Proximal vs. Distal LAD Lesions in ST-Elevation Myocardial Infarction: Insights from ECG and Coronary Angiography
Abstract
1. Introduction
2. Materials and Methods
- Chest pain over 30 min;
- Symptomatology debut of maximum 12 h before hospital presentation;
- A 4 mm or higher summed ST-elevation on the ECG, measured from the J point (over 2 mm in at least two leads from DI, aVL, V1–V6 or at least 1 mm elevation in all 4 leads DII, DIII, V5–V6 or over 2 mm in at least 2 leads DII, DIII, V5–V6).
- Fibrinolysis contraindication;
- Sepsis;
- Aortic aneurysm with mobile thrombus;
- Absence of femoral pulse or bilateral femoral vascular graft;
- Coronary artery bypass;
- Severe acute renal failure (creatinine over 250 µmol/L);
- Diabetics under metformin treatment in the last 48 h;
- Over 3 h interval from presentation to catheterization;
- Acute coronary syndrome (ACS) (with Q wave or not) in the last 30 days;
- High risk during ambulance transportation (cardiogenic shock or heart failure with severe hypotension–systolic arterial pressure over 65 mmHg);
- Severe cardiac arrhythmias;
- The existence of left bundle branch block (LBBB) or left ventricular hypertrophy (LVH) on the ECG;
- The existence of right bundle branch block (RBBB) with left anterior fascicular block (LAFB) on the ECG;
- Cardiac pacemaker.
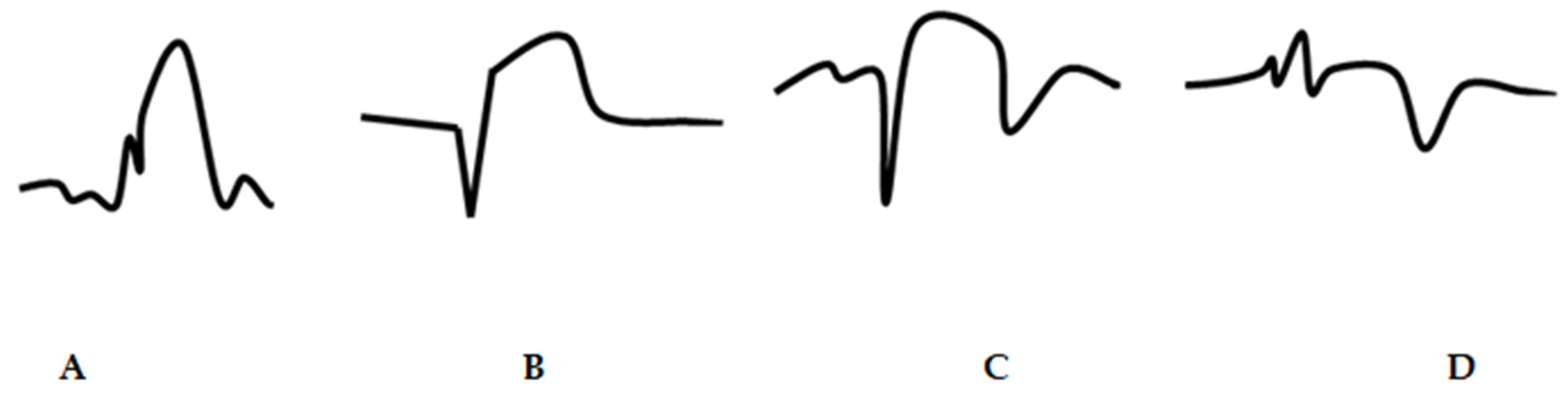
- ST-segment and T wave measurement method
- Q wave
- Any Q wave duration ≥ 30 ms in leads V1–V3;
- Q wave height ≥ 1 mm and duration ≥ 30 ms in leads DI, DII, aVL, aVF, V4–V6 in ≥2 adjacent leads;
- R-wave duration over 40 ms and R/S ratio over 1 (in the absence of pre-excitation syndrome, right ventricular hypertrophy, or RBBB in leads V1–V2);
- Localization of myocardial infarction.
2.1. The Culprit Artery and Occlusion Position
- (1)
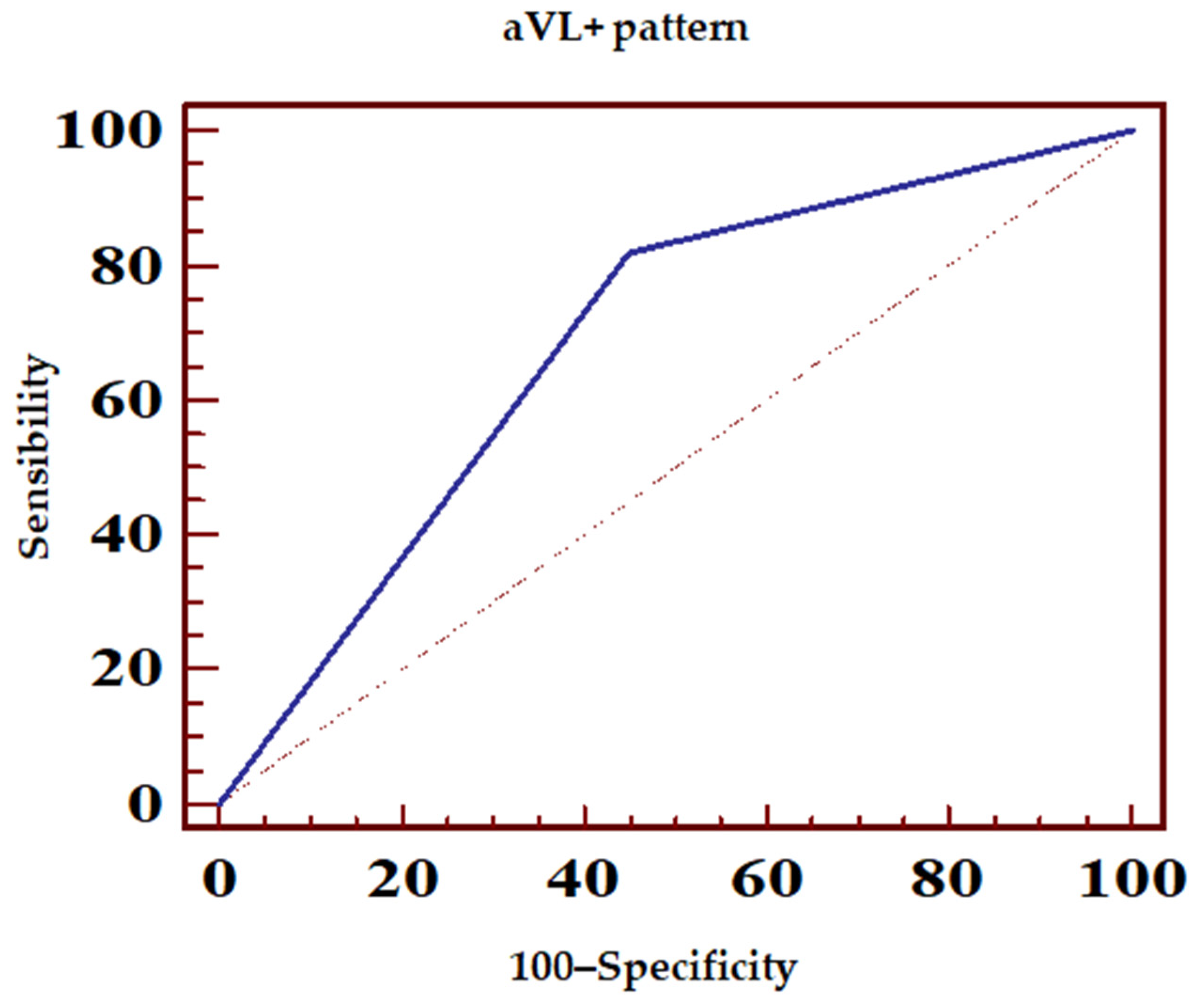
- Concomitant ST-segment elevation over 0.5 mm in lead aVL (aVL+ pattern) (Figure 1A);
- (2)
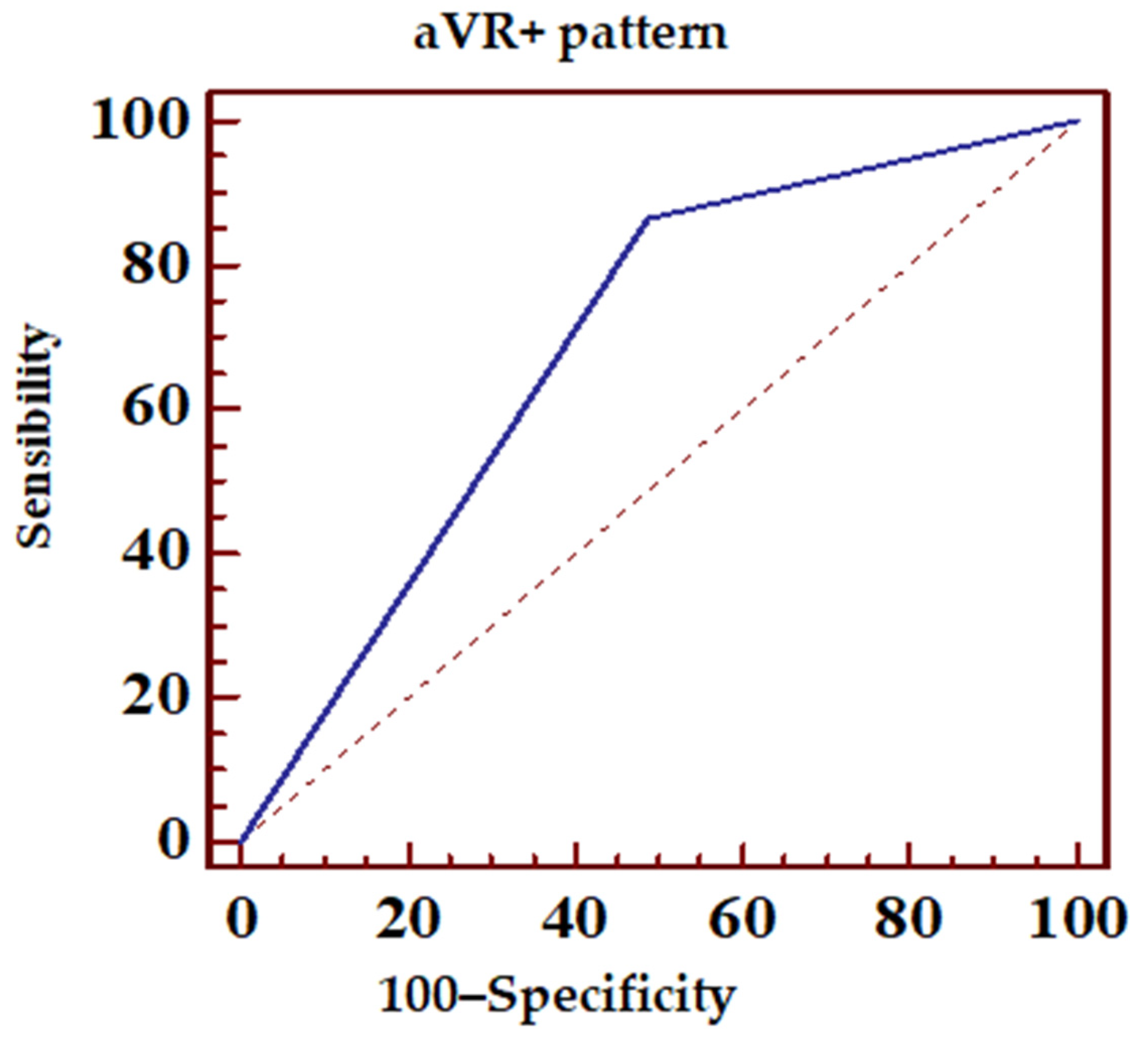
- ST-segment elevation over 0.5 mm in aVL or any ST-segment elevation in lead aVR (pattern + aVR) (Figure 1B).
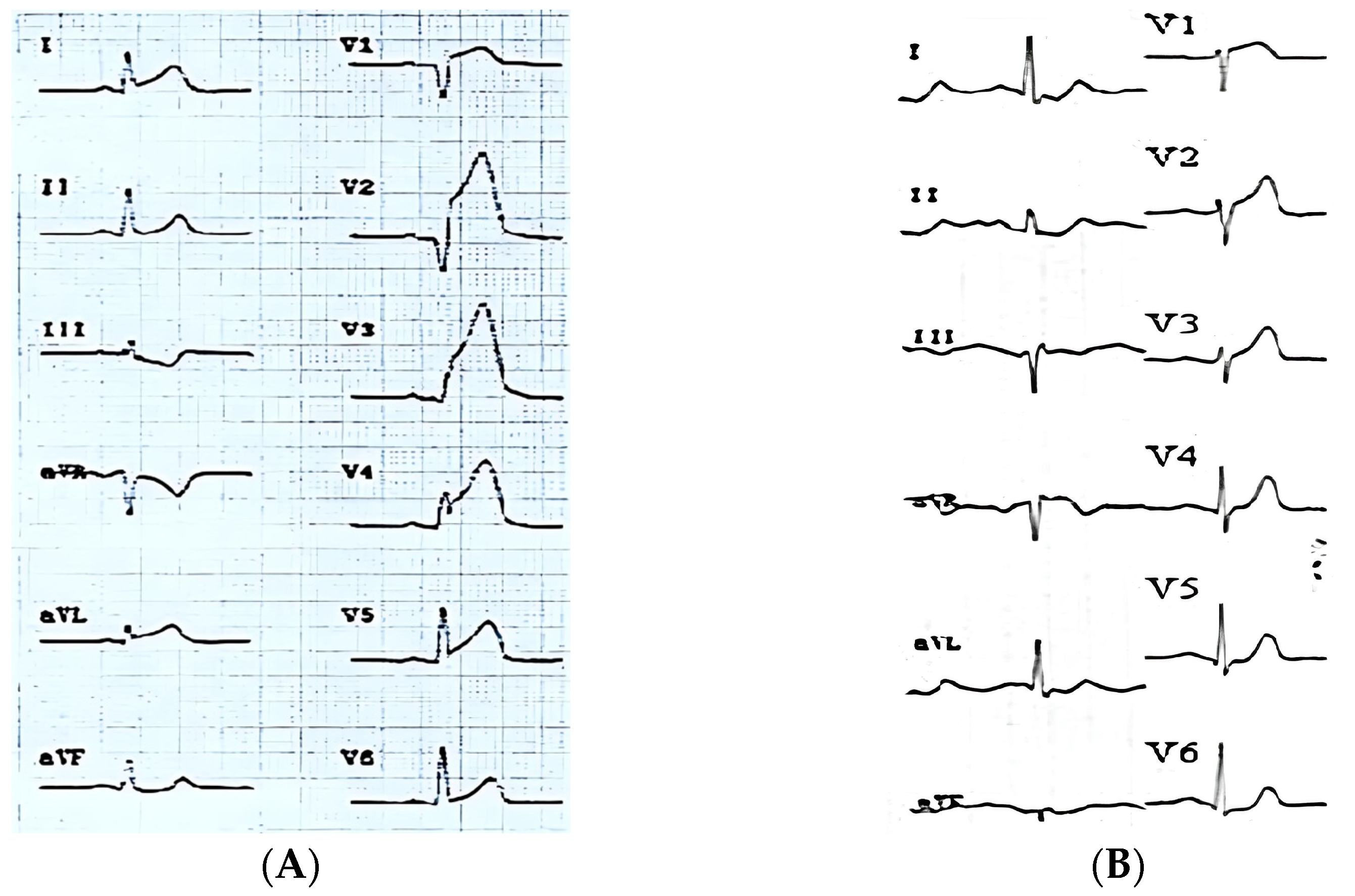
2.2. ECG Definitions of Evolving Myocardial Infarction and Clinically Established Myocardial Infarction
2.3. Coronary Angiography
2.4. Statistical Analysis
3. Results
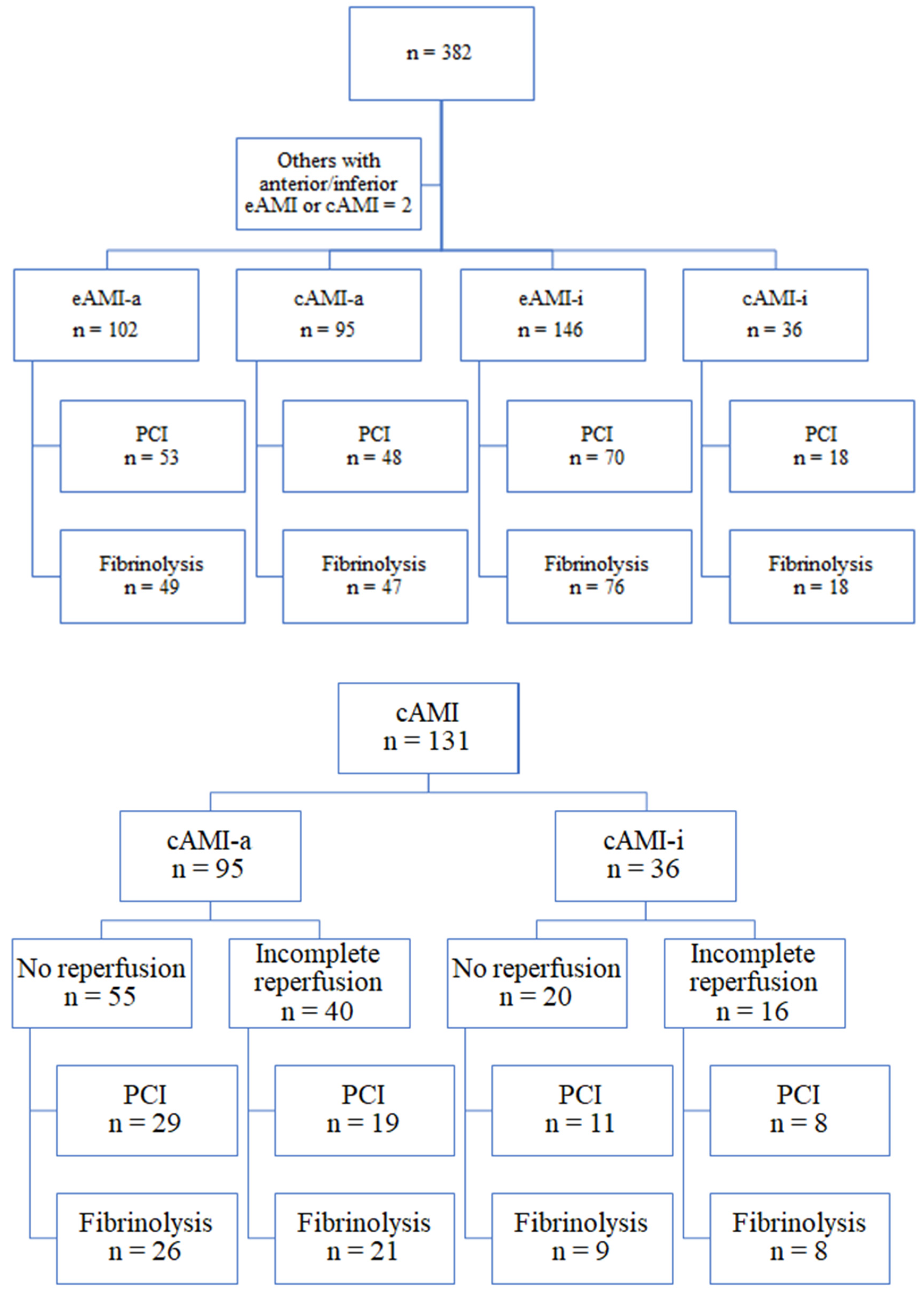
3.1. Coronary Angiography Correspondence
3.2. LAD Occlusion as Culprit Lesion
- ▪
- ▪
- ▪
3.3. Proximal LAD Occlusion—Effects on the Results
4. Discussions
4.1. LAD—The Culprit Artery in AMI
4.2. LAD Occlusion Location
- ST-elevation ≥ 0.5 mm;
- A smaller ST-segment elevation with T wave inversion;
- Isoelectric ST-segment symmetrically associated with T wave inversion and atypical Q wave when AMI was a consequence of obstruction proximal to the first diagonal branch.
4.3. Anticipating the Location of the Lesion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, N.; Maehara, A. Left anterior descending artery wrapping around the left ventricular apex predicts additional risk of future events after anterior myocardial infarction. Anatol. J. Cardiol. 2019, 21, 259–260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaspardone, C.; Romagnolo, D.; Fasolino, A.; Falasconi, G.; Beneduce, A.; Fiore, G.; Didelon, E.; Fortunato, F.; Galdieri, C.; Posteraro, G.A.; et al. A comprehensive and easy-to-use ECG algorithm to predict the coronary occlusion site in ST-segment elevation myocardial infarction. Am. Heart J. 2023, 255, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.S.; Macfarlane, P.; Wellens, H.; Josephson, M.; Gorgels, A.; Mirvis, D.M.; Pahlm, O.; Surawicz, B.; Kligfield, P.; Childers, R.; et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part VI: Acute ischemia/infarction: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: Endorsed by the International Society for Computerized Electrocardiology. Circulation 2009, 119, e262–e270. [Google Scholar] [CrossRef] [PubMed]
- Harhash, A.A.; Huang, J.J.; Reddy, S.; Natarajan, B.; Balakrishnan, M.; Shetty, R.; Hutchinson, M.D.; Kern, K.B. aVR ST Segment Elevation: Acute STEMI or Not? Incidence of an Acute Coronary Occlusion. Am. J. Med. 2019, 132, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, F.; Suryapranata, H.; de Boer, M.J. ST-segment elevation myocardial infarction: Historical perspective and new horizons. Neth. Heart J. 2020, 28, 93–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elsman, P.; van’t Hof, A.W.; Hoorntje, J.C.; de Boer, M.J.; Borm, G.F.; Suryapranata, H.; Ottervanger, J.P.; Gosselink, A.T.; Dambrink, J.H.; Zijlstra, F. Effect of coronary occlusion site on angiographic and clinical outcome in acute myocardial infarction patients treated with early coronary intervention. Am. J. Cardiol. 2006, 97, 1137–1141. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Tcheng, J.E.; Gersh, B.J.; Cox, D.A.; Stuckey, T.; Turco, M.; Mehran, R.; Garcia, E.; Zimetbaum, P.; McGlaughlin, M.G.; et al. Relation ship between infarct artery location, epicardial flow, and myocardial perfusion after primary percutaneous revascularization in acute myocardial infarction. Am. Heart J. 2006, 151, 1288–1295. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction. Eur. Heart J. 2018, 40, 237–269. [Google Scholar] [CrossRef]
- Kühl, J.T.; Berg, R.M. Utility of lead aVR for identifying the culprit lesion in acute myocardial infarction. Ann. Noninvasive Electrocardiol. 2009, 14, 219–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kireyev, D.; Arkhipov, M.V.; Zador, S.T.; Paris, J.A.; Boden, W.E. Clinical utility of aVR-The neglected electrocardiographic lead. Ann. Noninvasive Electrocardiol. 2010, 15, 175–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamura, A.; Kataoka, H.; Mikuriya, Y.; Nasu, M. Inferior ST segment depression as a useful marker for identifying proximal left anterior descending artery occlusion during acute anterior myocardial infarction. Eur. Heart J. 1995, 16, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; Nielsen, T.T.; Rasmussen, K.; Thuesen, L.; Kelbaek, H.; Thayssen, P.; Abildgaard, U.; Pedersen, F.; Madsen, J.K.; Grande, P.; et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N. Engl. J. Med. 2003, 349, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Zimarino, M.; Ausiello, A.; Contegiacomo, G.; Riccardi, I.; Renda, G.; Di Iorio, C.; De Caterina, R. Rapid decline of collateral circulation increases susceptibility to myocardial ischemia: The trade-off of successful percutaneous recanalization of chronic total occlusions. J. Am. Coll. Cardiol. 2006, 48, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; TamisHolland-, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. AHA/ACC Joint Committee on Clinical Practice Guidelines. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients With Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 151, e771–e862. [Google Scholar] [CrossRef]
- Brogan, R.A.; Malkin, C.J.; Batin, P.D.; Simms, A.D.; McLenachan, J.M.; Gale, C.P. Risk stratification for ST segment elevation myocardial infarction in the era of primary percutaneous coronary intervention. World J. Cardiol. 2014, 6, 865–873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Engelen, D.J.; Gorgels, A.P.; Cheriex, E.C.; De Muinck, E.D.; Ophuis, A.J.; Dassen, W.R.; Vainer, J.; van Ommen, V.G.; Wellens, H.J. Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J. Am. Coll. Cardiol. 1999, 34, 389–395. [Google Scholar] [CrossRef]
- Arbane, M.; Goy, J.J. Prediction of the site of total occlusion in the left anterior descending coronary artery using admission electrocardiogram in anterior wall acute myocardial infarction. Am. J. Cardiol. 2000, 85, 487–491. [Google Scholar] [CrossRef]
- Kim, T.Y.; Alturk, N.; Shaikh, N.; Kelen, G.; Salazar, M.; Grodman, R. An electrocardiographic algorithm for the prediction of the culprit lesion site in acute anterior myocardial infarction. Clin. Cardiol. 1999, 22, 77–83. [Google Scholar] [CrossRef]
- Koju, R.; Islam, N.; Rahman, A.; Mohsin, K.; Ali, A.; Islam, M.; Yadav, C. Electrocardiographic prediction of left anterior descending coronary artery occlusion site in acute anterior myocardial infarction. Nepal. Med. Coll. J. 2003, 5, 64–68. [Google Scholar]
- Birnbaum, Y.; Rankinen, J.; Jneid, H.; Atar, D.; Nikus, K. The Role of ECG in the Diagnosis and Risk Stratification of Acute Coronary Syndromes: An Old but Indispensable Tool. Curr. Cardiol. Rep. 2022, 24, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, M.; Kimura, K.; Ishikawa, T.; Endo, T.; Hongo, Y.; Shigemasa, T.; Iwasawa, Y.; Tochikubo, O.; Umemura, S. ST-segment depression in lead aVR predicts predischarge left ventricular dysfunction in patients with reperfused anterior acute myocardial infarction with anterolateral ST-segment elevation. Am. Heart J. 2001, 142, 51–57. [Google Scholar] [CrossRef]
- Sadanandan, S.; Hochman, J.S.; Kolodziej, A.; Criger, D.A.; Ross, A.; Selvester, R.; Wagner, G.S. Clinical and angiographic characteristics of patients with combined anterior and inferior ST-segment elevation on the initial electrocardiogram during acute myocardial infarction. Am. Heart J. 2003, 146, 653–661. [Google Scholar] [CrossRef]
- Sasaki, K.; Yotsukura, M.; Sakata, K.; Yoshino, H.; Ishikawa, K. Relation of ST-segment changes in inferior leads during anterior wall acute myocardial infarction to length and occlusion site of the left anterior descending coronary artery. Am. J. Cardiol. 2001, 87, 1340–1345. [Google Scholar] [CrossRef]
- Prasitlumkum, N.; Sirinvaravong, N.; Limpruttidham, N.; Rattanawong, P.; Tom, E.; Kanitsoraphan, C.; Chongsathidkiet, P.; Boondarikpornpant, T. Terminal QRS Distortion in ST Elevation Myocardial Infarction as a Prediction of Mortality: Systematic Review and Meta-Analysis. Acta Cardiol. Sin. 2019, 35, 445–458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nielsen, P.H.; Terkelsen, C.J.; Nielsen, T.T.; Thuesen, L.; Krusell, L.R.; Thayssen, P.; Kelbaek, H.; Abildgaard, U.; Villadsen, A.B.; Andersen, H.R.; et al. Danami-2 Investigators. System delay and timing of intervention in acute myocardial infarction (from the Danish Acute Myocardial Infarction-2 [DANAMI-2] trial). Am. J. Cardiol. 2011, 108, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Karha, J.; Murphy, S.A.; Kirtane, A.J.; de Lemos, J.A.; Aroesty, J.M.; Cannon, C.P.; Antman, E.M.; Braunwald, E.; Gibson, C.M.; TIMI Study Group. Evaluation of the association of proximal coronary culprit artery lesion location with clinical outcomes in acute myocardial infarction. Am. J. Cardiol. 2003, 92, 913–918. [Google Scholar] [CrossRef]


| Culprit Artery | eAMI-a n = 53 | cAMI-a n = 48 | eAMI-i n = 70 | cAMI-i n = 18 |
|---|---|---|---|---|
| LAD | 50 | 45 | 1 | 1 |
| LCX | 1 | - | 12 | 1 |
| RCA | - | 1 | 50 | 15 |
| Diagonal | 1 | 1 | 2 | - |
| Obtuse marginal | 1 | 1 | 4 | 1 |
| Left main | - | - | 1 | - |
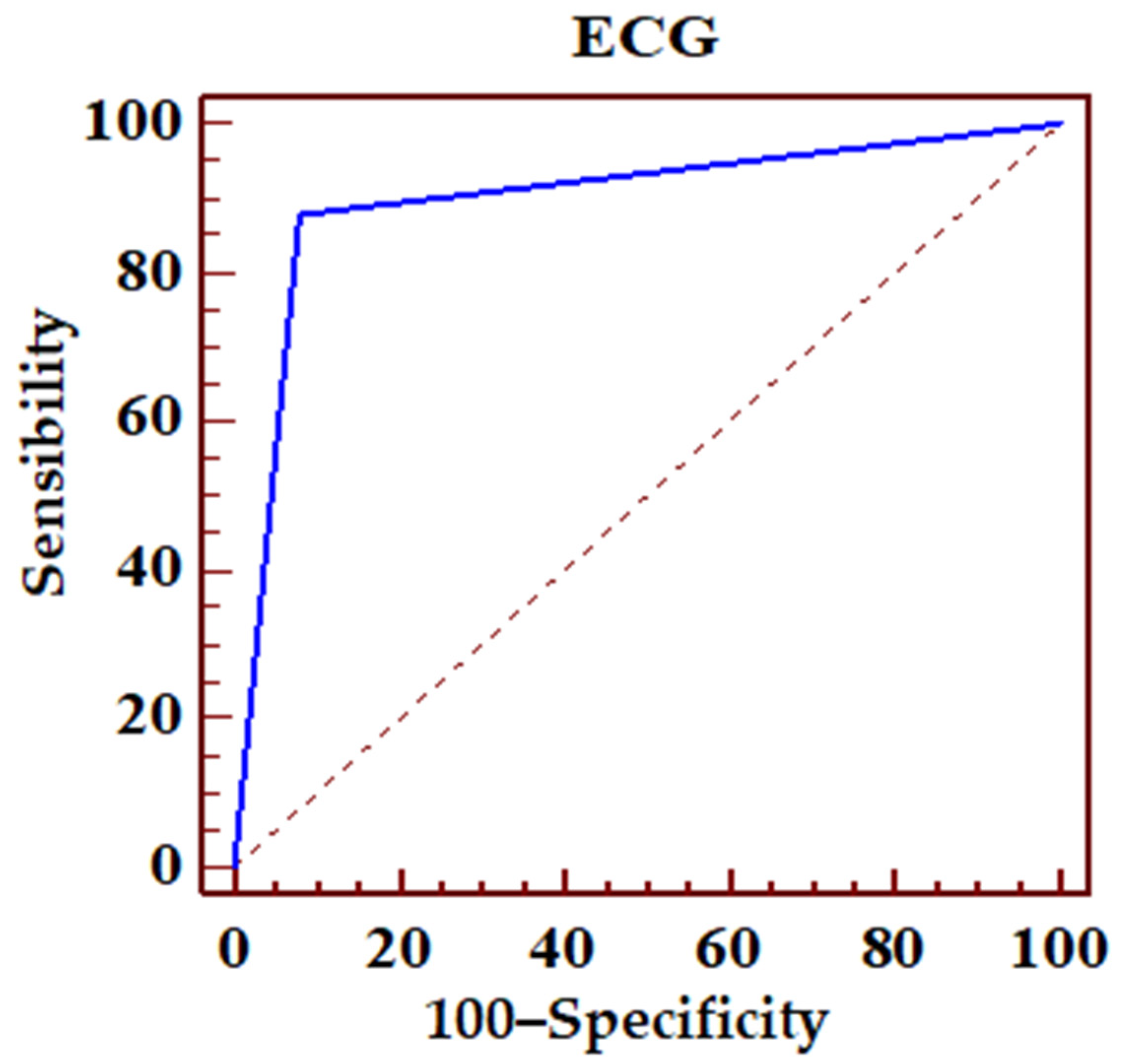
| ROC Area (AUC) | 0.899 | |||
| Standard error | 0.0239 | |||
| 95% confidence interval | 0.857–0.932 | |||
| z statistic s | 16.683 | |||
| P statistical significance (Area = 0.5) | 0.0001 | |||
| PPV | 95% CI | NPV | 95% CI | |
| 82.8 | 73.2–90.0 | 94.5 | 90.1–97.3 | |
| Sensibility | 95% CI | Specificity | 95% CI | |
| 87.80 | 78.7–94.0 | 91.98 | 87.1–95.4 | |
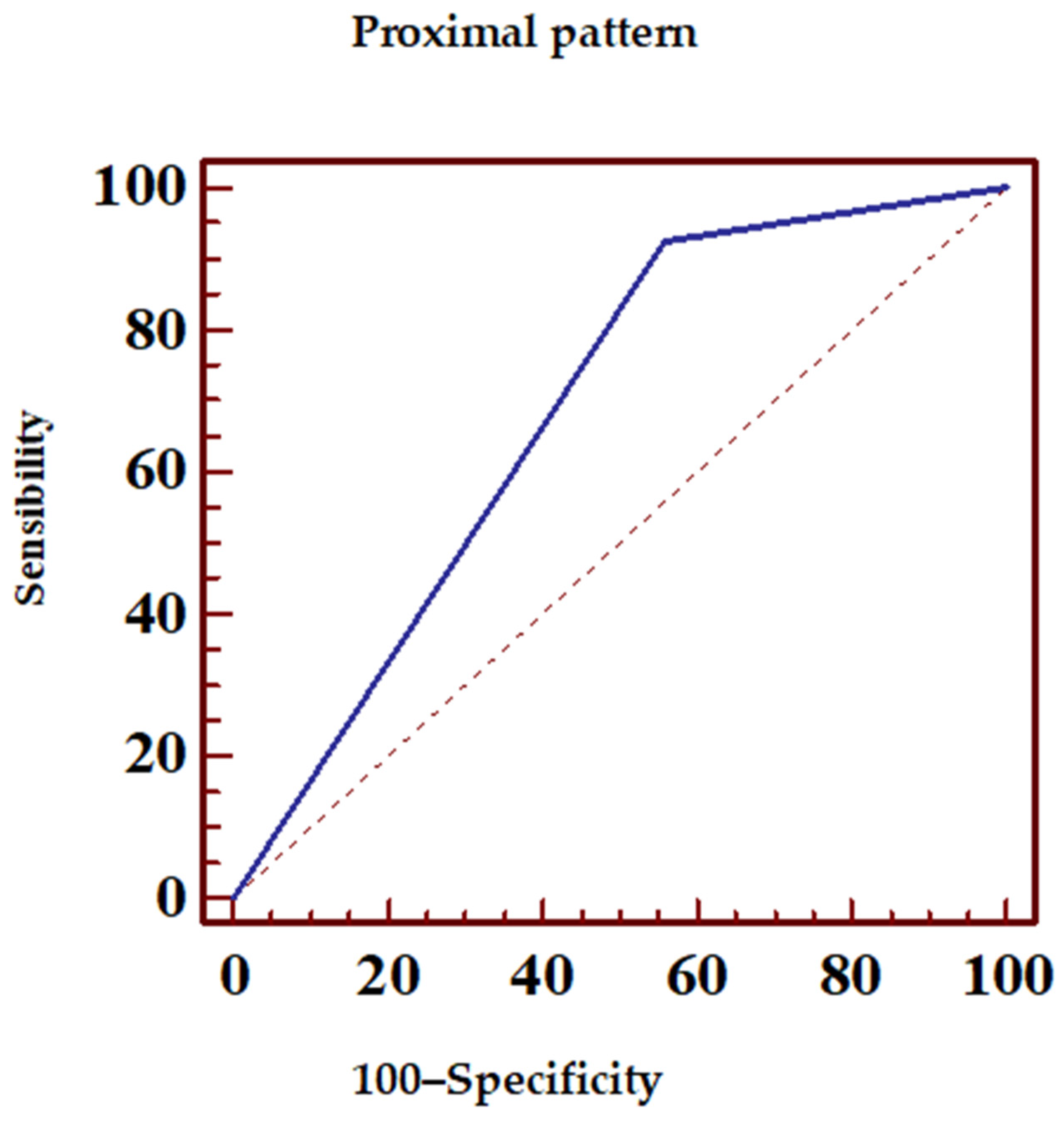
| ECG Pattern | Sensibility (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| aVL+ | 82.1 | 55.4 | 78 | 61.1 |
| aVR+ | 87 | 50 | 79.5 | 63.3 |
| Proximal | 92.5 | 44.1 | 83.8 | 65.2 |
| ROC Area (AUC) | 0.685 | |||
| Standard error | 0.0494 | |||
| 95% confidence interval | 0.593–0.768 | |||
| z statistics | 3.751 | |||
| p statistical significance (Area = 0.5) | 0.0002 | |||
| PPV | 95% CI | NPV | 95% CI | |
| 78 | 67.5–87.7 | 61.1 | 43.5–76.8 | |
| Sensibility | 95% CI | Specificity | 95% CI | |
| 82.05 | 71.8–89.8 | 55 | 38.5–70.7 | |
| ROC Area (AUC) | 0.689 | |||
| Standard error | 0.0498 | |||
| 95% confidence interval | 0.597–0.771 | |||
| z statistics | 3.793 | |||
| p statistical significance (Area = 0.5) | 0.0001 | |||
| PPV | 95% CI | NPV | 95% CI | |
| 79.5 | 69.6–87.4 | 63.3 | 43.9–80.0 | |
| Sensibility | 95% CI | Specificity | 95% CI | |
| 86.42 | 77.0–93.0 | 51.35 | 34.4–68.1 | |
| ROC Area (AUC) | 0.683 | |||
| Standard error | 0.0488 | |||
| 95% confidence interval | 0.599–0.758 | |||
| z statistics | 3.745 | |||
| p statistical significance (Area = 0.5) | 0.0002 | |||
| PPV | 95% CI | PPV | 95% CI | |
| 83.8 | 75.8–89.9 | 65.2 | 42.7–83.6 | |
| Sensibility | 95% CI | Specificity | 95% CI | |
| 85.7–96.7 | 44.12 | 27.2–62.1 | 85.7–96.7 | |
| Mean Time (min) | Standard Deviation | 95% CI | ||
|---|---|---|---|---|
| Time from symptoms onset to ECG recording (E time) | 141 | 52.76 | 136–147 | |
| Time from symptoms onset (E time) to PCI or fibrinolysis (PCI time/F time) | 215 | 58.42 | 208–220 | |
| Time from ECG recording (E time) to PCI or fibrinolysis (PCI time/F time) | 73 | 36.18 | 70–77 | |
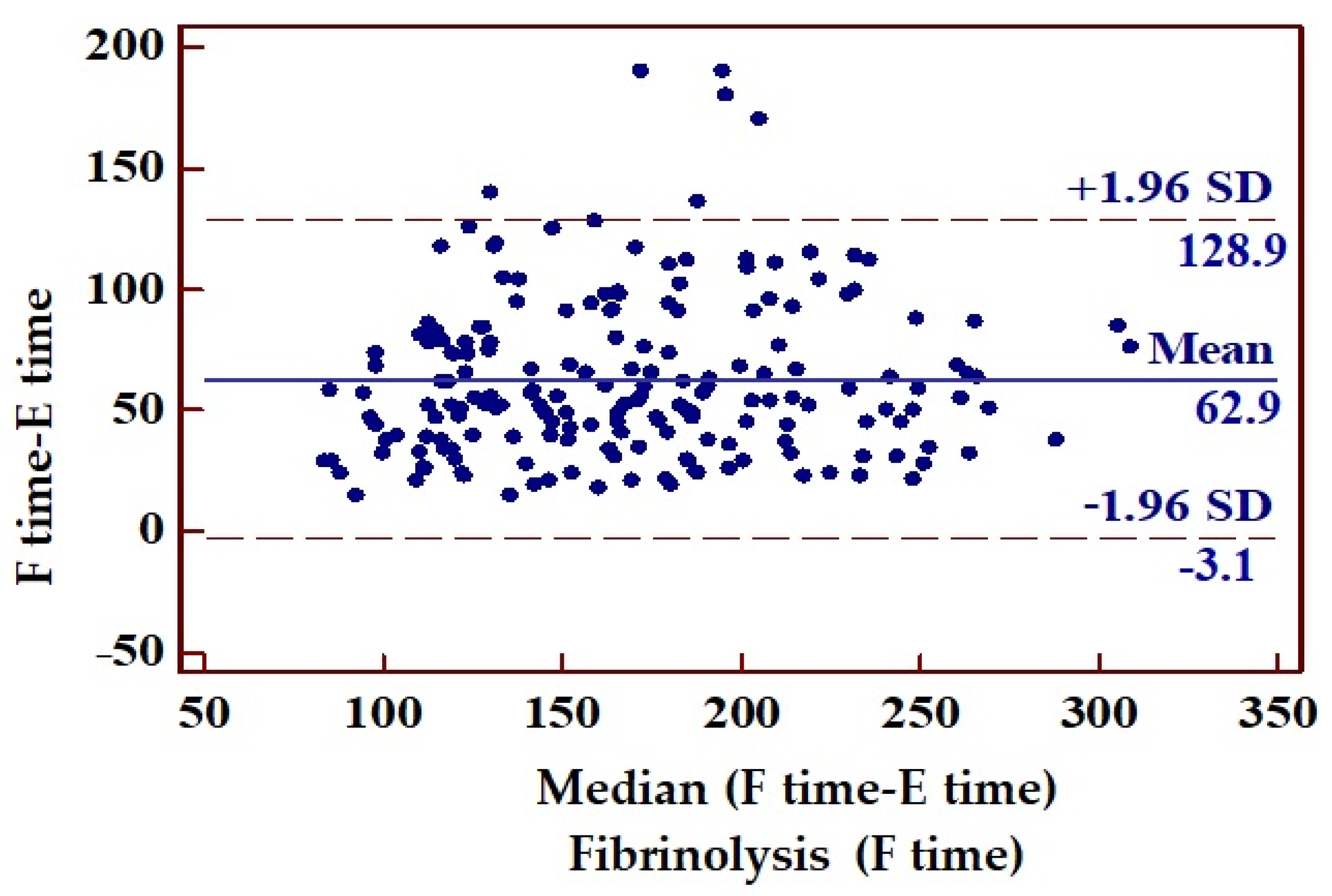
| Time from ECG recording (E time) to fibrinolysis (F time) | 63 | 33.66 | 58–67 | p < 0.0001 |
| Time from ECG recording (E time) to PCI (PCI time) | 84 | 35.63 | 78–89 | |
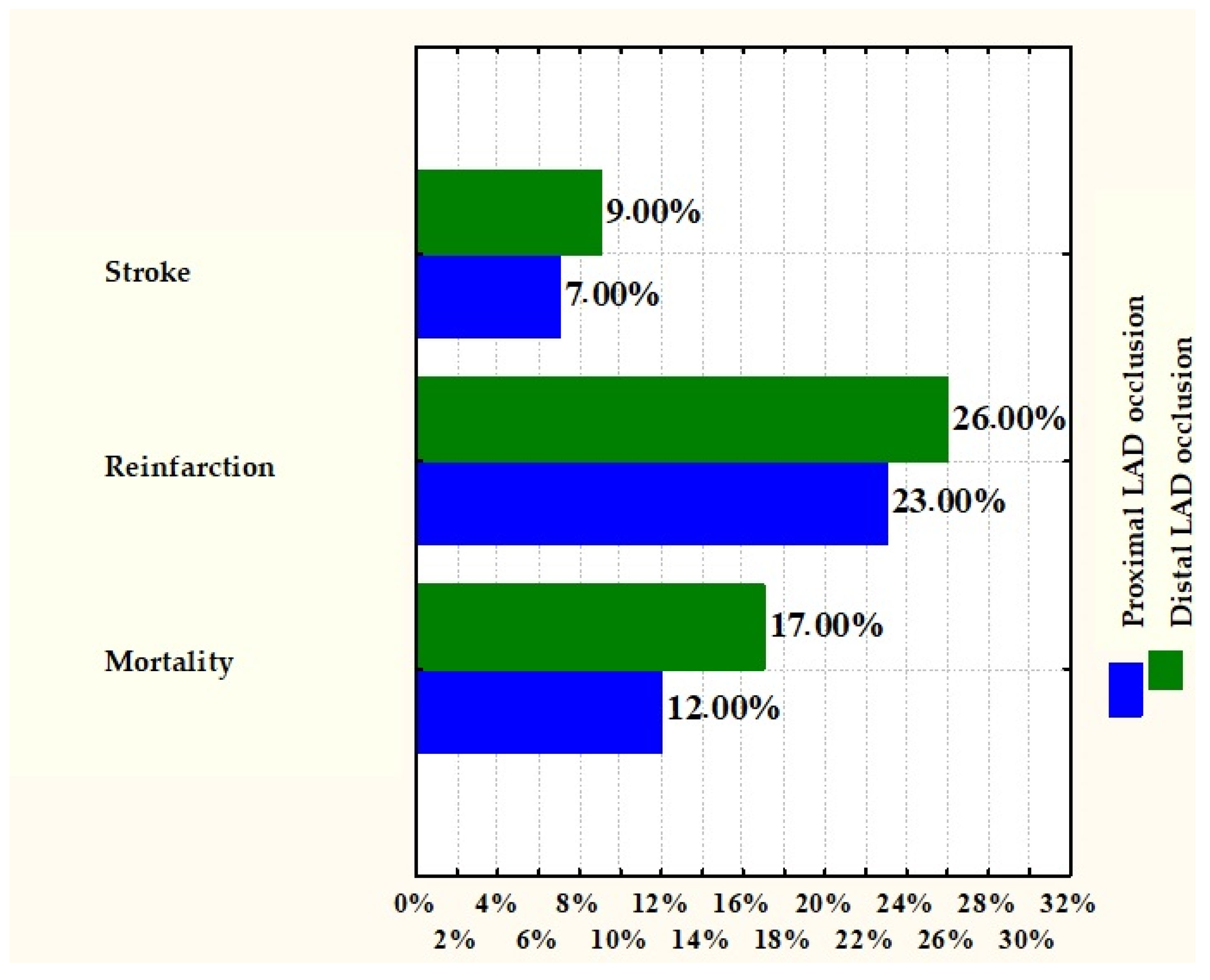
| LAD Proximal Occlusion | LAD Distal Occlusion | p Value | LAD Proximal Occlusion | |
|---|---|---|---|---|
| Mortality | 12% | 17% | 0.3832 | |
| Reinfarction | 23% | 26% | 0.7129 | |
| Stroke | 7% | 9% | 0.7604 | |
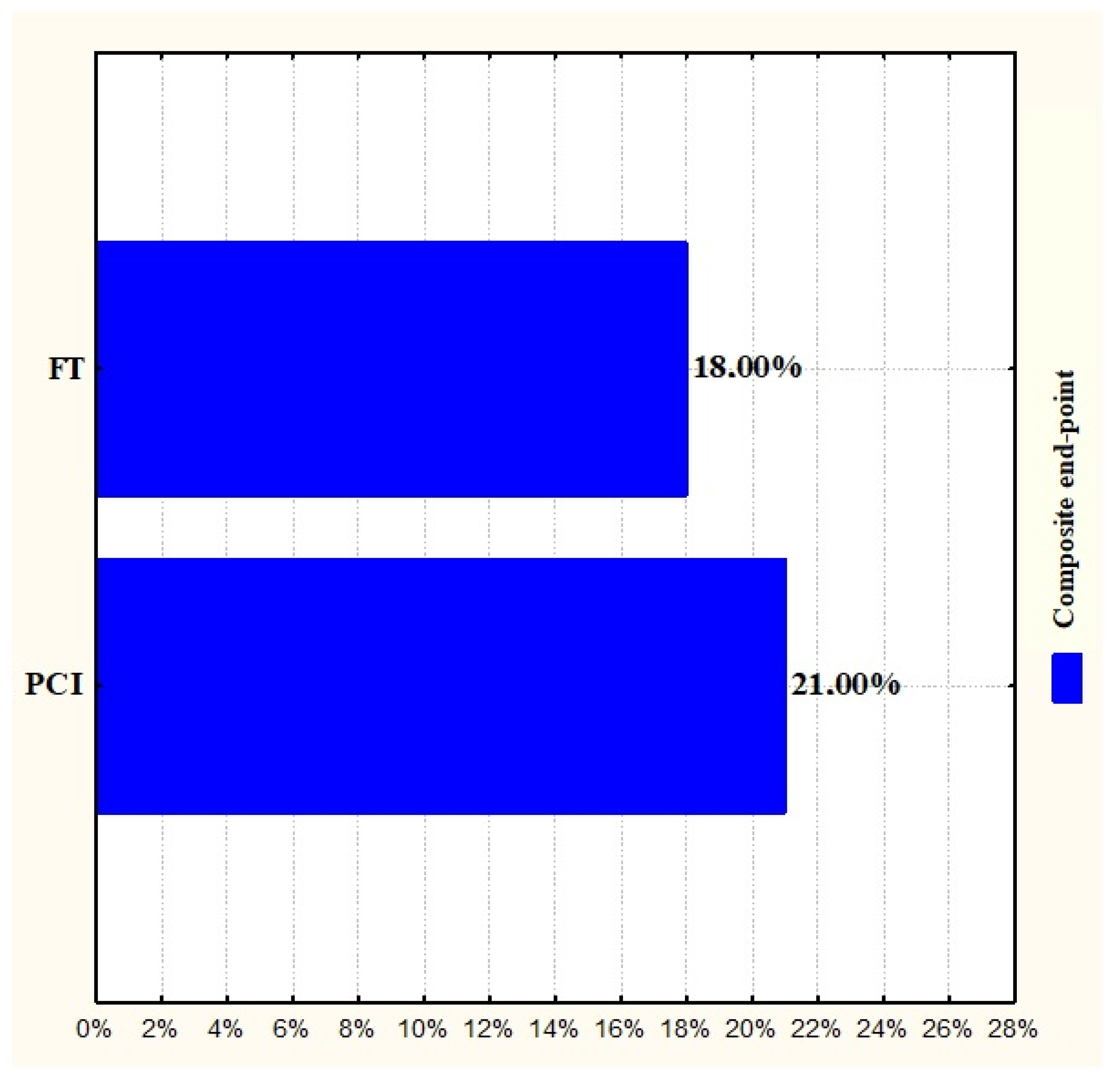
| Composite end-point | PCI | FT | - | 0.6862 |
| 21% | 18% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rus, M.; Negruțiu, B.M.; Andronie-Cioara, F.L.; Pasca, G.; Judea Pusta, C.T.; Sava, C.; Ardelean, A.I.; Popoviciu, M.-S.; Staniș, C.E. Proximal vs. Distal LAD Lesions in ST-Elevation Myocardial Infarction: Insights from ECG and Coronary Angiography. J. Clin. Med. 2025, 14, 5637. https://doi.org/10.3390/jcm14165637
Rus M, Negruțiu BM, Andronie-Cioara FL, Pasca G, Judea Pusta CT, Sava C, Ardelean AI, Popoviciu M-S, Staniș CE. Proximal vs. Distal LAD Lesions in ST-Elevation Myocardial Infarction: Insights from ECG and Coronary Angiography. Journal of Clinical Medicine. 2025; 14(16):5637. https://doi.org/10.3390/jcm14165637
Chicago/Turabian StyleRus, Marius, Bianca Maria Negruțiu, Felicia Liana Andronie-Cioara, Georgeta Pasca, Claudia Teodora Judea Pusta, Cristian Sava, Adriana Ioana Ardelean, Mihaela-Simona Popoviciu, and Claudia Elena Staniș. 2025. "Proximal vs. Distal LAD Lesions in ST-Elevation Myocardial Infarction: Insights from ECG and Coronary Angiography" Journal of Clinical Medicine 14, no. 16: 5637. https://doi.org/10.3390/jcm14165637
APA StyleRus, M., Negruțiu, B. M., Andronie-Cioara, F. L., Pasca, G., Judea Pusta, C. T., Sava, C., Ardelean, A. I., Popoviciu, M.-S., & Staniș, C. E. (2025). Proximal vs. Distal LAD Lesions in ST-Elevation Myocardial Infarction: Insights from ECG and Coronary Angiography. Journal of Clinical Medicine, 14(16), 5637. https://doi.org/10.3390/jcm14165637






