Predictive Tool for Tunnelled Central Venous Catheter Dysfunction in Haemodialysis
Abstract
1. Introduction
2. Methods
3. Results
3.1. Model Training
3.2. Stepwise Model (Forward and Backward)
3.3. Lasso Model
3.4. Bootstrapping Model
3.5. Internal Validation of the Predictive Model
3.6. Model Selection and Development of the Predictive Tool
4. Discussion
5. Limitations
5.1. Review of Traditional Approaches: Limitations and Opportunities
5.2. Predictive Variables: Consistency and Differences with the Literature
5.3. Clinical Relevance and Model Applicability
6. Conclusions
7. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffiths, R.I.; Newsome, B.B.; Block, G.A.; Herbert, R.J.; Danese, M.D. Patterns of hemodialysis catheter dysfunction defined according to National Kidney Foundation guidelines as blood flow <300 mL/min. Int. J. Nephrol. 2011, 2011, 891259. [Google Scholar] [CrossRef][Green Version]
- Lee, T.; Lok, C.; Vazquez, M.; Moist, L.; Maya, I.; Mokrzycki, M. Minimizing hemodialysis catheter dysfunction: An ounce of prevention. Int. J. Nephrol. 2012, 2012, 170857. [Google Scholar] [CrossRef]
- Brzosko, S.; Hryszko, T.; Malyszko, J.; Malyszko, J.S.; Mazerska, M.; Myśliwiec, M. Femoral localization and higher ultrafiltration rate but not concentration of heparin used for canal locking of hemodialysis catheter are negative predictors for its malfunction. Am. J. Nephrol. 2008, 28, 298–303. [Google Scholar] [CrossRef]
- Baskin, J.L.; Pui, C.H.; Reiss, U.; Wilimas, J.A.; Metzger, M.L.; Ribeiro, R.C.; Howard, S.C. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009, 374, 159–169. [Google Scholar] [CrossRef]
- Gallieni, M.; Giordano, A.; Rossi, U.; Cariati, M. Optimization of dialysis catheter function. J. Vasc. Access. 2016, 17 (Suppl. S1), S42–S46. [Google Scholar] [CrossRef]
- Villalobos, J.J.F.; Toro, M.P.M. Factores predictores para la pesquisa precoz en la disfunción de catéteres venosos tunelizados en hemodiálisis. Horiz. Enferm. 2019, 30, 6–15. [Google Scholar] [CrossRef][Green Version]
- Salvador García, J.; Gómez Valdés, J.; Casula, E.; Magán Martín, A.; Ruiz Guanter, A.; Lontejo Vicent, E. Disfunción tardía en catéteres de hemodiálisis: Claves diagnósticas y manejo terapéutico. Intervencionismo 2019, 19, 160–166. [Google Scholar] [CrossRef]
- Kennard, A.L.; Walters, G.D.; Jiang, S.H.; Talaulikar, G.S. Interventions for treating central venous haemodialysis catheter malfunction. Cochrane Database Syst. Rev. 2017, 10, CD011953. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Kappel, J.; MacRae, J.; Dipchand, C.; Hiremath, S.; Kiaii, M.; Lok, C.; Moist, L.; Oliver, M.; Miller, L.M.; et al. Practical aspects of nontunneled and tunneled hemodialysis catheters. Can. J. Kidney Health Dis. 2016, 3, 2054358116669128. [Google Scholar] [CrossRef]
- Zhao, X.; Niu, Q.; Gan, L.; Hou, F.F.; Liang, X.; Ni, Z.; Chen, X.; McCullough, K.; Zhao, J.; Robinson, B.; et al. Blood flow rate: An independent risk factor of mortality in Chinese hemodialysis patients. Semin. Dial. 2022, 35, 251–257. [Google Scholar] [CrossRef]
- Moya Mejía, C.; Fernández Ros, M.; Ibeas López, J.; Alcaraz Busqueta, J.; Mañé Buixó, N.; Yuste Jiménez, E.; García, M.G.; Falcó, J.; Perandreu, J.; Fortuño, J.R. Monitorización sistemática del catéter permanente: Una herramienta útil en el estudio de la tasa de infección y disfunción en dos tipos de catéteres tunelizados para hemodiálisis. Rev. Soc. Esp. Enferm. Nefrol. 2006, 9, 13–19. [Google Scholar] [CrossRef]
- Canaud, B.; Leray-Moragues, H.; Chenine, L.; Morena, M.; Miller, G.; Canaud, L.; Cristol, J.P. Comparative clinical performances of tunneled central venous catheters versus arterio-venous accesses in patients receiving high-volume hemodiafiltration: The case for High-Flow DualCath, a tunneled two-single-lumen silicone catheter. J. Clin. Med. 2023, 12, 4732. [Google Scholar] [CrossRef]
- Kosa, S.D.; Ye, C.; Thabane, L.; Gafni, A.; Lok, C.E. Predicting tissue plasminogen activator use and success in in-center hemodialysis patients. J. Vasc. Access 2018, 19, 146–152. [Google Scholar] [CrossRef]
- Vesely, T.M.; Ravenscroft, A. Hemodialysis catheter tip design: Observations on fluid flow and recirculation. J. Vasc. Access 2016, 17, 29–39. [Google Scholar] [CrossRef]
- Wang, Y.; Ivany, J.N.; Perkovic, V.; Gallagher, M.P.; Woodward, M.; Jardine, M.J. Anticoagulants and antiplatelet agents for preventing central venous haemodialysis catheter malfunction in patients with end-stage kidney disease. Cochrane Database Syst. Rev. 2016, 4, CD009631. [Google Scholar] [CrossRef] [PubMed]
- Tal, M.G.; Ni, N. Selecting optimal hemodialysis catheters: Material, design, advanced features, and preferences. Tech. Vasc. Interv. Radiol. 2008, 11, 186–191. [Google Scholar] [CrossRef]
- Ge, X.; Cavallazzi, R.; Li, C.; Pan, S.M.; Wang, Y.W.; Wang, F.L. Central venous access sites for the prevention of venous thrombosis, stenosis and infection. Cochrane Database Syst. Rev. 2012, 14, CD004084. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.; Glickman, M.H.; et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 update. Am. J. Kidney Dis. 2020, 75 (Suppl. S2), S1–S164. [Google Scholar] [CrossRef] [PubMed]
- Arribas Cobo, P.; Rodríguez Gayán, P.; Sáenz Santolaya, J.A.; Quesada Armenteros, M.T.; Molina Mejías, P.; Quero Lope, C. Estudio de la eficacia de sellado en el periodo interdialítico largo para catéteres de hemodiálisis disfuncionantes. Enferm. Nefrol. 2017, 20, 38–41. [Google Scholar] [CrossRef]
- González, A.M.; García, C.D.; Rodríguez, E.M.; María, A.; Rodríguez, M. Impacto de las disfunciones de catéter venoso central tunelizado para hemodiálisis: Eficacia y coste. Enferm Nefrol. 2017, 20 (Suppl. S1), 42. [Google Scholar]
- Besarab, A.; Pandey, R. Catheter management in hemodialysis patients: Delivering adequate flow. Clin. J. Am. Soc. Nephrol. 2011, 6, 227–234. [Google Scholar] [CrossRef][Green Version]
- Manzano-Angua, J.M.; Manzano-Angua, R.; Martín-Carrasco, M.A.; Cirera-Segura, F.; Márquez-Catalán, D.I. Influence of the orientation of the arterial line of the tunneled hemodialysis central venous catheter on early dysfunction. Enferm. Nefrol. 2022, 25, 319–328. [Google Scholar] [CrossRef]
- Szymańska, J.; Kakareko, K.; Rydzewska-Rosołowska, A.; Głowińska, I.; Hryszko, T. Locked away—Prophylaxis and management of catheter related thrombosis in hemodialysis. J. Clin. Med. 2021, 10, 2230. [Google Scholar] [CrossRef]
- Venegas Justiniano, J.Y.; Hurtado Aréstegui, A.; Loza Muñarriz, C. Características de la colocación y permanencia de catéteres venosos centrales temporales para hemodiálisis en un hospital público 2015–2019. Acta Med. Peru 2022, 39, 128–137. [Google Scholar] [CrossRef]
- Chouhani, B.A.; Kabbali, N.; Chiba Bennani, S.; El Bardai, G.; Sqalli Houssaini, T. Tunneled catheters in hemodialysis: Indications and complications. J. Med. Vasc. 2022, 47, 87–93. [Google Scholar] [CrossRef]
- Wang, K.; Wang, P.; Liang, X.; Lu, X.; Liu, Z. Epidemiology of haemodialysis catheter complications: A survey of 865 dialysis patients from 14 haemodialysis centres in Henan province in China. BMJ Open 2015, 5, e007136. [Google Scholar] [CrossRef]
- Fry, A.C.; Stratton, J.; Farrington, K.; Mahna, K.; Selvakumar, S.; Thompson, H.; Warwicker, P. Factors affecting long-term survival of tunnelled haemodialysis catheters: A prospective audit of 812 tunnelled catheters. Nephrol. Dial. Transplant. 2008, 23, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Moist, L.M.; Hemmelgarn, B.R.; Lok, C.E. Relationship between blood flow in central venous catheters and hemodialysis adequacy. Clin. J. Am. Soc. Nephrol. 2006, 1, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Pelayo Alonso, R.; Martínez Álvarez, P.; Cagigas Villoslada, M.J.; Villa Llamazares, C.; Cuadrado Mantecón, M.E.; Gándara Revuelta, M. Dosis de diálisis alcanzada en pacientes en hemodiálisis según el acceso vascular empleado. Enferm. Nefrol. 2017, 20, 324–329. [Google Scholar] [CrossRef]
- Delgado, Y.P.; Molina, Y.S.; Augier, M.M.; Sánchez, L.V.; López, E.L. Supervivencia y complicaciones de los catéteres para hemodiálisis: Nuestra experiencia. Rev. Cuba. Cir. 2006, 45, 3–4. [Google Scholar]
- García-Lopez, E. Factores Asociados con la Falla del Acceso Vascular de Hemodiálisis en Pacientes con Enfermedad Renal Crónica. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2019. [Google Scholar]
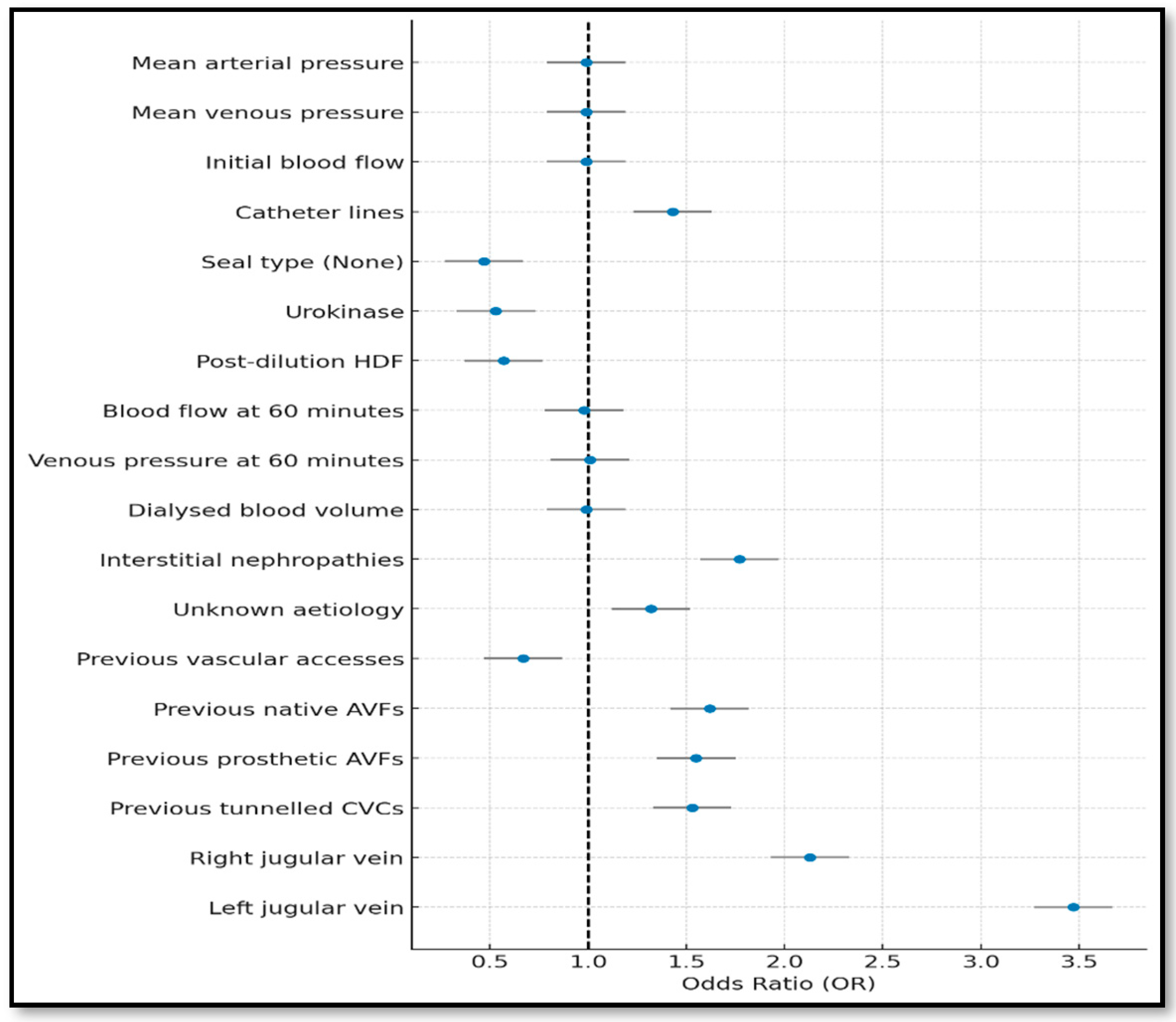
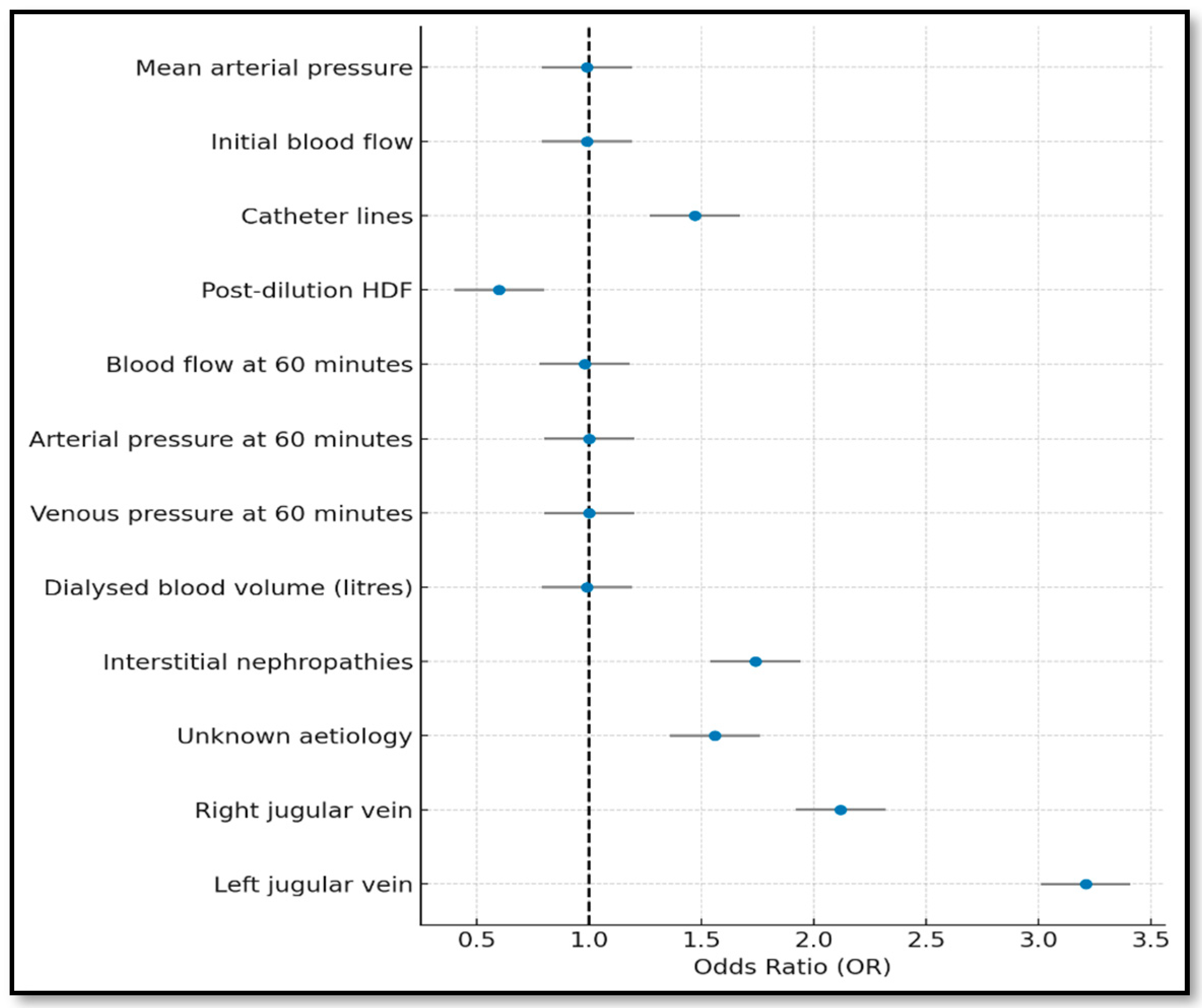
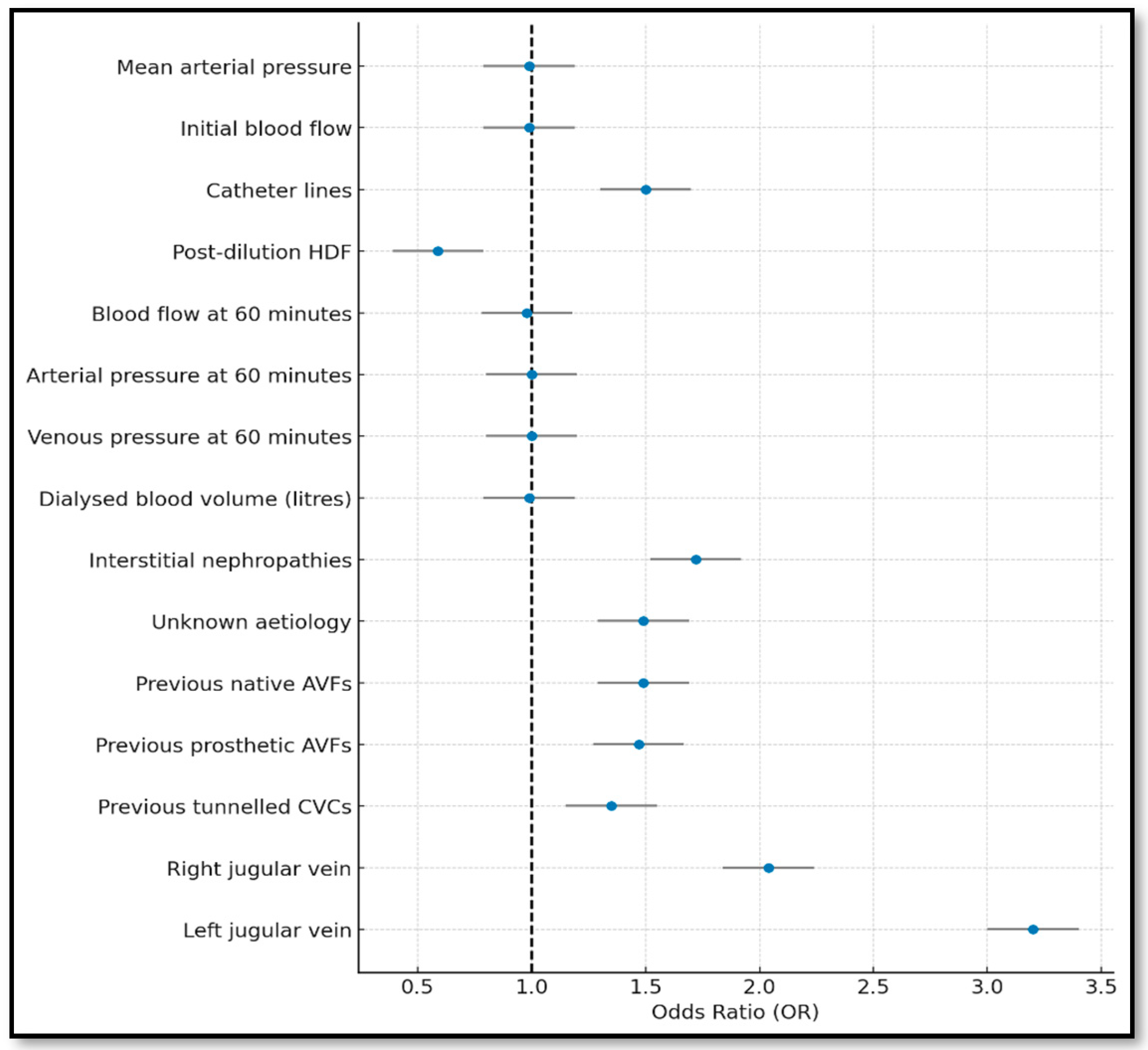
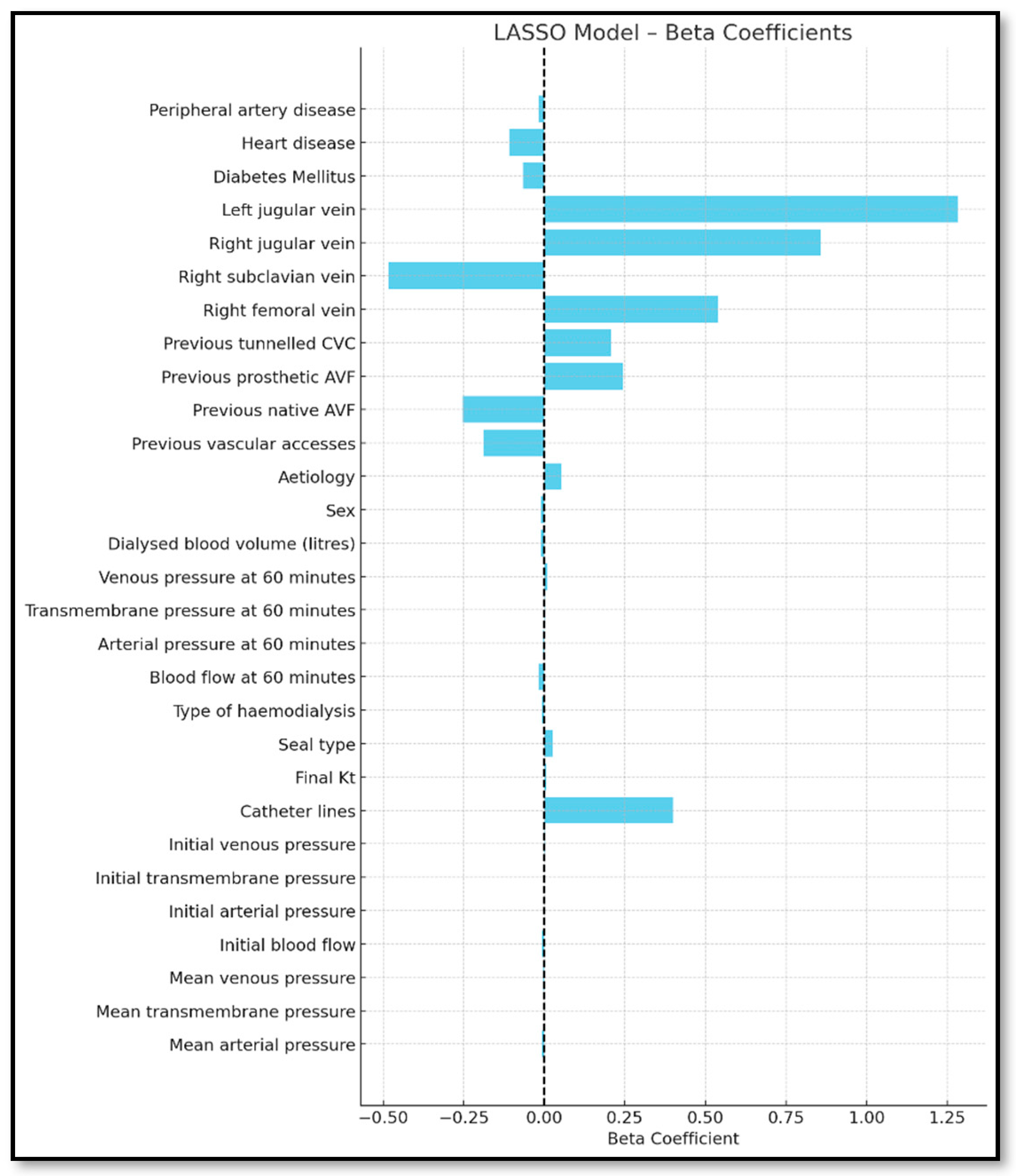
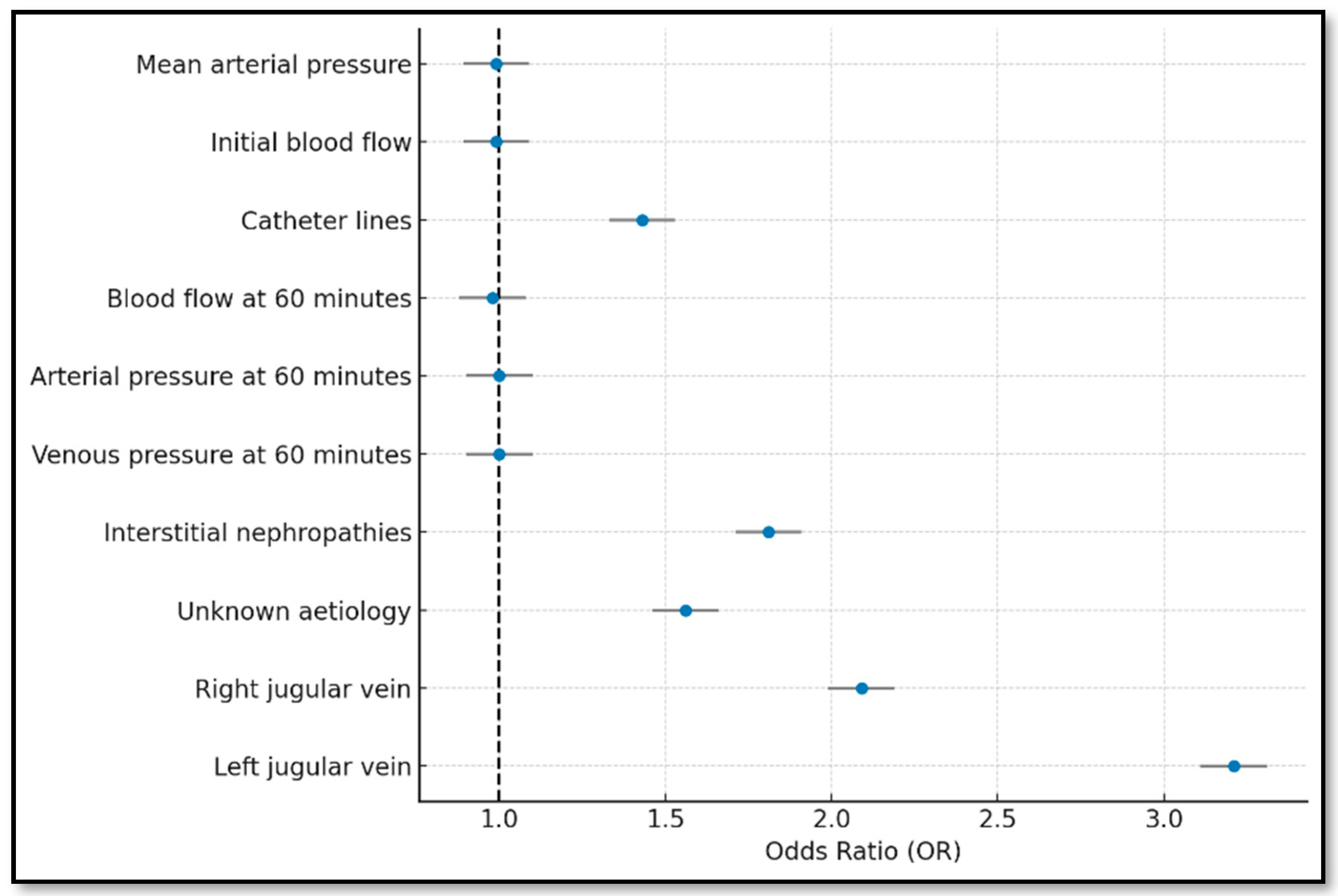
| Complete | Forward | Backward | Lasso | Bootstrapping | |
|---|---|---|---|---|---|
| AIC | 6873.113 | 7146.702 | 7144.51 | 6873.113 | 7192.647 |
| BIC | 7206.537 | 7326.752 | 7350.278 | 7206.537 | 7329.895 |
| Hosmer-Lemeshow | 0.0231 | 0.0013 | 0.0094 | 0.0231 | 0.0016 |
| Area Under the Curve | Standard Deviation | p-Value | Confidence Interval 95% | |
|---|---|---|---|---|
| Upper Limit | Lower Limit | |||
| 0.844 | 0.01 | 0.0000 | 0.82 | 0.86 |
| Cut-off Point | Sensitivity | 1-Specificity | Specificity |
|---|---|---|---|
| 0.019 | 0.816 | 0.291 | 0.709 |
| Bootstrapping | ||
|---|---|---|
| Dysfunction Indicator | Beta | |
| Mean arterial pressure | −0.007 | |
| Initial blood flow | −0.006 | |
| Catheter lines | 0.393 | |
| Blood flow at 60 min | −0.019 | |
| Arterial pressure at 60 min | −0.004 | |
| Venous pressure at 60 min | 0.005 | |
| Aetiology | Vascular disease | 0.162 |
| Glomerular disease | 0.231 | |
| Congenital and hereditary nephropathies | 0.076 | |
| Interstitial nephropathies | 0.596 | |
| Prolonged urinary tract obstruction | 0.423 | |
| Systemic diseases | 0.436 | |
| Unknown aetiology | 0.476 | |
| Tunnelled central venous catheter location | Right jugular vein | 0.739 |
| Left jugular vein | 1.167 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimeno-Hernán, V.; Herrero Calvo, J.A.; Beneit Montesinos, J.V.; Hernán Gascueña, D.; Serrano García, I.; Ortuño-Soriano, I. Predictive Tool for Tunnelled Central Venous Catheter Dysfunction in Haemodialysis. J. Clin. Med. 2025, 14, 5647. https://doi.org/10.3390/jcm14165647
Gimeno-Hernán V, Herrero Calvo JA, Beneit Montesinos JV, Hernán Gascueña D, Serrano García I, Ortuño-Soriano I. Predictive Tool for Tunnelled Central Venous Catheter Dysfunction in Haemodialysis. Journal of Clinical Medicine. 2025; 14(16):5647. https://doi.org/10.3390/jcm14165647
Chicago/Turabian StyleGimeno-Hernán, Verónica, Jose Antonio Herrero Calvo, Juan Vicente Beneit Montesinos, David Hernán Gascueña, Irene Serrano García, and Ismael Ortuño-Soriano. 2025. "Predictive Tool for Tunnelled Central Venous Catheter Dysfunction in Haemodialysis" Journal of Clinical Medicine 14, no. 16: 5647. https://doi.org/10.3390/jcm14165647
APA StyleGimeno-Hernán, V., Herrero Calvo, J. A., Beneit Montesinos, J. V., Hernán Gascueña, D., Serrano García, I., & Ortuño-Soriano, I. (2025). Predictive Tool for Tunnelled Central Venous Catheter Dysfunction in Haemodialysis. Journal of Clinical Medicine, 14(16), 5647. https://doi.org/10.3390/jcm14165647







