100) | 2 (20) | 0 | No | Single session (78%) Dual session (22%) |
| Kedia [57] | 2019 | Retrospective | 72/29 | 28 (96.5) | 28 (100) | 7 (24.1) | NA | Yes (LA-ERCP) | Dual session (100%) |
| Tyberg [16] | 2017 | Prospective | 16/16 | 16 (100) | 10/11 * (90.9) | 4 (25) | 1 (6.3) | No | Single session (40%) Dual session (60%) |
| Ngamruengphong [51] | 2017 | Retrospective | 13/13 | 13 (100) | 13 (100) | 0 | 0 | No | Single session (15.4%) Dual session (84.6%) |
| Attam [52] | 2015 | Retrospective | 10/10 | 9 (90) | 9 (100) | 0 | 0 | No | Single session (100%) |
| First Author | Year | Pooled Technical Success, % | Pooled Clinical Success, % | Pooled AEs Rates, % | Comparison with Other Approaches |
|---|---|---|---|---|---|
| Gellert [61] | 2025 | 96 | 93 | 20 | Yes, vs. LA-ERCP, EA-ERCP |
| Gangwani * [8] | 2024 | 94.8 | NA | 14.9 | Yes, vs. LA-ERCP, EA-ERCP |
| Gangwani [62] | 2024 | NA | NA | NA | Yes, vs. LA-ERCP |
| Su [63] | 2023 | 98 | 94 | 14 | No |
| Deliwala [64] | 2023 | 96 | 91 | 17 | Yes, vs. LA-ERCP, EA-ERCP |
| Gkolfakis [60] | 2022 | 97.9 | 97.9 | 13.1 | Yes, vs. LA-ERCP, EA-ERCP |
| de Oliveira [11] | 2022 | 97.8 | NA | 13 | Yes, vs. LA-ERCP |
| Dhindsa [7] | 2020 | 95.5 | 95.9 | 21.9 | Yes, vs. LA-ERCP and EA-ERCP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonini, F.; Rizzo, G.E.M.; Vanella, G.; Fuccio, L.; Lisotti, A.; Bronswijk, M.; Pérez-Cuadrado-Robles, E.; Binda, C.; Mazza, S.; Anderloni, A.; et al. Endoscopic Ultrasound-Directed Transgastric Endoscopic Retrograde Cholangiopancreatography (EDGE): Techniques, Outcomes and Safety Profiles. J. Clin. Med. 2025, 14, 5675. https://doi.org/10.3390/jcm14165675
Antonini F, Rizzo GEM, Vanella G, Fuccio L, Lisotti A, Bronswijk M, Pérez-Cuadrado-Robles E, Binda C, Mazza S, Anderloni A, et al. Endoscopic Ultrasound-Directed Transgastric Endoscopic Retrograde Cholangiopancreatography (EDGE): Techniques, Outcomes and Safety Profiles. Journal of Clinical Medicine. 2025; 14(16):5675. https://doi.org/10.3390/jcm14165675
Chicago/Turabian StyleAntonini, Filippo, Giacomo Emanuele Maria Rizzo, Giuseppe Vanella, Lorenzo Fuccio, Andrea Lisotti, Michiel Bronswijk, Enrique Pérez-Cuadrado-Robles, Cecilia Binda, Stefano Mazza, Andrea Anderloni, and et al. 2025. "Endoscopic Ultrasound-Directed Transgastric Endoscopic Retrograde Cholangiopancreatography (EDGE): Techniques, Outcomes and Safety Profiles" Journal of Clinical Medicine 14, no. 16: 5675. https://doi.org/10.3390/jcm14165675
APA StyleAntonini, F., Rizzo, G. E. M., Vanella, G., Fuccio, L., Lisotti, A., Bronswijk, M., Pérez-Cuadrado-Robles, E., Binda, C., Mazza, S., Anderloni, A., Fabbri, C., & Tarantino, I., on behalf of I-EUS Working Group. (2025). Endoscopic Ultrasound-Directed Transgastric Endoscopic Retrograde Cholangiopancreatography (EDGE): Techniques, Outcomes and Safety Profiles. Journal of Clinical Medicine, 14(16), 5675. https://doi.org/10.3390/jcm14165675






