Paroxysmal Supraventricular Tachycardia and Troponin Elevation: Insights into Mechanisms, Risk Factors, and Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
- ▪
- Insufficient data available in the medical records.
- ▪
- History of structural heart disease, including the following:
- ○
- Cardiomyopathy;
- ○
- Severe left ventricular hypertrophy;
- ○
- Significant valvular disease (moderate or severe stenosis or regurgitation);
- ○
- Left ventricular ejection fraction below 50% as documented by an echocardiogram performed within the past year or a bedside echocardiogram following cardioversion.
- ▪
- Chronically elevated troponin values.
- ▪
- Diagnosis of COVID-19 at presentation. During the study period, all patients admitted to the Cardiology Department were screened with rapid antigen tests for COVID-19, regardless of symptoms. PCR testing was selectively performed in patients with flu-like symptoms, elevated inflammatory markers, or known exposure to confirmed cases. Patients with a confirmed COVID-19 infection by either method were excluded from the study. No IgG or IgM serologic testing was performed, and a history of prior asymptomatic infection was not routinely available.
- ▪
- Presence of other potential underlying causes of PSVT, such as pulmonary embolism, infection, myocarditis, etc. Myocarditis was excluded based on clinical and anamnestic criteria. Patients with symptoms suggestive of viral illness (e.g., fever, myalgia), elevated inflammatory markers, electrocardiographic or echocardiographic abnormalities indicative of myocardial inflammation, or recent exposure to confirmed COVID-19 cases underwent further evaluation, including PCR testing. No additional imaging (e.g., cardiac magnetic resonance imaging) or serologic testing was routinely performed.
2.3. Methodology
2.4. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Risk Factors for Troponin Elevation
3.3. Coronary Artery Disease Evaluation
3.4. Outcomes Within One Year According to Troponin Status
3.5. Impact of CAD Evaluation on Clinical Outcomes in cTn (+) Patients
4. Discussion
4.1. Possible Mechanisms of Troponin Elevation
4.2. Absence of SVT History: A Marker of Susceptibility to Troponin Release
4.3. The Role of Antiarrhythmic Medication
4.4. Clinical Implications and CAD Evaluation
4.5. One-Year Follow-Up
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 95% CI | 95% confidence interval |
| ACS | Acute coronary syndrome |
| ANS | Autonomic nervous system |
| AT | Atrial tachycardia |
| AUC | Area under the curve |
| AVNRT | Atrioventricular nodal reentrant tachycardia |
| AVRT | Atrioventricular reentrant tachycardia |
| BPM | Beats per minute |
| CAD | Coronary artery disease |
| CCBs | Calcium channel blockers |
| CCTA | Coronary computed tomography angiography |
| cTn | Cardiac troponin |
| CVD | Cardiovascular disease |
| DM | Diabetes mellitus |
| ED | Emergency department |
| ESC | European Society of Cardiology |
| Hs-cTnI/T | High-sensitivity cardiac troponin I/T |
| HPA | Hypothalamic pituitary adrenal |
| HR | Heart rate |
| HTN | Hypertension |
| INOCA | Ischemia with no obstructive coronary artery disease |
| LVEF | Left ventricular ejection fraction |
| MACEs | Major adverse cardiovascular events |
| MI | Myocardial infarction |
| MSIMI | Mental stress-induced myocardial infarction |
| NSTE-ACS | Non-ST-elevation acute coronary syndrome |
| OR | Odds ratio |
| PSVT | Paroxysmal supraventricular tachycardia |
| ROC | Receiver operating curve |
| SBP | Systolic blood pressure |
| VT | Ventricular tachycardia |
| UNL | Upper normal limit |
| URL | Upper reference limit |
| WSS | Wall shear stress |
Appendix A
Appendix A.1. Comparison of Clinical Characteristics Between Male and Female Patients
| Parameter | Male (n = 44) | Female (n = 76) | p-Value |
|---|---|---|---|
| Age (years) | 60.5 (51–66) | 54 (47–69) | 0.336 |
| Symptoms: | |||
| Palpitations | 40 (90.9%) | 74 (97.4%) | 0.118 |
| Chest pain | 6 (13.6%) | 10 (13.2%) | 0.941 |
| Syncope/presyncope | 3 (6.8%) | 4 (5.3%) | 0.726 |
| Gastrointestinal disturbance | 3 (6.8%) | 0 (0%) | 0.021 |
| History and medication: | |||
| History of SVT * | 23 (52.3%) | 44 (57.9%) | 0.550 |
| Diabetes mellitus | 9 (20.5%) | 5 (6.6%) | 0.023 |
| Hypertension | 17 (38.6%) | 20 (26.3%) | 0.159 |
| Known coronary artery disease | 4 (9.1%) | 5 (6.6%) | 0.615 |
| Thyroid disorders | 7 (15.9%) | 17 (22.4%) | 0.394 |
| Dyslipidemia | 23 (52.3%) | 29 (38.2%) | 0.133 |
| Autoimmune disease | 1 (2.3%) | 6 (7.9%) | 0.205 |
| Antiarrhythmics (1 or more) | |||
| B-blockers | 10 (22.7%) | 18 (23.7%) | 0.905 |
| Calcium channel blockers | 1 (2.3%) | 1 (1.3%) | 0.693 |
| Class IC | 3 (6.8%) | 8 (10.5%) | 0.498 |
| Amiodarone | 1 (2.3%) | 2 (2.6%) | 0.903 |
| Duration of tachycardia and vital signs: | |||
| Duration of tachycardia (hours) | 2 (1–6) | 3 (1–6) | 0.078 |
| Admission heart rate (bpm #) | 160 (150–180) | 160 (141–180) | 0.171 |
| Admission systolic blood pressure (mmHg) | 130 (115–140) | 130 (120–150) | 0.599 |
| Admission laboratory parameters: | |||
| High-sensitivity cardiac troponin I (multiples above or below for UNL +) | 0.7 (0.15–3.11) | 0.99 (0.25–4.64) | 0.575 |
| Creatinine (mg/dL) | 1.05 (0.9–1.28) | 0.8 (0.7–0.95) | <0.001 |
| Urea (mg/dL) | 38 (30–47.5) | 34 (27–43) | 0.062 |
| Hospital admission | 18 (40.9%) | 33 (43.4%) | 0.789 |
| CAD ++ evaluation | 8 (18.2%) | 17 (22.3%) | 0.658 |
Appendix A.2. Analysis of Potential Predictors of SVT Recurrence
| Parameter | SVT *-Recurrence (+) (n = 28) | SVT *-Recurrence (−) (n = 90) | p-Value |
|---|---|---|---|
| Age (years) | 59 ± 15 | 56 ± 13 | 0.217 |
| Male sex | 9 (32.1%) | 35 (38.8%) | 0.570 |
| Symptoms: | |||
| Palpitations | 26 (92.8%) | 86 (95.5%) | 0.692 |
| Chest pain | 4 (14.3%) | 12 (13.3%) | 0.866 |
| Syncope/presyncope | 1 (3.6%) | 6 (6.7%) | 0.560 |
| Gastrointestinal disturbance | 1 (3.6%) | 2 (2.2%) | 0.678 |
| History and medication | |||
| History of SVT * | 17 (60.7%) | 50 (55.5%) | 0.553 |
| Diabetes mellitus | 3 (10.7%) | 11 (12.2%) | 0.858 |
| Hypertension | 10 (35.7%) | 27 (30%) | 0.523 |
| Known coronary artery disease | 1 (3.6%) | 8 (8.8%) | 0.367 |
| Thyroid disorders | 3 (10.7%) | 21 (23.3%) | 0.161 |
| Dyslipidemia | 13 (46.4%) | 39 (43.3%) | 0.706 |
| Autoimmune disease | 0 (0%) | 7 (7.7%) | 0.133 |
| Antiarrhythmics on admission (1 or more): | |||
| B-blockers | 6 (21.4%) | 22 (24.4%) | 0.785 |
| Calcium channel blockers | 0 (0%) | 2 (2.2%) | 0.431 |
| Class IC | 4 (14.3%) | 7 (7.7%) | 0.284 |
| Amiodarone | 0 (0%) | 3 (3.3%) | 0.333 |
| Duration of tachycardia and vital signs: | |||
| Duration of tachycardia (hours) | 3 (1–6) | 2 (1–6) | 0.531 |
| Admission heart rate (bpm #) | 150 (142–80) | 160 (150–180) | 0.361 |
| Admission systolic blood pressure (mmHg) | 130 (120–150) | 130 (119–140) | 0.524 |
| Hs-cTn-I @ (multiples above or below for UNL +) | 0.85 (0.14–2.34) | 0.94 (0.24–5.8) | 0.322 |
References
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.-P.; et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 655–720. [Google Scholar] [CrossRef]
- Page, R.L.; Joglar, J.A.; Caldwell, M.A.; Calkins, H.; Conti, J.B.; Deal, B.J.; Estes, N.A.M.; Field, M.E.; Goldberger, Z.D.; Hammill, S.C.; et al. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia. Circulation 2016, 133, e27–e115. [Google Scholar] [CrossRef]
- Murer, M.; Cuculi, F.; Toggweiler, S.; Weberndoerfer, V.; Young, M.; Kobza, R. Elevated high-sensitivity troponin does not indicate the presence of coronary artery disease in patients presenting with supraventricular tachycardia. Cardiol. J. 2017, 24, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Cucurachi, R.; Lamendola, P.; Candelli, M.; Pignataro, G.; Del Bono, G.; Franceschi, F. Troponin Testing in Adult Patients Presenting to the Emergency Department for Paroxysmal Supraventricular Tachycardia: A Review. Cardiol. Rev. 2023, 31, 265. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, A.; Checkeye, H.; Varghese, B.; Solomon, A.J.; Tran, Q.K. The Role of Troponin Testing in Patients with Supraventricular Tachycardia, Systematic Review and Meta-Analysis. J. Emerg. Med. 2024, 67, e402–e413. [Google Scholar] [CrossRef] [PubMed]
- 2023 ESC Guidelines for the Management of Acute Coronary Syndromes|European Heart Journal|Oxford Academic n.d. Available online: https://academic.oup.com/eurheartj/article/44/38/3720/7243210?login=true (accessed on 7 August 2024).
- Cardiac Troponin-an Overview|ScienceDirect Topics n.d. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/cardiac-troponin (accessed on 7 August 2024).
- Wu, A.H.B. Release of cardiac troponin from healthy and damaged myocardium. Front. Lab. Med. 2017, 1, 144–150. [Google Scholar] [CrossRef]
- Janssen, S.L.; Berge, K.; Luiken, T.; Aengevaeren, V.L.; Eijsvogels, T.M. Cardiac troponin release in athletes: What do we know and where should we go? Curr. Opin. Physiol. 2023, 31, 100629. [Google Scholar] [CrossRef]
- Hickman, P.E.; Potter, J.M.; Aroney, C.; Koerbin, G.; Southcott, E.; Wu, A.H.; Roberts, M.S. Cardiac troponin may be released by ischemia alone, without necrosis. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 318–323. [Google Scholar] [CrossRef]
- Aw, T.C.; van Wijk, X.M.R.; Wu, A.H.B.; Jaffe, A.S. Release of cardiac troponin using a high sensitivity assay after exercise: Type 2 acute myocardial infarction? Clin. Chim. Acta 2015, 44, 6–8. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Xue, F.; Jiang, T.B.; Jiang, B.; Cheng, X.J.; He, Y.M.; Li, X.; Yang, X.J. Cardiac troponin I elevation with supraventricular tachycardia: Two case reports and review of the literature. BMC Res. Notes 2014, 7, 136. [Google Scholar] [CrossRef][Green Version]
- Bukkapatnam, R.N.; Robinson, M.; Turnipseed, S.; Tancredi, D.; Amsterdam, E.; Srivatsa, U.N. Relationship of myocardial ischemia and injury to coronary artery disease in patients with supraventricular tachycardia. Am. J. Cardiol. 2010, 106, 374–377. [Google Scholar] [CrossRef]
- Yedder, N.B.; Roux, J.F.; Paredes, F.A. Troponin Elevation in Supraventricular Tachycardia: Primary Dependence on Heart Rate. Can. J. Cardiol. 2011, 27, 105–109. [Google Scholar] [CrossRef]
- Sayadnik, M.; Shafiee, A.; Jenab, Y.; Jalali, A.; Sadeghian, S. Predictors of High-Sensitivity Cardiac Troponin T Elevation in Patients with Acute Paroxysmal Supraventricular Tachycardia and Ischemic Heart Disease. Tex. Heart Inst. J. 2017, 44, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Matthew, M.W.M.; Kim, J.H.; Shah, A.B.; Phelan, D.; Emery, M.S.; Wasfy, M.M.; Fernandez, A.B.; Bunch, T.J.; Dean, P.; Danielian, A.; et al. Exercise-Induced Cardiovascular Adaptations and Approach to Exercise and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1453–1470. [Google Scholar] [CrossRef]
- Moulton, K.P.; Bhutta, B.S.; Mullin, J.C. Evaluation of Suspected Cardiac Arrhythmia; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Fortescue, E.B.; Shin, A.Y.; Greenes, D.S.; Mannix, R.C.; Agarwal, S.; Feldman, B.J.; Shah, M.I.; Rifai, N.; Newburger, J.W.; Almond, C.S.D.; et al. Cardiac troponin increases among runners in the Boston Marathon. Ann. Emerg. Med. 2007, 49, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mingels, A.; Jacobs, L.; Michielsen, E.; Swaanenburg, J.; Wodzig, W.; van Dieijen-Visser, M. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin. Chem. 2009, 55, 101–108. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Vancheri, E.; Henein, M.Y. Mental Stress and Cardiovascular Health—Part I. J. Clin. Med. 2022, 11, 3353. [Google Scholar] [CrossRef]
- Mehta, P.K.; Hermel, M.; Nelson, M.D.; Cook-Wiens, G.; Martin, E.A.; Alkhoder, A.A.; Wei, J.; Minissian, M.; Shufelt, C.L.; Marpuri, S.; et al. Mental stress peripheral vascular reactivity is elevated in women with coronary vascular dysfunction: Results from the NHLBI-sponsored Cardiac Autonomic Nervous System (CANS) study. Int. J. Cardiol. 2018, 251, 8–13. [Google Scholar] [CrossRef]
- Lyon, A.R.; Citro, R.; Schneider, B.; Morel, O.; Ghadri, J.R.; Templin, C.; Omerovic, E. Pathophysiology of Takotsubo Syndrome. J. Am. Coll. Cardiol. 2021, 77, 902–921. [Google Scholar] [CrossRef]
- Al Houri, H.N.; Jomaa, S.; Jabra, M.; Alhouri, A.N.; Latifeh, Y. Pathophysiology of stress cardiomyopathy: A comprehensive literature review. Ann. Med. Surg. 2022, 82, 104671. [Google Scholar] [CrossRef]
- Khan, O.; Patel, M.; Tomdio, A.N.; Beall, J.; Jovin, I.S. Beta-Blockers in the Prevention and Treatment of Ischemic Heart Disease: Evidence and Clinical Practice. Heart Views Off. J. Gulf Heart Assoc. 2023, 24, 41–49. [Google Scholar] [CrossRef]
- Enbergs, A.; Bürger, R.; Reinecke, H.; Borggrefe, M.; Breithardt, G.; Kerber, S. Prevalence of coronary artery disease in a general population without suspicion of coronary artery disease: Angiographic analysis of subjects aged 40 to 70 years referred for catheter ablation therapy. Eur. Heart J. 2000, 21, 45–52. [Google Scholar] [CrossRef]
- Noorvash, D.; Ramos, R.; Hatch, L.; Muck, A.; Olson, A.S. Assessment of the Utility of Ordering a Troponin in Low- and Intermediate-Risk Patients Presenting to the Emergency Department with Supraventricular Tachycardia: A Retrospective Chart Review. J. Emerg. Med. 2018, 55, 1–6. [Google Scholar] [CrossRef]
- Chen, J.-L.; Hsiao, C.-H.; Yen, C.-C. Prognostic value of cardiac troponin in elderly patients with paroxysmal supraventricular tachycardia: A multicenter study. Am. J. Emerg. Med. 2023, 69, 167–172. [Google Scholar] [CrossRef]
- Bandorski, D.; Höltgen, R.; Wieczorek, M.; Ghofrani, H.A.; Bogossian, H.; Iliodromitis, K. Evaluation of troponin I serum levels in patients with arrhythmias with and without coronary artery disease. Med. Klin. Intensiv. Notfallmedizin 2024, 119, 39–45. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Leutner, M.; Harreiter, J. Sex differences in type 2 diabetes. Diabetologia 2023, 66, 986–1002. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 120) | Elevated Troponin (n = 58) | Normal Troponin (n = 62) | p-Value |
|---|---|---|---|---|
| Demographics: | ||||
| Male sex | 44 (36.7%) | 20 (34.5%) | 24 (38.7%) | 0.631 |
| Age (years) | 57 ± 14 | 56 ± 12 | 57 ± 16 | 0.796 |
| Symptoms: | ||||
| Palpitations | 114 (95%) | 54 (93.1%) | 60 (96.8%) | 0.357 |
| Chest pain | 16 (13.3%) | 13 (22.4%) | 3 (4.8%) | 0.005 |
| Syncope/presyncope | 7 (5.8%) | 5 (8.6%) | 2 (3.2%) | 0.208 |
| Gastrointestinal tract disturbance | 3 (2.5%) | 2 (3.4%) | 1 (1.6%) | 0.520 |
| History and medication: | ||||
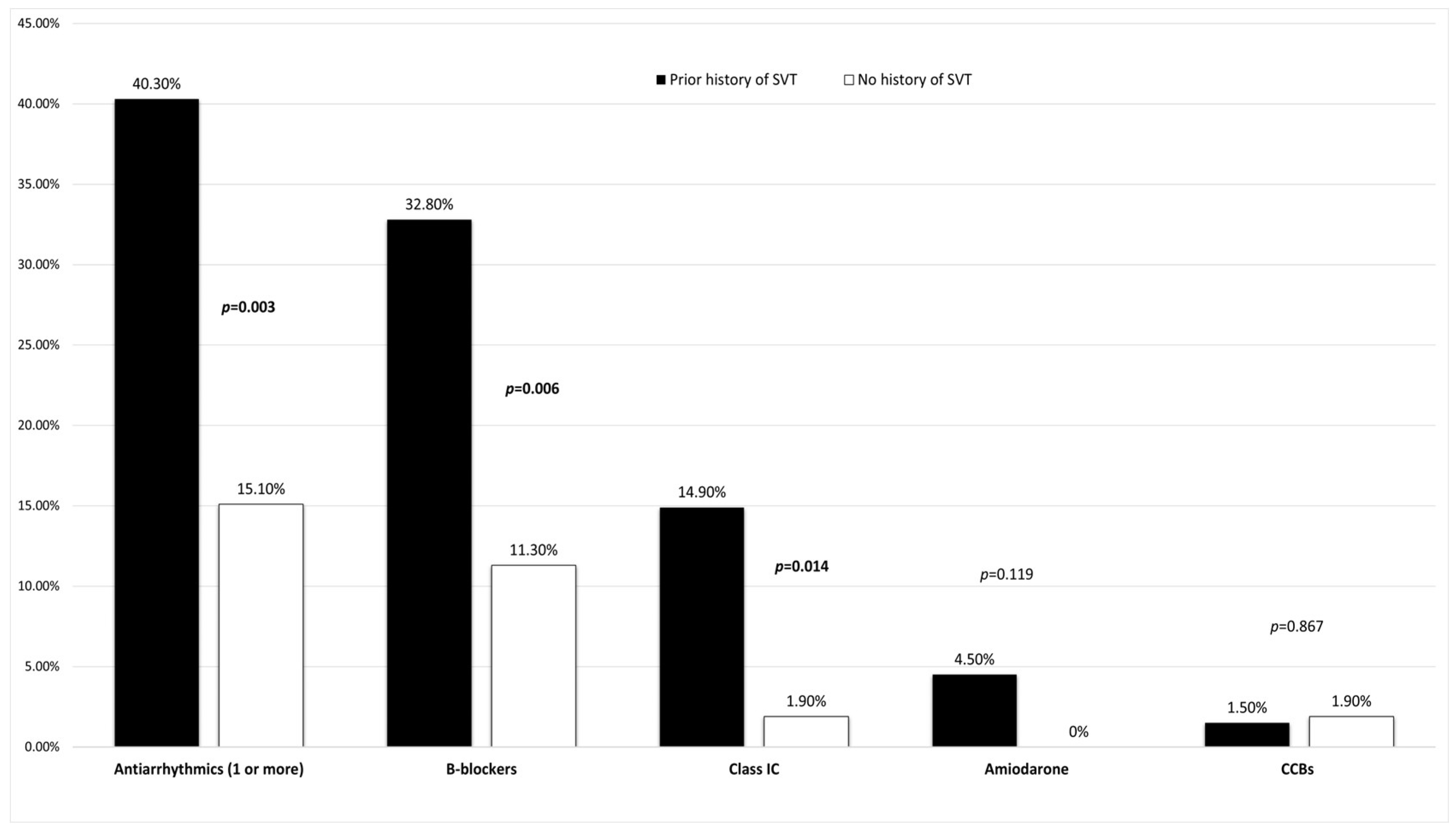
| History of SVT * | 67 (55.8%) | 26 (44.8%) | 41 (66.1%) | 0.019 |
| Diabetes mellitus | 14 (11.7%) | 7 (12.1%) | 7 (11.3%) | 0.894 |
| Hypertension | 37 (30.8%) | 15 (25.9%) | 22 (35.5%) | 0.254 |
| Known coronary artery disease | 9 (7.5%) | 5 (8.6%) | 4 (6.5%) | 0.652 |
| Thyroid disorders | 24 (20%) | 14 (24.1%) | 10 (16.1%) | 0.273 |
| Dyslipidemia | 52 (43.3%) | 28 (48.3%) | 24 (38.7%) | 0.291 |
| Autoimmune disease | 7 (5.8%) | 4 (6.9%) | 3 (4.8%) | 0.631 |
| Antiarrhythmics (1 or more) | 35 (29.2%) | 16 (27.6%) | 19 (30.6%) | 0.713 |
| B-blockers | 28 (23.3%) | 13 (22.4%) | 15 (24.2%) | 0.818 |
| Calcium channel blockers | 2 (1.7%) | 2 (3.4%) | 0 (0%) | 0.140 |
| Class IC | 11 (9.2%) | 2 (3.4%) | 9 (14.5%) | 0.056 |
| Amiodarone | 3 (2.5%) | 1 (1.7%) | 2 (3.2%) | 0.599 |
| Duration of tachycardia and vital signs: | ||||
| Duration of tachycardia (hours) | 2 (1–6) | 3 (1–6) | 2 (1–4.25) | 0.195 |
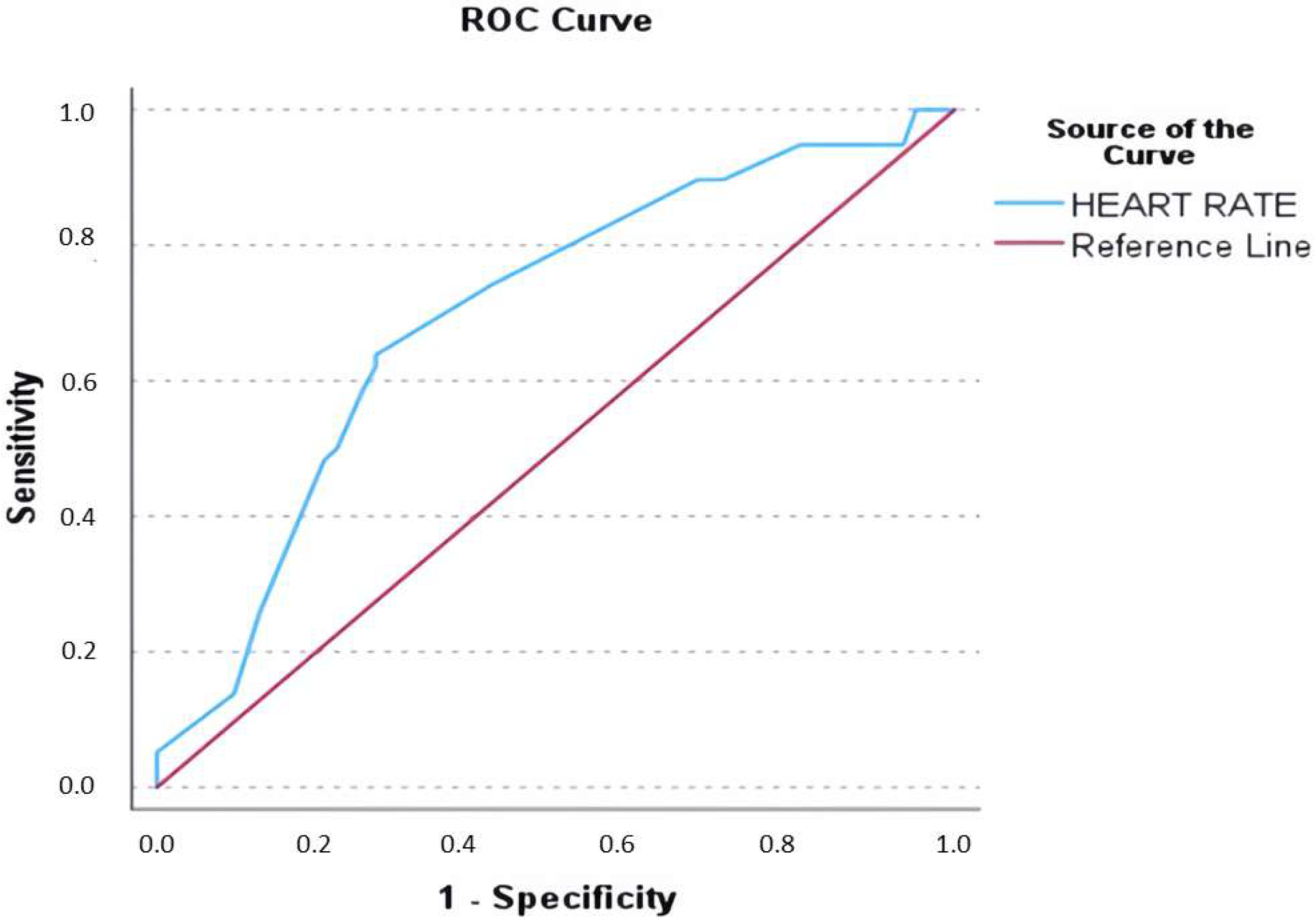
| Admission heart rate (beats per minute) | 160 (150–180) | 172.5 (150–190) | 150 (140–170) | <0.001 |
| Admission systolic blood pressure (mmHg) | 130 ± 20 | 126 ± 16 | 136 ± 22 | 0.007 |
| Cardioversion type: | ||||
| Vagal maneuvers | 13 (10.8%) | 8 (13.8%) | 5 (8.1%) | 0.313 |
| Intravenous adenosine | 83 (69.2%) | 44 (75.9%) | 39 (62.9%) | 0.125 |
| B-blockers/class IC | 8 (6.7%) | 2 (3.4%) | 6 (9.7%) | 0.172 |
| Spontaneous | 10 (8.3%) | 3 (5.2%) | 7 (11.3%) | 0.226 |
| Electrical | 4 (3.3%) | 1 (1.7%) | 3 (4.8%) | 0.342 |
| Calcium channel blockers | 2 (1.7%) | 0 (0%) | 2 (3.2%) | 0.168 |
| Admission laboratory parameters: | ||||
| High-sensitivity cardiac troponin I (multiples above or below for UNL +) | 0.88 (0.23–4.11) | 4.28 (2.15–13.33) | 0.24 (0.1–0.48) | <0.001 |
| Creatinine (mg/dL) | 0.9 (0.78–1.06) | 0.92 (0.79–1.08) | 0.88 (0.76–1.06) | 0.390 |
| Urea (mg/dL) | 35 (28–45) | 35 (27–46) | 35 (28.5–43.5) | 0.838 |
| In-hospital admission | 51 (42.5%) | 41 (70.7%) | 10 (16.1%) | <0.001 |
| Outcomes within a year (n = 118): | ||||
| SVT recurrence | 28 (23.7%) | 12 (21%) | 16 (26.2%) | 0.508 |
| Rehospitalization (any cause) | 13 (11%) | 5 (8.7%) | 8 (13.1%) | 0.451 |
| Ablation | 24 (20.3%) | 14 (24.5%) | 10 (16.3%) | 0.273 |
| Death | 1 (0.8%) | 0 (0%) | 1 (1.6%) | 0.336 |
| Parameters | Antiarrhythmics (+) [n = 35] | Antiarrhythmics (−) [n = 85] | p-Value |
|---|---|---|---|
| Heart rate (beats per minute) | 150 (135–180) | 165 (150–180) | 0.011 |
| Retrosternal chest pain | 5 (14.3%) | 11 (12.9%) | 0.844 |
| History of SVT * | 27 (77.1%) | 40 (47.1%) | 0.003 |
| Systolic BP + (mmHg) | 130 (110–141) | 130 (120–145) | 0.924 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Parameters | +OR | +95% CI | p | +OR | +95% CI | p |
| Retrosternal chest pain | 5.681 | 1.527–21.140 | 0.01 | 4.761 | 1.123–20.190 | 0.034 |
| History of SVT | 0.416 | 0.199–0.870 | 0.020 | 0.431 | 0.187–0.994 | 0.048 |
| Heart rate (beats per minute) | 1.030 | 1.013–1.048 | 0.001 | 1.030 | 1.012–1.049 | 0.001 |
| Systolic blood pressure (mmHg) | 0.974 | 0.955–0.994 | 0.01 | 0.977 | 0.956–0.999 | 0.042 |
| Outcome: | CAD + Evaluation (+) (n = 25) | CAD + Evaluation (−) (n = 32) | p-Value |
|---|---|---|---|
| SVT * recurrence | 4 (16%) | 8 (25%) | 0.303 |
| Rehospitalization (all-cause) | 3 (12%) | 2 (6.3%) | 0.528 |
| Ablation | 6 (24%) | 8 (25%) | 0.750 |
| Death | 0 (0%) | 0 (0%) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aletras, G.; Koutalas, E.; Bachlitzanaki, M.; Stratinaki, M.; Bachlitzanaki, I.; Stavratis, S.; Garidas, G.; Pitarokoilis, M.; Foukarakis, E. Paroxysmal Supraventricular Tachycardia and Troponin Elevation: Insights into Mechanisms, Risk Factors, and Outcomes. J. Clin. Med. 2025, 14, 5644. https://doi.org/10.3390/jcm14165644
Aletras G, Koutalas E, Bachlitzanaki M, Stratinaki M, Bachlitzanaki I, Stavratis S, Garidas G, Pitarokoilis M, Foukarakis E. Paroxysmal Supraventricular Tachycardia and Troponin Elevation: Insights into Mechanisms, Risk Factors, and Outcomes. Journal of Clinical Medicine. 2025; 14(16):5644. https://doi.org/10.3390/jcm14165644
Chicago/Turabian StyleAletras, Georgios, Emmanuel Koutalas, Maria Bachlitzanaki, Maria Stratinaki, Irene Bachlitzanaki, Spyridon Stavratis, Gerasimos Garidas, Michael Pitarokoilis, and Emmanuel Foukarakis. 2025. "Paroxysmal Supraventricular Tachycardia and Troponin Elevation: Insights into Mechanisms, Risk Factors, and Outcomes" Journal of Clinical Medicine 14, no. 16: 5644. https://doi.org/10.3390/jcm14165644
APA StyleAletras, G., Koutalas, E., Bachlitzanaki, M., Stratinaki, M., Bachlitzanaki, I., Stavratis, S., Garidas, G., Pitarokoilis, M., & Foukarakis, E. (2025). Paroxysmal Supraventricular Tachycardia and Troponin Elevation: Insights into Mechanisms, Risk Factors, and Outcomes. Journal of Clinical Medicine, 14(16), 5644. https://doi.org/10.3390/jcm14165644







