Beyond the Plate: Patient Perspectives on Diet and Daily Life with Crohn’s Disease—A National Survey
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics
3.2. Characteristics of the Disease
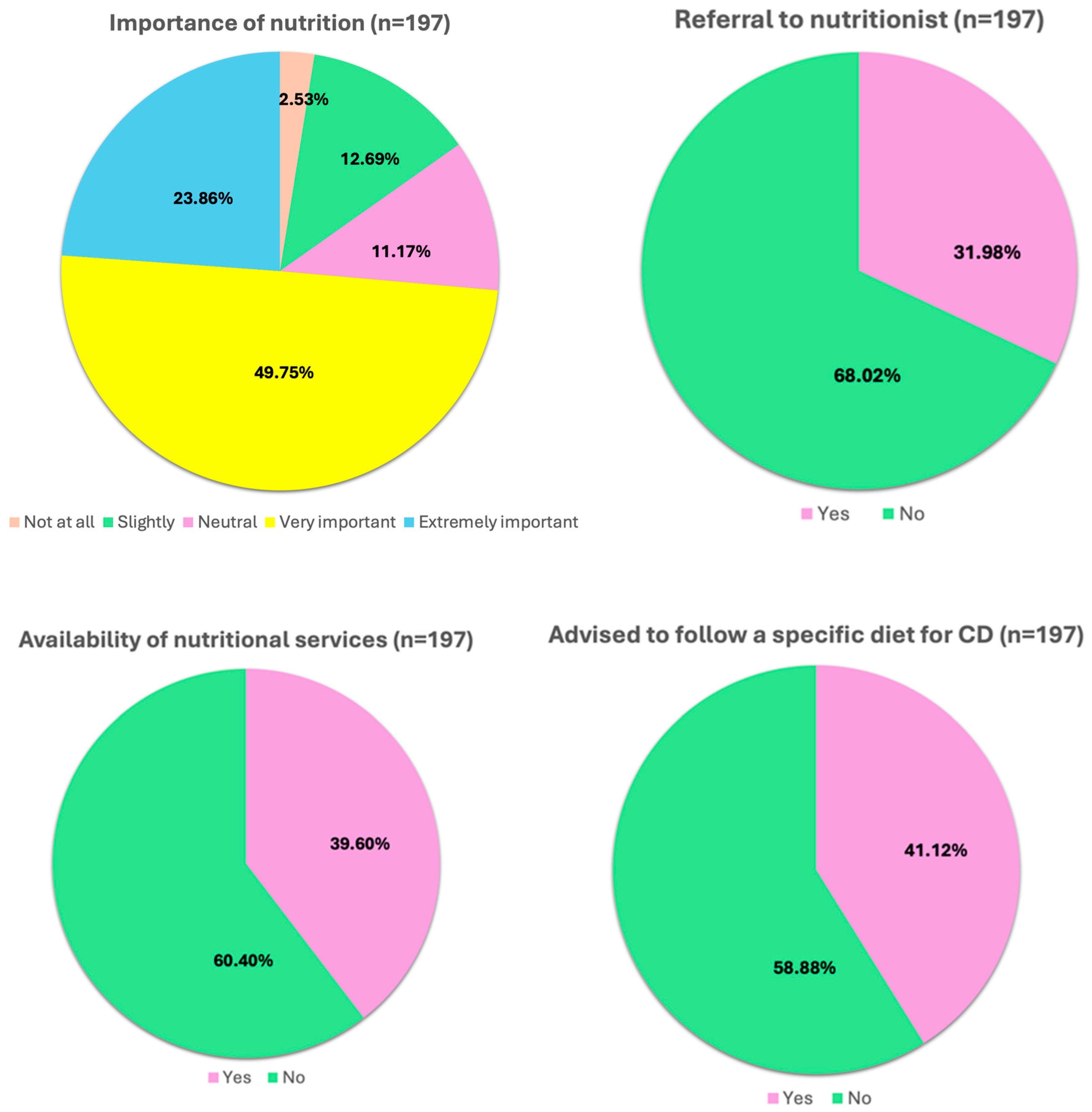
3.3. Role of Diet
3.3.1. Perceived Role of Nutrition and Daily Management in the Remission Phase
3.3.2. Perceived Role of Nutrition and Daily Management During Flare-Ups
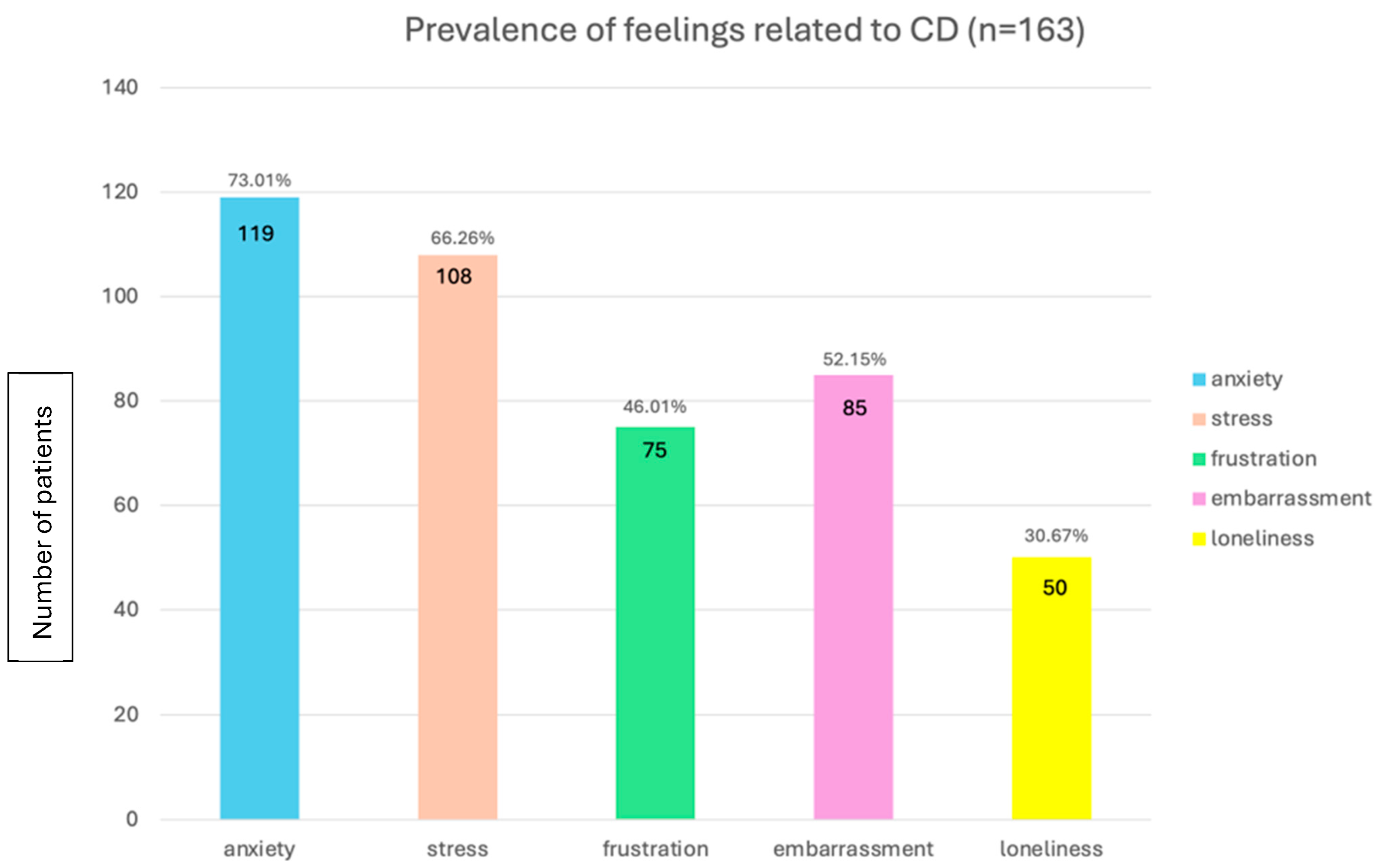
3.4. Psychological Impact: Navigating the Mental Health Challenges of Crohn’s Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laudisi, F.; Stolfi, C.; Monteleone, G. Impact of Food Additives on Gut Homeostasis. Nutrients 2019, 11, 2334. [Google Scholar] [CrossRef]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients with Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef]
- Norton, C.; Czuber-Dochan, W.; Bassett, P.; Berliner, S.; Bredin, F.; Darvell, M.; Forbes, A.; Gay, M.; Ream, E.; Terry, H. Assessing Fatigue in Inflammatory Bowel Disease: Comparison of Three Fatigue Scales. Aliment. Pharmacol. Ther. 2015, 42, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Minh Duc, N.T.; Luu Lam Thang, T.; Nam, N.H.; Ng, S.J.; Abbas, K.S.; Huy, N.T.; Marušić, A.; Paul, C.L.; Kwok, J.; et al. A Consensus-Based Checklist for Reporting of Survey Studies (CROSS). J. Gen. Intern. Med. 2021, 36, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Stam, A.; Kleiner, B. Data Anonymisation: Legal, Ethical, and Strategic Considerations. In FORS Guide N°11; Version 1.0; Swiss Centre of Expertise in the Social Sciences FORS: Lausanne, Switzerland, 2020. [Google Scholar] [CrossRef]
- D’Amico, F.; Gomollón, F.; Bamias, G.; Magro, F.; Targownik, L.; Leitner, C.; Heatta-Speicher, T.; Michelena, N.; Kolterer, S.; Lapthorn, J.; et al. Proportion of Inflammatory Bowel Diseases Patients with Suboptimal Disease Control in Daily Clinical Practice-Real-World Evidence from the Inflammatory Bowel Diseases-Podcast Study. United Eur. Gastroenterol. J. 2024, 12, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Chavarría, C.; Casanova, M.J.; Chaparro, M.; Barreiro-de Acosta, M.; Ezquiaga, E.; Bujanda, L.; Rivero, M.; Argüelles-Arias, F.; Martín-Arranz, M.D.; Martínez-Montiel, M.P.; et al. Prevalence and Factors Associated with Fatigue in Patients with Inflammatory Bowel Disease: A Multicentre Study. J. Crohns Colitis 2019, 13, 996–1002. [Google Scholar] [CrossRef]
- D’Silva, A.; Fox, D.E.; Nasser, Y.; Vallance, J.K.; Quinn, R.R.; Ronksley, P.E.; Raman, M. Prevalence and Risk Factors for Fatigue in Adults with Inflammatory Bowel Disease: A Systematic Review with Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 995–1009.e7. [Google Scholar] [CrossRef]
- van Langenberg, D.R.; Gibson, P.R. Systematic Review: Fatigue in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2010, 32, 131–143. [Google Scholar] [CrossRef]
- Gong, S.-S.; Fan, Y.-H.; Lv, B.; Zhang, M.-Q.; Xu, Y.; Zhao, J. Fatigue in Patients with Inflammatory Bowel Disease in Eastern China. World J. Gastroenterol. 2021, 27, 1076–1089. [Google Scholar] [CrossRef]
- Grimstad, T.; Norheim, K.B.; Isaksen, K.; Leitao, K.; Hetta, A.K.; Carlsen, A.; Karlsen, L.N.; Skoie, I.M.; Gøransson, L.; Harboe, E.; et al. Fatigue in Newly Diagnosed Inflammatory Bowel Disease. J. Crohns Colitis 2015, 9, 725–730. [Google Scholar] [CrossRef]
- Nocerino, A.; Nguyen, A.; Agrawal, M.; Mone, A.; Lakhani, K.; Swaminath, A. Fatigue in Inflammatory Bowel Diseases: Etiologies and Management. Adv. Ther. 2020, 37, 97–112. [Google Scholar] [CrossRef]
- Borren, N.Z.; van der Woude, C.J.; Ananthakrishnan, A.N. Fatigue in IBD: Epidemiology, Pathophysiology and Management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 247–259. [Google Scholar] [CrossRef]
- Weisshof, R.; Chermesh, I. Micronutrient Deficiencies in Inflammatory Bowel Disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef]
- Charlebois, A.; Rosenfeld, G.; Bressler, B. The Impact of Dietary Interventions on the Symptoms of Inflammatory Bowel Disease: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1370–1378. [Google Scholar] [CrossRef]
- Gerasimidis, K.; McGrogan, P.; Edwards, C.A. The Aetiology and Impact of Malnutrition in Paediatric Inflammatory Bowel Disease. J. Hum. Nutr. Diet. 2011, 24, 313–326. [Google Scholar] [CrossRef]
- Levenstein, S.; Prantera, C.; Luzi, C.; D’Ubaldi, A. Low Residue or Normal Diet in Crohn’s Disease: A Prospective Controlled Study in Italian Patients. Gut 1985, 26, 989–993. [Google Scholar] [CrossRef]
- Faggiani, I.; Fanizza, J.; Massironi, S.; D’Amico, F.; Allocca, M.; Furfaro, F.; Parigi, T.L.; Fiorino, G.; Danese, S.; Zilli, A. The Role of Diet in Inflammatory Bowel Disease: A Comprehensive Review of the Literature. Best Pract. Res. Clin. Gastroenterol. 2025, 77, 101995. [Google Scholar] [CrossRef]
- Gordon, H.; Minozzi, S.; Kopylov, U.; Verstockt, B.; Chaparro, M.; Buskens, C.; Warusavitarne, J.; Agrawal, M.; Allocca, M.; Atreya, R.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2024, 18, 1531–1555. [Google Scholar] [CrossRef]
- Parigi, T.L. Impact of Diet on the Effectiveness of Anti-TNF Therapy in Inflammatory Bowel Disease: A Prospective, Double Arm, Open-Label Study; Clinicaltrials.gov: 2025. Available online: https://clinicaltrials.gov/study/NCT0689630522 (accessed on 6 August 2025).
- Wolfson Medical Center. Stelara and CDED Diet Trial for Crohn’s Disease; Clinicaltrials.gov; Wolfson Medical Center: Holon, Israel, 2021. [Google Scholar]
- Universitaire Ziekenhuizen KU Leuven. COmbinAtion Therapy of dieT with biologicalS for Crohn’s Disease: The OATS Study; Clinicaltrials.gov; Universitaire Ziekenhuizen KU Leuven: Leuven, Belgium, 2024. Available online: https://clinicaltrials.gov/study/NCT04946448 (accessed on 6 August 2025).
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; Cohen, N.A.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. The Crohn’s Disease Exclusion Diet for Induction and Maintenance of Remission in Adults with Mild-to-Moderate Crohn’s Disease (CDED-AD): An Open-Label, Pilot, Randomised Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 49–59. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN Guideline on Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef]
- Massironi, S.; Pigoni, A.; Vegni, E.A.M.; Keefer, L.; Dubinsky, M.C.; Brambilla, P.; Delvecchio, G.; Danese, S. The Burden of Psychiatric Manifestations in Inflammatory Bowel Diseases: A Systematic Review with Meta-Analysis. Inflamm. Bowel Dis. 2025, 31, 1441–1459. [Google Scholar] [CrossRef]
- Tarar, Z.I.; Zafar, M.U.; Farooq, U.; Ghous, G.; Aslam, A.; Inayat, F.; Ghouri, Y.A. Burden of Depression and Anxiety among Patients with Inflammatory Bowel Disease: Results of a Nationwide Analysis. Int. J. Color. Dis. 2022, 37, 313–321. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, G.; Stapersma, L.; Vlug, L.E.; Rizopolous, D.; Bodelier, A.G.; van Wering, H.; Hurkmans, P.C.W.M.; Stuyt, R.J.L.; Hendriks, D.M.; van der Burg, J.A.T.; et al. Clinical Disease Activity Is Associated with Anxiety and Depressive Symptoms in Adolescents and Young Adults with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2018, 48, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Stroie, T.; Preda, C.; Meianu, C.; Croitoru, A.; Gheorghe, L.; Gheorghe, C.; Diculescu, M. Health-Related Quality of Life in Patients with Inflammatory Bowel Disease in Clinical Remission: What Should We Look For? Medicina 2022, 58, 486. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Durante, T.; Palladino, G.; D’Onofrio, R.; Mammone, S.; Arboretto, G.; Auletta, S.; Imperio, G.; Ventura, A.; et al. Telemedicine in Inflammatory Bowel Diseases: A New Brick in the Medicine of the Future? World J. Methodol. 2023, 13, 194–209. [Google Scholar] [CrossRef]
- Rashed, R.; Valcheva, R.; Dieleman, L.A. Manipulation of Gut Microbiota as a Key Target for Crohn’s Disease. Front. Med. 2022, 9, 887044. [Google Scholar] [CrossRef] [PubMed]

| Patient (n = 222) | |
| Sex | |
| Male | 89 (40.09%) |
| Female | 132 (59.46%) |
| Others | 1 (0.45%) |
| Age (years) | |
| 18–19 y | 9 (4.05%) |
| 20–29 y | 57 (25.68%) |
| 30–39 y | 58 (26.13%) |
| 40–49 y | 47 (21.17%) |
| >50 y | 51 (22.97%) |
| Place of residence | |
| Northern Italy | 143 (64.41%) |
| Central Italy | 26 (11.71%) |
| Southern Italy | 53 (23.87%) |
| Age at diagnosis (years) | |
| 1–9 y | 2 (0.90%) |
| 10–19 y | 51 (22.97%) |
| 20–29 y | 82 (36.94%) |
| 30–39 y | 46 (20.72%) |
| 40–49 y | 29 (13.06%) |
| >50 y | 12 (5.41%) |
| Location | |
| Ileal | 181/218 (83.03%) |
| Colonic | 69/218 (31.65%) |
| Perianal | 28/218 (12.84%) |
| Gastro-duodenal | 15/218 (6.88%) |
| Symptoms | |
| Fatigue | 159 (71.62%) |
| Abdominal pain | 102 (45.95%) |
| Diarrhea | 101 (45.50%) |
| Joint pain | 87 (39.19%) |
| Gastroesophageal reflux | 50 (22.52%) |
| Rectal bleeding | 42 (18.92%) |
| Weight loss | 33 (14.86%) |
| Nausea | 26 (11.71%) |
| Vomiting | 10 (4.50%) |
| Therapy | |
| Biologic therapy | 153/199 (76.88%) |
| Systemic corticosteroids | 16/199 (8.04%) |
| Mesalamine | 19/199 (9.55%) |
| Experimental trial protocols | 2/199 (1.01%) |
| Small-molecule drugs | 3/199 (1.51%) |
| Budesonide | 2/199 (1.01%) |
| Immunosuppressants | 3/199 (1.51%) |
| Not specified | 1/199 (0.50%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bencardino, S.; D’Amico, F.; Ciliberto, A.; Danese, S. Beyond the Plate: Patient Perspectives on Diet and Daily Life with Crohn’s Disease—A National Survey. J. Clin. Med. 2025, 14, 5648. https://doi.org/10.3390/jcm14165648
Bencardino S, D’Amico F, Ciliberto A, Danese S. Beyond the Plate: Patient Perspectives on Diet and Daily Life with Crohn’s Disease—A National Survey. Journal of Clinical Medicine. 2025; 14(16):5648. https://doi.org/10.3390/jcm14165648
Chicago/Turabian StyleBencardino, Sarah, Ferdinando D’Amico, Ambra Ciliberto, and Silvio Danese. 2025. "Beyond the Plate: Patient Perspectives on Diet and Daily Life with Crohn’s Disease—A National Survey" Journal of Clinical Medicine 14, no. 16: 5648. https://doi.org/10.3390/jcm14165648
APA StyleBencardino, S., D’Amico, F., Ciliberto, A., & Danese, S. (2025). Beyond the Plate: Patient Perspectives on Diet and Daily Life with Crohn’s Disease—A National Survey. Journal of Clinical Medicine, 14(16), 5648. https://doi.org/10.3390/jcm14165648







