Defining the Chronic Complexities of hEDS and HSD: A Global Survey of Diagnostic Challenges, Life-Long Comorbidities, and Unmet Needs
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Survey Design
2.3. Statistical Analyses
3. Results
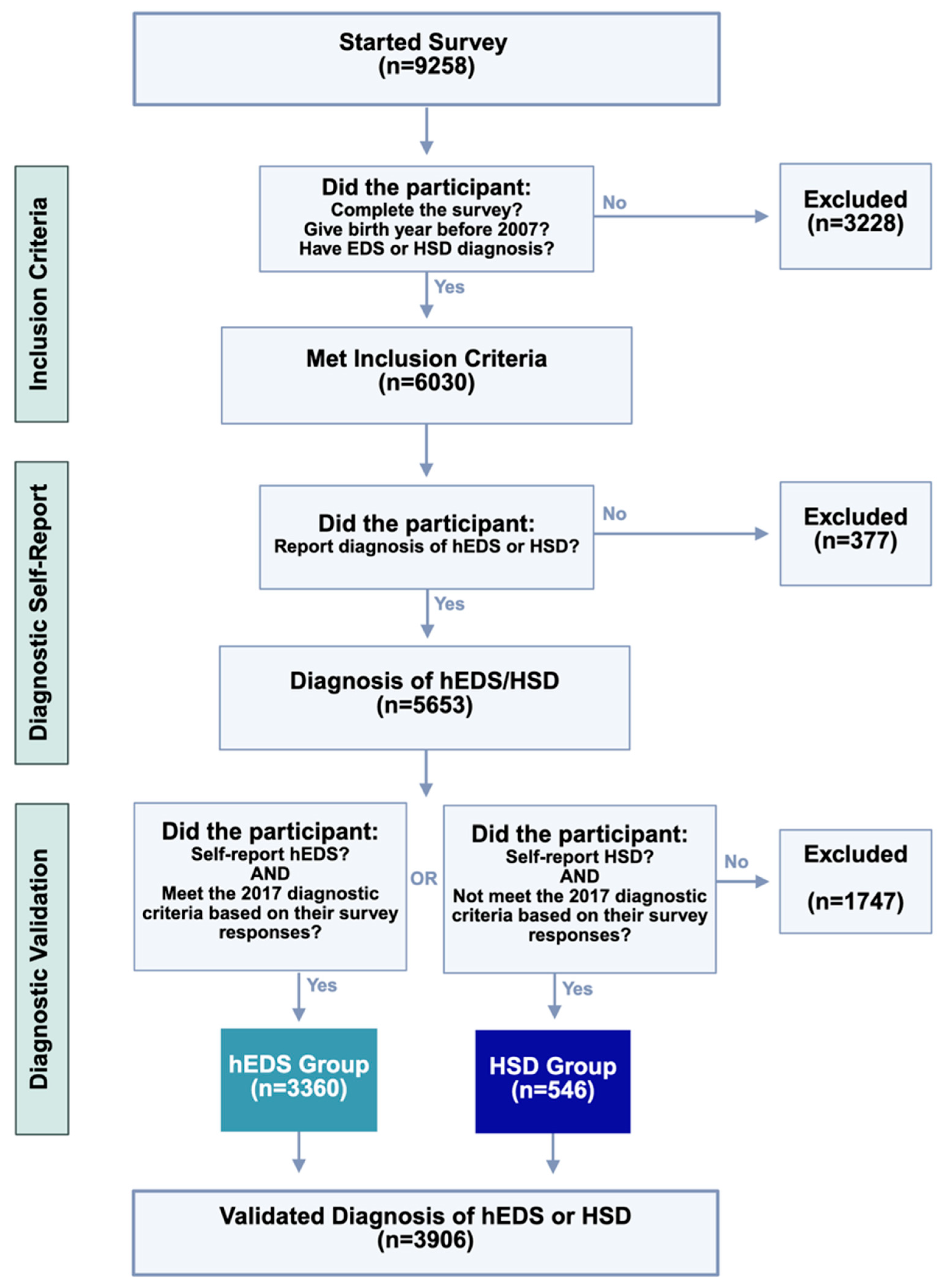
3.1. Study Cohort
3.1.1. Eligibility Criteria
3.1.2. Demographics
3.2. Diagnostic Journey
3.3. Multisystemic Clinical Presentation
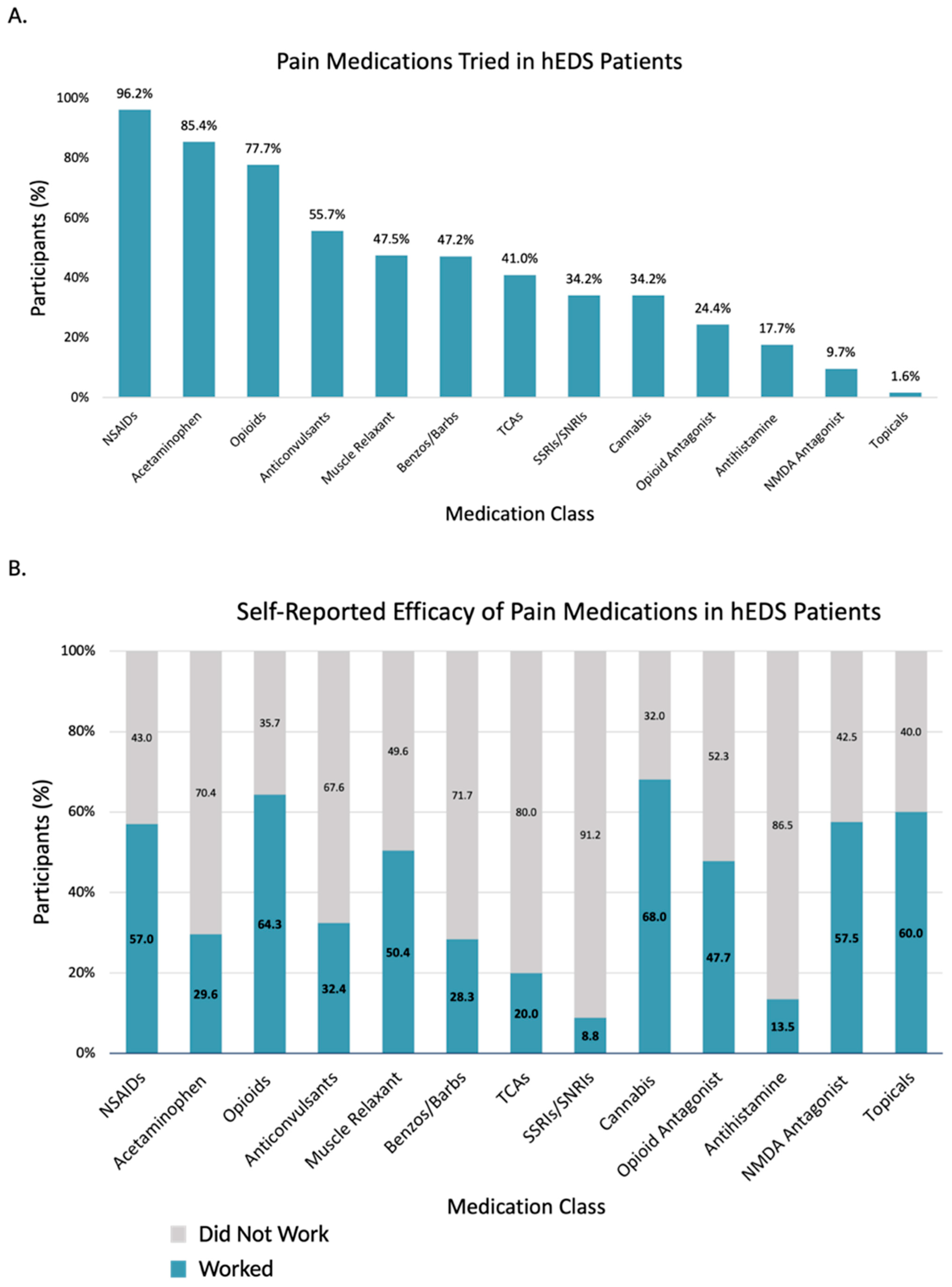
3.3.1. Pain
3.3.2. Orthopedic
3.3.3. Neurological
3.3.4. Autonomic
3.3.5. Gastrointestinal
3.3.6. Cardiopulmonary
3.3.7. Endocrine
3.3.8. Hematologic
3.3.9. Reproductive
3.3.10. Urological
3.3.11. Dermatological
3.3.12. Allergic and Immunologic
3.3.13. Ocular
3.3.14. Dental
3.3.15. Mental Health and Sleep
3.3.16. Neurodivergence
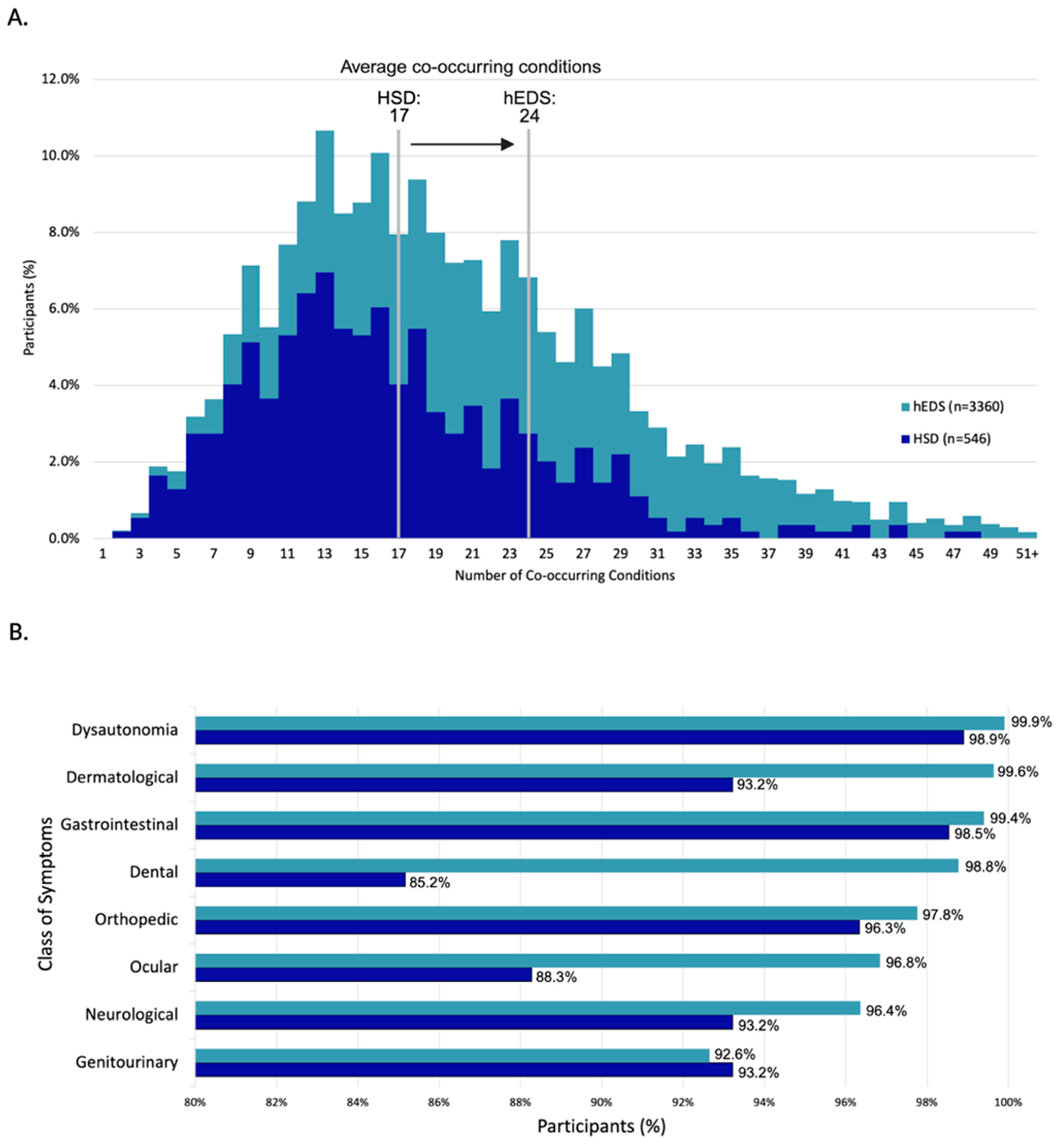
3.3.17. Multimorbidity in hEDS
4. Discussion
4.1. Multisystemic Manifestations
4.2. hEDS, HSD, and Rare Diseases
4.3. Challenges of the Current Diagnostic Criteria
4.4. Triggering Events
4.5. Limitations and Future Directions
4.6. Reconsidering Etiology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackburn, P.R.; Xu, Z.; Tumelty, K.E.; Zhao, R.W.; Monis, W.J.; Harris, K.G.; Gass, J.M.; Cousin, M.A.; Boczek, N.J.; Mitkov, M.V.; et al. Bi-allelic Alterations in AEBP1 Lead to Defective Collagen Assembly and Connective Tissue Structure Resulting in a Variant of Ehlers-Danlos Syndrome. Am. J. Hum. Genet. 2018, 102, 696–705. [Google Scholar] [CrossRef]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers–Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef] [PubMed]
- De Wandele, I.; Rombaut, L.; Malfait, F.; De Backer, T.; De Paepe, A.; Calders, P. Clinical heterogeneity in patients with the hypermobility type of Ehlers-Danlos Syndrome. Res. Dev. Disabil. 2013, 34, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.B. Hypermobility spectrum disorders: A review. Rheumatol. Immunol. Res. 2023, 4, 60–68. [Google Scholar] [CrossRef]
- Aubry-Rozier, B.; Schwitzguebel, A.; Valerio, F.; Tanniger, J.; Paquier, C.; Berna, C.; Hügle, T.; Benaim, C. Are patients with hypermobile Ehlers–Danlos syndrome or hypermobility spectrum disorder so different? Rheumatol. Int. 2021, 41, 1785–1794. [Google Scholar] [CrossRef]
- Castori, M.; Tinkle, B.; Levy, H.; Grahame, R.; Malfait, F.; Hakim, A. A framework for the classification of joint hypermobility and related conditions. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 148–157. [Google Scholar] [CrossRef]
- Tinkle, B.; Castori, M.; Berglund, B.; Cohen, H.; Grahame, R.; Kazkaz, H.; Levy, H. Hypermobile Ehlers–Danlos syndrome (a.k.a. Ehlers–Danlos syndrome Type III and Ehlers–Danlos syndrome hypermobility type): Clinical description and natural history. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 48–69. [Google Scholar] [CrossRef]
- Pezaro, S.; Brock, I.; Buckley, M.; Callaway, S.; Demirdas, S.; Hakim, A.; Harris, C.; High Gross, C.; Karanfil, M.; Le Ray, I.; et al. Management of childbearing with hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders: A scoping review and expert co-creation of evidence-based clinical guidelines. PLoS ONE 2024, 19, e0302401. [Google Scholar] [CrossRef]
- Xiong, T.; Baker, J.; Chey, W.D.; Nojkov, B. 1182 Small Intestinal Bacterial Overgrowth (SIBO) Is Common in Patients With Ehlers-Danlos Syndrome (EDS). Am. J. Gastroenterol. 2019, 114, S663–S664. [Google Scholar] [CrossRef]
- Tinkle, B.T.; Levy, H.P. Symptomatic Joint Hypermobility: The Hypermobile Type of Ehlers-Danlos Syndrome and the Hypermobility Spectrum Disorders. Med. Clin. N. Am. 2019, 103, 1021–1033. [Google Scholar] [CrossRef]
- Gensemer, C.; Burks, R.; Kautz, S.; Judge, D.P.; Lavallee, M.; Norris, R.A. Hypermobile Ehlers-Danlos syndromes: Complex phenotypes, challenging diagnoses, and poorly understood causes. Dev. Dyn. 2021, 250, 318–344. [Google Scholar] [CrossRef] [PubMed]
- Halverson, C.M.E.; Clayton, E.W.; Garcia Sierra, A.; Francomano, C. Patients with Ehlers–Danlos syndrome on the diagnostic odyssey: Rethinking complexity and difficulty as a hero’s journey. Am. J. Med. Genet. Part C Semin. Med. Genet. 2021, 187, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Rashed, E.R.; Ruiz Maya, T.; Black, J.; Fettig, V.; Kadian-Dodov, D.; Olin, J.W.; Mehta, L.; Gelb, B.D.; Kontorovich, A.R. Cardiovascular manifestations of hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. Vasc. Med. 2022, 27, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Gensemer, C.; Daylor, V.; Nix, J.; Norris, R.A.; Patel, S. Co-occurrence of tethered cord syndrome and cervical spine instability in hypermobile Ehlers-Danlos syndrome. Front. Neurol. 2024, 15, 1441866. [Google Scholar] [CrossRef]
- Petrucci, T.; Barclay, S.J.; Gensemer, C.; Morningstar, J.; Daylor, V.; Byerly, K.; Bistran, E.; Griggs, M.; Elliott, J.M.; Kelechi, T.; et al. Phenotypic Clusters and Multimorbidity in Hypermobile Ehlers-Danlos Syndrome. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 253–262. [Google Scholar] [CrossRef]
- Hakimi, A.; Bergoin, C.; Mucci, P. What are the most important symptoms to assess in hypermobile Ehlers-Danlos syndrome? A questionnaire study based on the Delphi technique. Disabil. Rehabil. 2022, 44, 8325–8331. [Google Scholar] [CrossRef]
- Nelson, A.D.; Mouchli, M.A.; Valentin, N.; Deyle, D.; Pichurin, P.; Acosta, A.; Camilleri, M. Ehlers Danlos syndrome and gastrointestinal manifestations: A 20-year experience at Mayo Clinic. Neurogastroenterol. Motil. 2015, 27, 1657–1666. [Google Scholar] [CrossRef]
- Anderson, L.K.; Lane, K.R. The diagnostic journey in adults with hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. J. Am. Assoc. Nurse Pract. 2022, 34, 639–648. [Google Scholar] [CrossRef]
- Black, W.R.; Black, L.L.; Jones, J.T. Barriers to the Diagnosis, Care, and Management of Pediatric Patients With Ehlers-Danlos Syndrome in the United States: A Qualitative Analysis. Glob. Pediatr. Health 2023, 10, 2333794X231212081. [Google Scholar] [CrossRef]
- Knight, D.R.T.; Bruno, K.A.; Singh, A.; Munipalli, B.; Gajarawala, S.; Solomon, M.; Kocsis, S.C.; Darakjian, A.A.; Jain, A.; Whelan, E.R.; et al. Cardiac defects of hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders: A retrospective cohort study. Front. Cardiovasc. Med. 2024, 11, 1332508. [Google Scholar] [CrossRef]
- Kim, M.J.; Choe, J.; Lee, B.H.; Song, J.W. Ehlers–Danlos syndrome presenting as cystic lung disease with recurrent pneumothorax: A case report. Respirol. Case Rep. 2021, 9, e00747. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Stecco, A. Fascial thickness and stiffness in hypermobile Ehlers-Danlos syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2021, 187, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Bańkowski, Z.; Levine, R.J.; Council for International Organizations of Medical Sciences; World Health Organization. Ethics and Research on Human Sujects: International Guidelines, Proceedings of the XXVIth CIOMS Conference, Geneva, Switzerland, 5–7 February 1992; CIOMS: Geneva, Switzerland, 1993. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inf. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Hanna, R.; Porter, E.; Romero, S.; Wildenhain, P.; Beasley, W.; Kadauke, S. REDCapTidieR: Extracting complex REDCap databases into tidy tables. J. Open Source Softw. 2024, 9, 6277. [Google Scholar] [CrossRef]
- Rosner, B. Fundamentals of Biostatistics; Brooks/Cole, Cengage Learning: Boston, MA, USA, 2011. [Google Scholar]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Gu, Z. Complex heatmap visualization. Imeta 2022, 1, e43. [Google Scholar] [CrossRef]
- Glayzer, J.E.; Bray, B.C.; Kobak, W.H.; Steffen, A.D.; Schlaeger, J.M. Lack of Diversity in Research on Females with Ehlers-Danlos Syndromes: Recruitment Protocol for a Quantitative Online Survey. JMIR Res. Protoc. 2024, 13, e53646. [Google Scholar] [CrossRef]
- Malfait, F.; Castori, M.; Francomano, C.A.; Giunta, C.; Kosho, T.; Byers, P.H. The Ehlers-Danlos syndromes. Nat. Rev. Dis. Primers 2020, 6, 64. [Google Scholar] [CrossRef]
- Schubart, J.R.; Schaefer, E.W.; Knight, D.R.T.; Mills, S.E.; Francomano, C.A. Estimates of the excess cost burden of Ehlers-Danlos syndromes: A United States MarketScan® claims database analysis. Front. Public Health 2024, 12, 1365712. [Google Scholar] [CrossRef]
- Fikree, A.; Aktar, R.; Morris, J.K.; Grahame, R.; Knowles, C.H.; Aziz, Q. The association between Ehlers-Danlos syndrome-hypermobility type and gastrointestinal symptoms in university students: A cross-sectional study. Neurogastroenterol. Motil. 2017, 29, e12942. [Google Scholar] [CrossRef] [PubMed]
- Oaklander, A.L.; Klein, M.M. Evidence of small-fiber polyneuropathy in unexplained, juvenile-onset, widespread pain syndromes. Pediatrics 2013, 131, e1091–e1100. [Google Scholar] [CrossRef]
- Leone, C.M.; Celletti, C.; Gaudiano, G.; Puglisi, P.A.; Fasolino, A.; Cruccu, G.; Camerota, F.; Truini, A. Pain due to Ehlers-Danlos Syndrome Is Associated with Deficit of the Endogenous Pain Inhibitory Control. Pain. Med. 2020, 21, 1929–1935. [Google Scholar] [CrossRef]
- De Wandele, I.; Calders, P.; Peersman, W.; Rimbaut, S.; De Backer, T.; Malfait, F.; De Paepe, A.; Rombaut, L. Autonomic symptom burden in the hypermobility type of Ehlers-Danlos syndrome: A comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin. Arthritis Rheum. 2014, 44, 353–361. [Google Scholar] [CrossRef]
- Chelimsky, G.; Simpson, P.; Feng, M.; Willis, E. Does Unconscious Bias Affect. How Pediatricians Manage Their Patients? WMJ 2022, 121, 18–25. [Google Scholar]
- Zhou, W.; Zikos, T.A.; Halawi, H.; Sheth, V.R.; Gurland, B.; Nguyen, L.A.; Neshatian, L. Anorectal manometry for the diagnosis of pelvic floor disorders in patients with hypermobility spectrum disorders and hypermobile Ehlers-Danlos syndrome. BMC Gastroenterol. 2022, 22, 538. [Google Scholar] [CrossRef]
- Chopra, P.; Tinkle, B.; Hamonet, C.; Brock, I.; Gompel, A.; Bulbena, A.; Francomano, C. Pain management in the Ehlers–Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.; O’Callaghan, C.; De Wandele, I.; Stiles, L.; Pocinki, A.; Rowe, P. Cardiovascular autonomic dysfunction in Ehlers-Danlos syndrome-Hypermobile type. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 168–174. [Google Scholar] [CrossRef]
- Malek, S.; Reinhold, E.J.; Pearce, G.S. The Beighton Score as a measure of generalised joint hypermobility. Rheumatol. Int. 2021, 41, 1707–1716. [Google Scholar] [CrossRef] [PubMed]



| hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | |
|---|---|---|---|
| Yes, event preceded symptoms of hEDS/HSD | 2355 (70.1) | 355 (65) | 1.08 (1.01–1.15), ns |
| No | 1005 (29.9) | 191 (35) | 0.86 (0.75–0.97), ns |
| Puberty | 1009 (30) | 118 (21.6) | 1.39 (1.17–1.64), 0.0293 |
| Viral or bacterial infection | 860 (25.6) | 126 (23.1) | 1.11 (0.94–1.31), ns |
| Physical accident | 551 (16.4) | 75 (13.7) | 1.19 (0.95–1.49), ns |
| Psychological or emotional event | 481 (14.3) | 86 (15.8) | 0.91 (0.74–1.12), ns |
| Pregnancy | 477 (14.2) | 50 (9.2) | 1.55 (1.18–2.04), ns |
| Other | 470 (14) | 68 (12.5) | 1.12 (0.89–1.42), ns |
| Diagnosed with Long COVID | 221 (6.6) | 34 (6.2) | 1.06 (0.74–1.50), ns |
| Diagnosed with Long COVID after hEDS/HSD | 142 (4.2) | 17 (3.1) | 1.36 (0.83–2.23), ns |
| Diagnosed with Long COVID prior to hEDS/HSD | 79 (2.4) | 17 (3.1) | 0.76 (0.45–1.27), ns |
| hEDS Most Severe Symptoms | hEDS | HSD Most Severe Symptoms | HSD |
|---|---|---|---|
| Chronic pain | 32.6% | Chronic pain | 34.2% |
| Gastrointestinal Symptoms | 13.5% | Joint Manifestations | 14.1% |
| Autonomic dysfunction | 12.8% | Autonomic dysfunction | 10.0% |
| Joint Manifestations | 12.2% | Gastrointestinal Symptoms | 9.6% |
| Neurological Symptoms | 7.2% | Neurological Symptoms | 7.1% |
| Mental health | 4.1% | Mental health | 5.0% |
| Allergic Symptoms | 3.5% | Sleep Issues | 4.0% |
| Sleep Issues | 3.3% | Allergic Symptoms | 3.1% |
| Other | 2.8% | Other | 2.8% |
| Gynecological Symptoms | 1.7% | Gynecological Symptoms | 2.2% |
| Neurodiversity | 0.9% | Neurodiversity | 1.3% |
| Urinary Symptoms | 0.9% | Urinary Symptoms | 1.1% |
| Dental Manifestations | 0.6% | Dental Manifestations | 0.9% |
| Vision Dysfunction | 0.5% | Vision Dysfunction | 0.7% |
| Dermatological Symptoms | 0.4% | Endocrine Dysfunction | 0.7% |
| Endocrine Dysfunction | 0.4% | Dermatological Symptoms | 0.5% |
| Medical Care | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Average hours per week coordinating care | |||
| Less than 5 h | 1741 (51.8) | 365 (66.8) | 0.78 (0.72–0.83), <0.0001 |
| 5 to 10 h | 1068 (31.8) | 136 (24.9) | 1.28 (1.09–1.49), ns |
| 11 to 15 h | 305 (9.1) | 32 (5.9) | 1.55 (1.09–2.20), ns |
| 16 to 20 h | 121 (3.6) | 8 (1.5) | 2.46 (1.21–5.00), ns |
| More than 20 h | 125 (3.7) | 5 (0.9) | 4.06 (1.67–9.89), ns |
| Care Coordination | |||
| Self | 3092 (92) | 494 (90.5) | 1.02 (0.99–1.05), ns |
| Medical provider | 553 (16.5) | 83 (15.2) | 1.08 (0.88–1.34), ns |
| Family member | 417 (12.4) | 63 (11.5) | 1.08 (0.84–1.38), ns |
| Other | 19 (0.6) | 1 (0.2) | - |
| Medical specialists seen in last year ‡ | 6.5 (±3.7) | 5.2 (±3.2) | (−1.64 to −1.04), <0.0001 |
| Medical specialists seen in the past year | |||
| Family medicine | 2150 (64) | 287 (52.6) | 1.22 (1.12–1.32), 0.0002 |
| Cardiology | 1918 (57.1) | 221 (40.5) | 1.41 (1.27–1.57), <0.0001 |
| Gastroenterology | 1500 (44.6) | 155 (28.4) | 1.57 (1.37–1.81), <0.0001 |
| Neurology | 1418 (42.2) | 172 (31.5) | 1.34 (1.18–1.53), 0.0012 |
| Emergency medicine | 1383 (41.2) | 140 (25.6) | 1.61 (1.38–1.86), <0.0001 |
| Psychiatry | 1287 (38.3) | 190 (34.8) | 1.10 (0.97–1.24), ns |
| Rheumatology | 1284 (38.2) | 205 (37.5) | 1.02 (0.91–1.14), ns |
| Dermatology | 1129 (33.6) | 139 (25.5) | 1.32 (1.13–1.54), ns |
| Ophthalmology | 1091 (32.5) | 120 (22) | 1.48 (1.25–1.74), 0.0005 |
| Radiology | 1089 (32.4) | 110 (20.1) | 1.61 (1.35–1.91), <0.0001 |
| Internal medicine | 956 (28.5) | 115 (21.1) | 1.35 (1.14–1.60), ns |
| Sports medicine | 870 (25.9) | 140 (25.6) | 1.01 (0.87–1.18), ns |
| Immunology | 673 (20) | 78 (14.3) | 1.40 (1.13–1.74), ns |
| Endocrinology | 596 (17.7) | 94 (17.2) | 1.03 (0.85–1.26), ns |
| Urology | 507 (15.1) | 39 (7.1) | 2.11 (1.54–2.89), 0.0004 |
| Otolaryngology | 241 (7.2) | 36 (6.6) | 1.09 (0.78–1.53), ns |
| Nephrology | 118 (3.5) | 11 (2) | 1.74 (0.95–3.21), ns |
| Hepatology | 81 (2.4) | 5 (0.9) | 2.63 (1.07–6.47), ns |
| Geriatric | 26 (0.8) | 1 (0.2) | - |
| Other | 1503 (44.7) | 232 (42.5) | 1.05 (0.95–1.17), ns |
| None of the above | 71 (2.1) | 19 (3.5) | 0.61 (0.37–1.00), ns |
| Admitted inpatient for hEDS/HSD or related condition | 1395 (41.5) | 108 (19.8) | 2.10 (1.76–2.50), <0.0001 |
| Admissions in the last year | |||
| 0 | 581 (17.3) | 47 (8.6) | 2.01 (1.51–2.67), 0.0002 |
| 1 | 403 (12) | 27 (4.9) | 2.43 (1.66–3.54), 0.0006 |
| 2 | 185 (5.5) | 21 (3.8) | 1.43 (0.92–2.23), ns |
| 3+ | 226 (6.7) | 13 (2.4) | 2.83 (1.63–4.90), ns |
| N/A | 1965 (58.5) | 438 (80.2) | - |
| Farthest distance traveled for treatment | |||
| Less than 300 miles | 2309 (68.7) | 458 (83.9) | 0.82 (0.78–0.86), <0.0001 |
| 300 to 599 miles | 503 (15) | 46 (8.4) | 1.78 (1.33–2.37), 0.0249 |
| 600 to 999 miles | 219 (6.5) | 13 (2.4) | 2.74 (1.58–4.75), ns |
| 1000 to 2000 miles | 169 (5) | 11 (2) | 2.50 (1.37–4.56), ns |
| More than 2000 miles | 160 (4.8) | 18 (3.3) | 1.44 (0.89–2.33), ns |
| Paid out-of-pocket for medical expenses | 2041 (60.7) | 245 (44.9) | 1.35 (1.23–1.49), 0.0103 |
| Chronic Pain | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Experience chronic pain | 3318 (98.8) | 506 (92.7) | 1.07 (1.04–1.09), <0.0001 |
| Chronic Pain Sites | |||
| Neck | 2684 (79.9) | 367 (67.2) | 1.19 (1.12–1.26), <0.0001 |
| Lower back | 2616 (77.9) | 359 (65.8) | 1.18 (1.11–1.26), <0.0001 |
| Shoulder | 2578 (76.7) | 360 (65.9) | 1.16 (1.09–1.24), <0.0001 |
| Hip | 2523 (75.1) | 342 (62.6) | 1.20 (1.12–1.28), <0.0001 |
| Knee | 2397 (71.3) | 324 (59.3) | 1.20 (1.12–1.29), <0.0001 |
| Fingers | 2162 (64.3) | 276 (50.5) | 1.27 (1.17–1.39), <0.0001 |
| Upper back | 2132 (63.5) | 291 (53.3) | 1.19 (1.10–1.29), 0.0030 |
| Wrist | 1959 (58.3) | 269 (49.3) | 1.18 (1.08–1.29), 0.0387 |
| Head | 1894 (56.4) | 219 (40.1) | 1.41 (1.26–1.56), <0.0001 |
| Jaw | 1880 (56) | 233 (42.7) | 1.31 (1.18–1.45), <0.0001 |
| Ankle | 1861 (55.4) | 245 (44.9) | 1.23 (1.12–1.36), 0.0025 |
| Widespread | 1842 (54.8) | 232 (42.5) | 1.29 (1.16–1.43), <0.0001 |
| Abdominal | 1774 (52.8) | 188 (34.4) | 1.53 (1.36–1.73), <0.0001 |
| Ribs | 1479 (44) | 153 (28) | 1.57 (1.37–1.81), <0.0001 |
| Elbow | 1149 (34.2) | 145 (26.6) | 1.29 (1.11–1.49), ns |
| Toes | 1079 (32.1) | 136 (24.9) | 1.29 (1.11–1.50), ns |
| Other | 283 (8.4) | 47 (8.6) | 0.98 (0.73–1.31), ns |
| Alternative Medicine | hEDS n (%) | HSD n (%) |
|---|---|---|
| Tried alternative medicine | 2858 (85.1) | 445 (81.5) |
| Alternative medicine type | ||
| Massage | 2366 (70.4) | 369 (67.6) |
| Chiropractic | 1846 (54.9) | 250 (45.8) |
| Meditation | 1553 (46.2) | 218 (39.9) |
| Acupuncture | 1491 (44.4) | 232 (42.5) |
| Functional medicine | 745 (22.2) | 99 (18.1) |
| Naturopathy | 632 (18.8) | 77 (14.1) |
| Homeopathy | 596 (17.7) | 76 (13.9) |
| Chinese medicine | 423 (12.6) | 69 (12.6) |
| Hypnosis | 220 (6.5) | 35 (6.4) |
| Ayurveda | 167 (5) | 19 (3.5) |
| Other | 239 (7.1) | 44 (8.1) |
| Anesthesia Complications | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Anesthesia Complications | 1660 (49.4) | 155 (28.4) | 1.74 (1.52–2.00), <0.0001 |
| Anesthesia type | |||
| Local anesthesia only | 1287 (38.3) | 105 (19.2) | 1.99 (1.67–2.38), ns |
| General anesthesia only | 1191 (35.4) | 95 (17.4) | 2.04 (1.69–2.46), ns |
| Both general and local anesthesia | 844 (25.1) | 47 (8.6) | 2.92 (2.21–3.86), 0.0007 |
| Complication Type | |||
| Shortened effect | 1270 (37.8) | 103 (18.9) | 2.00 (1.67–2.40), ns |
| Insufficient pain control | 1017 (30.3) | 62 (11.4) | 2.67 (2.10–3.39), 0.0002 |
| Intubation complication | 193 (5.7) | 15 (2.7) | 2.09 (1.25–3.51), ns |
| Anaphylaxis or allergic reaction | 137 (4.1) | 8 (1.5) | 2.78 (1.37–5.64), ns |
| Other | 400 (11.9) | 46 (8.4) | 1.41 (1.06–1.89), ns |
| Orthopedic Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Tendonitis | 1631 (48.5) | 215 (39.4) | 1.23 (1.10–1.38), 0.0352 | 23,000 (6.5) | 7.48 (7.21–7.76), <0.0001 |
| Bursitis | 1199 (35.7) | 157 (28.8) | 1.24 (1.08–1.43), ns | 29,060 (8.2) | 4.35 (4.15–4.56), <0.0001 |
| Ligament tear | 1090 (32.4) | 127 (23.3) | 1.39 (1.19–1.64), 0.0091 | 61,560 (17.4) | 1.87 (1.78–1.96), <0.0001 |
| Tendon rupture | 543 (16.2) | 63 (11.5) | 1.40 (1.10–1.79), ns | 1320 (0.4) | 43.39 (39.50–47.66), <0.0001 |
| Slipping rib syndrome | 383 (11.4) | 22 (4) | 2.83 (1.86–4.31), 0.0001 | 7040 (2) | 5.74 (5.21–6.32), <0.0001 |
| Congenital hip dislocation | 161 (4.8) | 12 (2.2) | 2.18 (1.22–3.89), ns | 100 (0) | 169.82 (132.62–217.45), <0.0001 |
| Bowing of legs | 159 (4.7) | 17 (3.1) | 1.52 (0.93–2.49), ns | 940 (0.3) | 17.84 (15.13–21.03), <0.0001 |
| Osgood-Schlatter disease | 155 (4.6) | 19 (3.5) | 1.33 (0.83–2.12), ns | - | - |
| Pectus excavatum | 125 (3.7) | 10 (1.8) | 2.03 (1.07–3.84), ns | 340 (0.1) | 38.78 (31.68–47.47), <0.0001 |
| Joint contracture | 70 (2.1) | 7 (1.3) | 1.63 (0.75–3.52), ns | 180 (0.1) | 41.02 (31.19–53.95), <0.0001 |
| Club foot | 70 (2.1) | 5 (0.9) | 2.28 (0.92–5.61), ns | 40 (0) | 184.58 (125.35–271.81), <0.0001 |
| Congenital muscle hypotonia | 42 (1.3) | 3 (0.5) | - | - | - |
| None of the above | 885 (26.3) | 205 (37.5) | 0.70 (0.62–0.79), <0.0001 | - | - |
| Orthopedic Symptoms | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Joint subluxations | 3254 (96.8) | 467 (85.5) | 1.13 (1.09–1.17), <0.0001 |
| Joint dislocations | 2159 (64.3) | 196 (35.9) | 1.79 (1.60–2.01), <0.0001 |
| Unexplained fractures | 666 (19.8) | 52 (9.5) | 2.08 (1.59–2.72), <0.0001 |
| None of the above | 75 (2.2) | 64 (11.7) | 0.19 (0.14–0.26), <0.0001 |
| Joints Affected | |||
| Shoulder | 2704 (80.5) | 328 (60.1) | 1.34 (1.25–1.44), <0.0001 |
| Hip | 2428 (72.3) | 256 (46.9) | 1.54 (1.41–1.69), <0.0001 |
| Knee | 2345 (69.8) | 275 (50.4) | 1.39 (1.27–1.51), <0.0001 |
| Fingers | 2313 (68.8) | 258 (47.3) | 1.46 (1.33–1.60), <0.0001 |
| Ribs | 2022 (60.2) | 198 (36.3) | 1.66 (1.48–1.86), <0.0001 |
| Ankle | 2003 (59.6) | 221 (40.5) | 1.47 (1.33–1.64), <0.0001 |
| Wrist | 1872 (55.7) | 180 (33) | 1.69 (1.49–1.91), <0.0001 |
| Toes | 1387 (41.3) | 124 (22.7) | 1.82 (1.55–2.13), <0.0001 |
| Elbow | 1315 (39.1) | 139 (25.5) | 1.54 (1.32–1.79), <0.0001 |
| Spine | 1288 (38.3) | 129 (23.6) | 1.62 (1.39–1.90), <0.0001 |
| Other | 454 (13.5) | 47 (8.6) | 1.57 (1.18–2.09), ns |
| Anatomical Abnormalities | hEDS n = 3360 n (%) |
|---|---|
| Uterus | 549 (16.3) |
| Ribs | 40 (1.2) |
| Lumbar/sacral | 38 (1.1) |
| Extra or missing vertebrae | 9 (0.3) |
| Neurological Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Migraine | 2162 (64.3) | 255 (46.7) | 1.38 (1.26–1.51), <0.0001 | 49,740 (14) | 4.58 (4.46–4.71), <0.0001 |
| Scoliosis | 1145 (34.1) | 114 (20.9) | 1.63 (1.38–1.93), <0.0001 | 11,680 (3.3) | 10.34 (9.83–10.87), <0.0001 |
| Raynaud’s phenomenon | 1130 (33.6) | 104 (19) | 1.77 (1.48–2.11), <0.0001 | 3060 (0.9) | 38.95 (36.71–41.32), <0.0001 |
| Tinnitus | 1046 (31.1) | 121 (22.2) | 1.40 (1.19–1.66), 0.0113 | 26,620 (7.5) | 4.14 (3.94–4.36), <0.0001 |
| Other nerve entrapments | 910 (27.1) | 100 (18.3) | 1.48 (1.23–1.78), 0.0076 | - | - |
| Herniated disk(s) | 882 (26.3) | 97 (17.8) | 1.48 (1.22–1.79), 0.0117 | 37,360 (10.5) | 2.49 (2.35–2.64), <0.0001 |
| CCI/AAI | 742 (22.1) | 41 (7.5) | 2.94 (2.18–3.97), <0.0001 | 180 (0.1) | 434.80 (370.78–509.86), <0.0001 |
| ME/CFS | 568 (16.9) | 78 (14.3) | 1.18 (0.95–1.47), ns | 12,920 (3.6) | 4.64 (4.29–5.01), <0.0001 |
| Spinal stenosis | 443 (13.2) | 36 (6.6) | 2.00 (1.44–2.77), 0.0077 | 37,140 (10.5) | 1.26 (1.15–1.37), <0.0001 |
| Other spinal instability | 396 (11.8) | 42 (7.7) | 1.53 (1.13–2.08), ns | 15,640 (4.4) | 2.67 (2.43–2.93), <0.0001 |
| Occipital neuralgia | 394 (11.7) | 23 (4.2) | 2.78 (1.85–4.20), <0.0001 | 3160 (0.9) | 13.15 (11.91–14.52), <0.0001 |
| SFN | 337 (10) | 24 (4.4) | 2.28 (1.52–3.42), 0.0148 | 80 (0) | 444.32 (349.03–565.62), <0.0001 |
| Thoracic outlet syndrome | 292 (8.7) | 30 (5.5) | 1.58 (1.10–2.28), ns | 160 (0) | 192.49 (159.22–232.72), <0.0001 |
| Hearing impairment | 291 (8.7) | 33 (6) | 1.43 (1.01–2.03), ns | 52,120 (14.7) | 0.59 (0.53–0.66), <0.0001 |
| CRPS | 236 (7) | 17 (3.1) | 2.26 (1.39–3.66), ns | 1660 (0.5) | 15.00 (13.14–17.11), <0.0001 |
| Trigeminal neuralgia | 228 (6.8) | 18 (3.3) | 2.06 (1.28–3.30), ns | 2360 (0.7) | 10.19 (8.93–11.62), <0.0001 |
| Chiari malformation | 211 (6.3) | 5 (0.9) | 6.86 (2.84–16.57), 0.0003 | 360 (0.1) | 61.82 (52.34–73.02), <0.0001 |
| Dystonia | 208 (6.2) | 16 (2.9) | 2.11 (1.28–3.48), ns | 3080 (0.9) | 7.12 (6.22–8.16), <0.0001 |
| Kyphoscoliosis | 199 (5.9) | 9 (1.6) | 3.59 (1.85–6.96), 0.0240 | 240 (0.1) | 87.46 (72.70–105.21), <0.0001 |
| CSF leak | 177 (5.3) | 13 (2.4) | 2.21 (1.27–3.86), ns | 60 (0) | 311.15 (232.63–416.18), <0.0001 |
| TCS | 154 (4.6) | 5 (0.9) | 5.01 (2.06–12.14), 0.0393 | 40 (0) | 406.08 (287.26–574.05), <0.0001 |
| Voice disorder | 134 (4) | 6 (1.1) | 3.63 (1.61–8.18), ns | 11,320 (3.2) | 1.25 (1.06–1.48), ns |
| Intracranial hypotension | 126 (3.8) | 9 (1.6) | 2.28 (1.16–4.45), ns | - | - |
| Syringomyelia | 53 (1.6) | 3 (0.5) | - | - | - |
| Transverse sinus stenosis | 24 (0.7) | 5 (0.9) | 0.78 (0.30–2.04), ns | - | - |
| Multiple sclerosis | 23 (0.7) | 0 (0) | - | - | - |
| Myasthenia gravis | 14 (0.4) | 0 (0) | - | - | - |
| None of the above | 341 (10.1) | 131 (24) | 0.42 (0.35–0.51), <0.0001 | - | - |
| Neurological Symptoms | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Weakness | 2676 (79.6) | 391 (71.6) | 1.11 (1.05–1.18), 0.0121 |
| Muscle spasm | 2632 (78.3) | 367 (67.2) | 1.17 (1.10–1.24), <0.0001 |
| Tingling | 2555 (76) | 366 (67) | 1.13 (1.07–1.21), 0.0037 |
| Numbness | 2365 (70.4) | 288 (52.7) | 1.33 (1.23–1.45), <0.0001 |
| None of the above | 122 (3.6) | 37 (6.8) | 0.54 (0.38–0.77), ns |
| Autonomic Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| POTS | 1772 (52.7) | 142 (26) | 2.03 (1.75–2.34), <0.0001 | 660 (0.2) | 283.19 (260.72–307.59), <0.0001 |
| General dysautonomia | 906 (27) | 80 (14.7) | 1.84 (1.49–2.27), <0.0001 | 5080 (1.4) | 18.81 (17.68–20.01), <0.0001 |
| Orthostatic hypotension | 545 (16.2) | 44 (8.1) | 2.01 (1.50–2.70), 0.0004 | 10,840 (3.1) | 5.30 (4.90–5.74), <0.0001 |
| Orthostatic intolerance | 512 (15.2) | 43 (7.9) | 1.93 (1.44–2.61), 0.0028 | - | - |
| Autonomic neuropathy | 247 (7.4) | 13 (2.4) | 3.09 (1.78–5.35), 0.0098 | 2520 (0.7) | 10.34 (9.11–11.73), <0.0001 |
| Hyperadrenergic POTS | 178 (5.3) | 14 (2.6) | 2.07 (1.21–3.53), ns | - | - |
| Hypovolemic POTS | 82 (2.4) | 7 (1.3) | 1.90 (0.88–4.10), ns | 6500 (1.8) | 1.33 (1.07–1.65), <0.0001 |
| Pure autonomic failure | 14 (0.4) | 0 (0) | - | - | - |
| None of the above | 962 (28.6) | 326 (59.7) | 0.48 (0.44–0.52), <0.0001 | - | - |
| Autonomic Symptoms | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Fatigue | 3312 (98.6) | 516 (94.5) | 1.04 (1.02–1.06), <0.0001 |
| Dizziness | 3216 (95.7) | 482 (88.3) | 1.08 (1.05–1.12), <0.0001 |
| Brain fog | 3211 (95.6) | 491 (89.9) | 1.06 (1.03–1.09), <0.0001 |
| Heart palpitations | 3177 (94.6) | 468 (85.7) | 1.10 (1.06–1.14), <0.0001 |
| Thermoregulatory dysfunction | 3090 (92) | 440 (80.6) | 1.14 (1.09–1.19), <0.0001 |
| Vertigo | 2782 (82.8) | 371 (67.9) | 1.22 (1.15–1.29), <0.0001 |
| Trouble swallowing | 2171 (64.6) | 251 (46) | 1.41 (1.28–1.54), <0.0001 |
| Fainting | 2166 (64.5) | 239 (43.8) | 1.47 (1.33–1.62), <0.0001 |
| None of the above | 3 (0.1) | 6 (1.1) | - |
| Gastrointestinal Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Irritable bowel syndrome (IBS) | 1627 (48.4) | 201 (36.8) | 1.32 (1.17–1.48), 0.0002 | 21,220 (6) | 8.09 (7.79–8.39), <0.0001 |
| Gastroesophageal reflux disease (GERD) | 1601 (47.6) | 168 (30.8) | 1.55 (1.36–1.76), <0.0001 | 110,180 (31.1) | 1.53 (1.48–1.59), <0.0001 |
| Constipation | 1467 (43.7) | 153 (28) | 1.56 (1.35–1.79), <0.0001 | 63,800 (18) | 2.43 (2.33–2.52), <0.0001 |
| Dysmotility | 880 (26.2) | 57 (10.4) | 2.51 (1.95–3.23), <0.0001 | - | - |
| Gastritis | 818 (24.3) | 81 (14.8) | 1.64 (1.33–2.02), 0.0005 | 35,320 (10) | 2.44 (2.30–2.59), <0.0001 |
| Obesity | 730 (21.7) | 106 (19.4) | 1.12 (0.93–1.34), ns | 111,560 (31.5) | 0.69 (0.65–0.74), <0.0001 |
| Non-celiac gluten sensitivity | 514 (15.3) | 60 (11) | 1.39 (1.08–1.79), ns | - | - |
| Hiatal hernia | 501 (14.9) | 43 (7.9) | 1.89 (1.41–2.55), 0.0060 | 700 (0.2) | 75.49 (67.66–84.23), <0.0001 |
| Abdominal hernia | 341 (10.1) | 18 (3.3) | 3.08 (1.93–4.90), 0.0002 | 48,640 (13.7) | 0.74 (0.67–0.82), <0.0001 |
| Gastric ulcer | 330 (9.8) | 18 (3.3) | 2.98 (1.87–4.75), 0.0004 | 7420 (2.1) | 4.69 (4.22–5.21), <0.0001 |
| Appendicitis | 322 (9.6) | 45 (8.2) | 1.16 (0.86–1.57), ns | 4200 (1.2) | 8.09 (7.26–9.01), <0.0001 |
| Small intestine bacterial overgrowth (SIBO) | 312 (9.3) | 31 (5.7) | 1.64 (1.14–2.34), ns | - | - |
| Colorectal polyp(s) | 283 (8.4) | 30 (5.5) | 1.53 (1.06–2.21), ns | 26,580 (7.5) | 1.12 (1.00–1.26), ns |
| Diverticulosis/diverticulitis | 262 (7.8) | 18 (3.3) | 2.37 (1.48–3.78), ns | 9320 (2.6) | 2.97 (2.64–3.34), <0.0001 |
| Functional dyspepsia | 190 (5.7) | 17 (3.1) | 1.82 (1.12–2.96), ns | 4860 (1.4) | 4.12 (3.58–4.75), <0.0001 |
| Celiac disease | 150 (4.5) | 14 (2.6) | 1.74 (1.01–2.99), ns | 2760 (0.8) | 5.73 (4.88–6.73), <0.0001 |
| Feeding intolerance | 125 (3.7) | 8 (1.5) | 2.54 (1.25–5.16), ns | 1360 (0.4) | 9.69 (8.10–11.61), <0.0001 |
| Cholelithiasis | 118 (3.5) | 9 (1.6) | 2.13 (1.09–4.17), ns | 22,980 (6.5) | 0.54 (0.45–0.65), <0.0001 |
| Liver disease | 105 (3.1) | 10 (1.8) | 1.71 (0.90–3.24), ns | 60,580 (17.1) | 0.18 (0.15–0.22), <0.0001 |
| Eosinophilic esophagitis (EoE) | 102 (3) | 10 (1.8) | 1.66 (0.87–3.15), ns | 1300 (0.4) | 8.28 (6.78–10.09), <0.0001 |
| Median arcuate ligament syndrome (MALS) | 96 (2.9) | 4 (0.7) | - | - | - |
| Duodenal ulcer | 78 (2.3) | 3 (0.5) | - | - | - |
| Superior mesenteric artery syndrome (SMAS) | 62 (1.8) | 2 (0.4) | - | - | - |
| Ulcerative colitis | 59 (1.8) | 6 (1.1) | 1.60 (0.69–3.68), ns | 4060 (1.1) | 1.53 (1.19–1.98), ns |
| Crohn’s disease | 43 (1.3) | 5 (0.9) | 1.40 (0.56–3.51), ns | 4040 (1.1) | 1.12 (0.83–1.51), <0.0001 |
| Visceroptosis | 26 (0.8) | 1 (0.2) | - | - | - |
| Intestinal malrotation | 22 (0.7) | 1 (0.2) | - | - | - |
| Gastrointestinal organ rupture | 17 (0.5) | 1 (0.2) | - | - | - |
| None of the above | 529 (15.7) | 169 (31) | 0.51 (0.44–0.59), ns | - | - |
| Types of dysmotility | |||||
| Gastric | 711 (21.2) | 43 (7.9) | 2.69 (2.00–3.61), <0.0001 | 5260 (1.5) | 14.26 (13.29–15.30), <0.0001 |
| Esophageal | 232 (6.9) | 13 (2.4) | 2.90 (1.67–5.03), 0.0329 | - | - |
| Colon | 230 (6.8) | 17 (3.1) | 2.20 (1.35–3.57), ns | 4000 (1.1) | 6.06 (5.33–6.90), <0.0001 |
| Small bowel | 202 (6) | 8 (1.5) | 4.10 (2.04–8.27), 0.0083 | - | - |
| Types of IBS | |||||
| Mixed | 911 (27.1) | 89 (16.3) | 1.66 (1.36–2.03), <0.0001 | - | - |
| Constipation | 435 (12.9) | 46 (8.4) | 1.54 (1.15–2.05), ns | - | - |
| Diarrhea | 357 (10.6) | 48 (8.8) | 1.21 (0.91–1.61), ns | - | - |
| Undifferentiated | 233 (6.9) | 42 (7.7) | 0.90 (0.66–1.24), ns | - | - |
| Gastrointestinal Symptoms | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Abdominal pain | 3120 (92.9) | 439 (80.4) | 1.15 (1.11–1.20), <0.0001 |
| Nausea | 3044 (90.6) | 425 (77.8) | 1.16 (1.11–1.22), <0.0001 |
| Bloating | 3012 (89.6) | 439 (80.4) | 1.11 (1.07–1.16), <0.0001 |
| Constipation | 2925 (87.1) | 412 (75.5) | 1.15 (1.10–1.21), <0.0001 |
| Diarrhea | 2778 (82.7) | 391 (71.6) | 1.15 (1.09–1.22), <0.0001 |
| Reflux | 2640 (78.6) | 350 (64.1) | 1.23 (1.15–1.31), <0.0001 |
| Heartburn | 2488 (74) | 337 (61.7) | 1.20 (1.12–1.29), <0.0001 |
| Vomiting | 1968 (58.6) | 211 (38.6) | 1.52 (1.36–1.69), <0.0001 |
| Rectal bleeding | 1523 (45.3) | 166 (30.4) | 1.49 (1.31–1.70), <0.0001 |
| None of the above | 20 (0.6) | 8 (1.5) | 0.41 (0.18–0.92), ns |
| Feeding Devices and Ostomies | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Have used a feeding device | 240 (7.1) | 11 (2) | 3.55 (1.95–6.44), 0.0038 |
| Type of Feeding Device | |||
| Nasogastric (NG) tube | 130 (3.9) | 4 (0.7) | - |
| Nasojejunal (NJ) tube | 110 (3.3) | 4 (0.7) | - |
| Gastrojejunostomy (GJ) tube | 74 (2.2) | 3 (0.5) | - |
| Total parenteral nutrition (TPN) | 74 (2.2) | 2 (0.4) | - |
| Jejunostomy (JEJ, PEJ, or RIJ) tube | 55 (1.6) | 0 (0) | - |
| Gastric (G) tube | 48 (1.4) | 3 (0.5) | - |
| Peripheral parenteral nutrition (PPN) | 28 (0.8) | 0 (0) | - |
| Nasoduodenal (ND) tube | 26 (0.8) | 1 (0.2) | - |
| Orogastric (OG) tube | 3 (0.1) | 0 (0) | - |
| Oroenteric tube | 0 (0) | 0 (0) | - |
| Ostomies | |||
| Colostomy | 51 (1.5) | 10 (1.8) | - |
| Ileostomy | 38 (1.1) | 2 (0.4) | - |
| None of the above | 3287 (97.8) | 535 (98) | - |
| Cardiopulmonary Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Mitral valve defect | 547 (16.3) | 30 (5.5) | 2.96 (2.07–4.23), <0.0001 | 2880 (0.8) | 20.03 (18.40–21.81), <0.0001 |
| Other arrhythmia | 485 (14.4) | 53 (9.7) | 1.49 (1.14–1.95), ns | - | - |
| Supraventricular tachycardia (SVT) | 263 (7.8) | 20 (3.7) | 2.14 (1.37–3.34), ns | 32,080 (9.1) | 0.86 (0.77–0.97), ns |
| Tricuspid valve defect | 201 (6) | 13 (2.4) | 2.51 (1.44–4.37), ns | 15,340 (4.3) | 1.38 (1.21–1.58), 0.0005 |
| Aortic valve defect | 111 (3.3) | 3 (0.5) | - | - | - |
| Lung disease | 99 (2.9) | 11 (2) | 1.46 (0.79–2.71), ns | 83,600 (23.6) | 0.12 (0.10–0.15), <0.0001 |
| Atrial fibrillation | 99 (2.9) | 7 (1.3) | 2.30 (1.07–4.92), ns | 26,160 (7.4) | 0.40 (0.33–0.48), <0.0001 |
| Stroke | 80 (2.4) | 5 (0.9) | 2.60 (1.06–6.39), ns | 160 (0) | 52.74 (40.41–68.82), <0.0001 |
| May-Thurner syndrome | 62 (1.8) | 6 (1.1) | 1.68 (0.73–3.86), ns | - | - |
| Nutcracker syndrome | 61 (1.8) | 4 (0.7) | - | - | - |
| Pulmonary valve defect | 60 (1.8) | 3 (0.5) | - | - | - |
| Patent foramen ovale (PFO) | 51 (1.5) | 4 (0.7) | - | - | - |
| Heart failure | 44 (1.3) | 2 (0.4) | - | - | - |
| Coronary artery disease | 36 (1.1) | 2 (0.4) | - | - | - |
| Aortic aneurysm | 32 (1) | 0 (0) | - | - | - |
| Atrial septal defect (ASD) (not PFO) | 24 (0.7) | 1 (0.2) | - | - | - |
| Myocardial infarction | 17 (0.5) | 2 (0.4) | - | - | - |
| Hypertrophic cardiomyopathy | 15 (0.4) | 2 (0.4) | - | - | - |
| Rheumatic heart disease | 5 (0.1) | 0 (0) | - | - | - |
| Spontaneous Coronary Artery Dissection (SCAD) | 4 (0.1) | 0 (0) | - | - | - |
| Ebstein’s anomaly | 2 (0.1) | 1 (0.2) | - | - | - |
| None of the above | 2083 (62) | 431 (78.9) | 0.79 (0.75–0.83), <0.0001 | - | - |
| Endocrinological Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Hypothyroidism (non-autoimmune) | 389 (11.6) | 49 (9) | 1.29 (0.97–1.71), ns | 36,920 (10.4) | 1.11 (1.01–1.22), ns |
| Osteopenia | 354 (10.5) | 42 (7.7) | 1.37 (1.01–1.86), ns | 3220 (0.9) | 11.60 (10.45–12.87), <0.0001 |
| Hashimoto’s disease | 231 (6.9) | 30 (5.5) | 1.25 (0.86–1.81), ns | 2880 (0.8) | 8.46 (7.43–9.63), <0.0001 |
| Osteoporosis | 189 (5.6) | 18 (3.3) | 1.71 (1.06–2.74), ns | 32,160 (9.1) | 0.62 (0.54–0.71), <0.0001 |
| Adrenal insufficiency (non-autoimmune) | 104 (3.1) | 18 (3.3) | 0.94 (0.57–1.54), ns | 2240 (0.6) | 4.90 (4.03–5.94), <0.0001 |
| Hyperthyroidism (non-autoimmune) | 83 (2.5) | 6 (1.1) | 2.25 (0.99–5.12), ns | 740 (0.2) | 11.83 (9.45–14.81), <0.0001 |
| Diabetes, type 2 | 82 (2.4) | 14 (2.6) | 0.95 (0.54–1.67), ns | 74,920 (21.1) | 0.12 (0.09–0.14), <0.0001 |
| Diabetes, gestational | 77 (2.3) | 7 (1.3) | 1.79 (0.83–3.85), ns | 3180 (0.9) | 2.55 (2.04–3.19), <0.0001 |
| Pineal cyst | 62 (1.8) | 5 (0.9) | 2.02 (0.81–4.99), ns | 40 (0) | 163.49 (110.02–242.93), <0.0001 |
| Pituitary tumor/adenoma | 56 (1.7) | 6 (1.1) | 1.52 (0.66–3.50), ns | 200 (0.1) | 29.53 (22.00–39.64), <0.0001 |
| Grave’s disease | 33 (1) | 6 (1.1) | 0.89 (0.38–2.12), ns | 300 (0.1) | 11.60 (8.11–16.59), <0.0001 |
| Hyperparathyroidism | 27 (0.8) | 5 (0.9) | 0.88 (0.34–2.27), ns | 10,560 (3) | 0.27 (0.19–0.39), <0.0001 |
| Diabetes, type 1 | 23 (0.7) | 1 (0.2) | - | - | - |
| Cushing disease | 22 (0.7) | 1 (0.2) | - | - | - |
| Addison’s disease | 12 (0.4) | 1 (0.2) | - | - | - |
| Congenital adrenal hyperplasia | 9 (0.3) | 1 (0.2) | - | - | - |
| Multiple endocrine neoplasia | 2 (0.1) | 0 (0) | - | - | - |
| None of the above | 2485 (74) | 435 (79.7) | - | - | - |
| Hematological Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Pernicious anemia | 155 (4.6) | 14 (2.6) | 1.80 (1.05–3.09), ns | 1180 (0.3) | 13.85 (11.76–16.32), <0.0001 |
| Deep vein thrombosis (DVT) | 116 (3.5) | 6 (1.1) | 3.14 (1.39–7.10), ns | 11,440 (3.2) | 1.07 (0.89–1.28), ns |
| Pulmonary embolism (PE) | 71 (2.1) | 8 (1.5) | 1.44 (0.70–2.98), ns | 9940 (2.8) | 0.75 (0.60–0.95), ns |
| Lymphedema | 63 (1.9) | 6 (1.1) | 1.71 (0.74–3.92), ns | 6560 (1.9) | 1.01 (0.79–1.30), ns |
| Thrombocytosis | 41 (1.2) | 5 (0.9) | 1.33 (0.53–3.36), ns | 3700 (1) | 1.17 (0.86–1.59), ns |
| Antiphospholipid antibody syndrome (APLS) | 37 (1.1) | 1 (0.2) | - | - | - |
| Von Willebrand disease | 33 (1) | 4 (0.7) | - | - | - |
| Hemophilia | 15 (0.4) | 2 (0.4) | - | - | - |
| Arterial thrombosis | 6 (0.2) | 0 (0) | - | - | - |
| None of the above | 2971 (88.4) | 510 (93.4) | - | - | - |
| Easy or severe bruising | 2696 (80.2) | 337 (61.7) | 1.30 (1.21–1.39), <0.0001 | - | - |
| Reproductive Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Endometriosis | 622 (18.5) | 66 (12.1) | 1.53 (1.21–1.94), ns | 7620 (2.2) | 8.61 (7.99–9.27), <0.0001 |
| Polycystic ovary syndrome (PCOS) | 571 (17) | 80 (14.7) | 1.16 (0.93–1.44), ns | 5000 (1.4) | 12.05 (11.12–13.04), <0.0001 |
| Pelvic organ prolapse | 455 (13.5) | 19 (3.5) | 3.89 (2.48–6.10), <0.0001 | 10,020 (2.8) | 4.79 (4.39–5.23), <0.0001 |
| Vaginismus | 246 (7.3) | 28 (5.1) | 1.43 (0.98–2.09), ns | 320 (0.1) | 81.08 (68.91–95.41), <0.0001 |
| Vulvodynia | 231 (6.9) | 30 (5.5) | 1.25 (0.86–1.81), ns | 620 (0.2) | 39.30 (33.92–45.53), <0.0001 |
| Infertility | 198 (5.9) | 36 (6.6) | 0.89 (0.63–1.26), ns | 7340 (2.1) | 2.85 (2.48–3.26), <0.0001 |
| Pelvic congestion syndrome | 101 (3) | 10 (1.8) | 1.64 (0.86–3.12), ns | 260 (0.1) | 40.97 (32.64–51.43), <0.0001 |
| Hypogonadism | 11 (0.3) | 3 (0.5) | - | - | - |
| Erectile dysfunction | 10 (0.3) | 3 (0.5) | - | - | - |
| Enlarged prostate gland | 7 (0.2) | 0 (0) | - | - | - |
| Premature ejaculation | 3 (0.1) | 2 (0.4) | - | - | - |
| Peyronie’s disease | 1 (0) | 0 (0) | - | - | - |
| Testicular torsion | 1 (0) | 0 (0) | - | - | - |
| Penile fracture | 1 (0) | 0 (0) | - | - | - |
| Cryptorchidism | 0 (0) | 1 (0.2) | - | - | - |
| Reproductive Symptoms, Menstruation, and Pregnancy | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Pelvic pain | 2701 (80.4) | 361 (66.1) | 1.22 (1.14–1.29), <0.0001 |
| Irregular periods | 2363 (70.3) | 310 (56.8) | 1.24 (1.15–1.34), <0.0001 |
| Pain during sex | 2130 (63.4) | 274 (50.2) | 1.26 (1.16–1.38), <0.0001 |
| Bleeding during sex | 1285 (38.2) | 139 (25.5) | 1.50 (1.29–1.75), <0.0001 |
| Genital overstimulation | 594 (17.7) | 65 (11.9) | 1.49 (1.17–1.89), ns |
| Tight foreskin | 17 (0.5) | 4 (0.7) | - |
| None of the above | 247 (7.4) | 81 (14.8) | 0.50 (0.39–0.63), <0.0001 |
| Age of first menstrual period *‡ | 12.3 (±1.7) | 12.4 (±1.5) | (−0.11–0.18), ns |
| Have been pregnant * | 1463 (44.5) | 186 (35.1) | - |
| Average number of pregnancies ** | 2.5 (±1.4) | 2.3 (±1.2) | - |
| Had pregnancy complications ** | 983 (67.2) | 102 (54.8) | 1.23 (1.07–1.40), <0.0001 |
| Pregnancy complication types ** | |||
| Preterm labor | 339 (23.2) | 24 (12.9) | 1.80 (1.22–2.64), 0.0111 |
| Spontaneous abortion | 583 (39.8) | 57 (30.6) | 1.30 (1.04–1.63), 0.0247 |
| Prelabor membrane rupture | 178 (23.2) | 20 (10.8) | 1.13 (0.73–1.75), ns |
| Failure to progress in labor | 320 (21.9) | 38 (20.4) | 1.07 (0.79–1.44), <0.0001 |
| Stillbirth | 40 (2.7) | 4 (2.2) | - |
| None of the above | 480 (32.8) | 84 (45.2) | 0.73 (0.61–0.87), <0.0001 |
| Average number of spontaneous abortions *** | 1.5 (±0.7) | 1.4 (±0.7) | (−0.28–0.10), ns |
| Urinary Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Recurrent urinary tract infections (UTIs) | 980 (29.2) | 98 (17.9) | 1.63 (1.35–1.96), <0.0001 | 740 (0.2) | 139.68 (127.76–152.72), <0.0001 |
| Urinary incontinence | 711 (21.2) | 61 (11.2) | 1.89 (1.48–2.42), <0.0001 | 38,020 (10.7) | 1.97 (1.85–2.11), <0.0001 |
| Voiding dysfunction | 500 (14.9) | 43 (7.9) | 1.89 (1.40–2.55), 0.0065 | 60 (0) | 878.97 (673.93–1146.39), <0.0001 |
| Kidney stone(s) | 450 (13.4) | 39 (7.1) | 1.88 (1.37–2.57), 0.0240 | 25,060 (7.1) | 1.89 (1.74–2.07), <0.0001 |
| Overactive bladder syndrome | 441 (13.1) | 39 (7.1) | 1.84 (1.34–2.52), 0.0439 | 8580 (2.4) | 5.42 (4.96–5.93), <0.0001 |
| Bladder pain syndrome | 215 (6.4) | 20 (3.7) | 1.75 (1.11–2.74), ns | 1520 (0.4) | 14.92 (12.99–17.14), <0.0001 |
| Urinary hesitancy | 196 (5.8) | 18 (3.3) | 1.77 (1.10–2.84), ns | 3500 (1) | 5.91 (5.14–6.79), <0.0001 |
| Kidney disease | 85 (2.5) | 9 (1.6) | 1.53 (0.78–3.03), ns | 87,560 (24.7) | 0.10 (0.08–0.13), <0.0001 |
| Vesicoureteral reflux | 27 (0.8) | 1 (0.2) | - | - | - |
| None of the above | 1597 (47.5) | 353 (64.7) | 0.74 (0.68–0.79), <0.0001 | - | - |
| Dermatological Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Atopic dermatitis (eczema) | 1052 (31.3) | 158 (28.9) | 1.08 (0.94–1.25), ns | 9540 (2.7) | 11.63 (11.02–12.27), <0.0001 |
| Hyperhidrosis | 223 (6.6) | 21 (3.8) | 1.73 (1.11–2.67), ns | 12,280 (3.5) | 1.92 (1.69–2.18), <0.0001 |
| Hidradenitis suppurativa | 93 (2.8) | 10 (1.8) | 1.51 (0.79–2.88), ns | 2640 (0.7) | 3.72 (3.03–4.56), <0.0001 |
| Hypohidrosis | 36 (1.1) | 3 (0.5) | - | - | - |
| Acrogeria | 1 (0) | 0 (0) | - | - | - |
| None of the above | 1993 (59.3) | 352 (64.5) | 0.92 (0.86–0.99), ns | - | - |
| Dermatological Symptoms | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Soft and velvety skin | 3059 (91) | 332 (60.8) | 1.50 (1.40–1.60), <0.0001 |
| Abnormally stretchy skin | 2602 (77.4) | 178 (32.6) | 2.38 (2.10–2.68), <0.0001 |
| Unexplained stretch marks | 2473 (73.6) | 205 (37.5) | 1.96 (1.76–2.19), <0.0001 |
| Poor wound healing | 2445 (72.8) | 266 (48.7) | 1.49 (1.37–1.63), <0.0001 |
| Atrophic scarring | 2114 (62.9) | 149 (27.3) | 2.31 (2.01–2.65), <0.0001 |
| Keratosis pilaris | 1820 (54.2) | 250 (45.8) | 1.18 (1.07–1.30), ns |
| Recurrent Hives (urticaria) | 1636 (48.7) | 182 (33.3) | 1.46 (1.29–1.65), <0.0001 |
| Hypertrophic scarring | 1331 (39.6) | 147 (26.9) | 1.47 (1.27–1.70), <0.0001 |
| Recurrent folliculitis or abscesses | 1212 (36.1) | 132 (24.2) | 1.49 (1.28–1.74), <0.0001 |
| Acne (if 30+ years old) | 979 (29.1) | 133 (24.4) | 1.20 (1.02–1.40), ns |
| Keloid scarring | 751 (22.4) | 67 (12.3) | 1.82 (1.44–2.30), <0.0001 |
| None of the above | 12 (0.4) | 20 (3.7) | 0.10 (0.05–0.20), <0.0001 |
| Allergy and Immune-Related Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Allergies | 2707 (80.6) | 379 (69.4) | 1.16 (1.10–1.23), <0.0001 | 24,160 (6.8) | 11.82 (11.58–12.06), <0.0001 |
| Asthma | 1415 (42.1) | 163 (29.9) | 1.41 (1.23–1.61), <0.0001 | 65,180 (18.4) | 2.29 (2.20–2.38), <0.0001 |
| Mast cell activation syndrome (MCAS) | 1003 (29.9) | 66 (12.1) | 2.47 (1.96–3.11), <0.0001 | 100 (0) | 1057.93 (863.81–1295.66), <0.0001 |
| Chronic sinusitis | 899 (26.8) | 94 (17.2) | 1.55 (1.28–1.88), 0.0011 | 40,320 (11.4) | 2.35 (2.22–2.49), <0.0001 |
| Chronic urticaria | 493 (14.7) | 26 (4.8) | 3.08 (2.10–4.52), <0.0001 | 220 (0.1) | 236.36 (202.38–276.06), <0.0001 |
| Histamine intolerance | 365 (10.9) | 36 (6.6) | 1.65 (1.18–2.29), ns | - | - |
| Immune deficiency | 243 (7.2) | 24 (4.4) | 1.65 (1.09–2.48), ns | - | - |
| Nasal polyps | 236 (7) | 20 (3.7) | 1.92 (1.23–3.00), ns | 880 (0.2) | 28.29 (24.60–32.52), <0.0001 |
| Cold urticaria | 170 (5.1) | 10 (1.8) | 2.76 (1.47–5.19), ns | 160 (0) | 112.07 (90.55–138.70), <0.0001 |
| Systemic mastocytosis | 19 (0.6) | 1 (0.2) | - | - | - |
| None of the above | 346 (10.3) | 120 (22) | 0.47 (0.39–0.56), <0.0001 | - | - |
| Types of Allergies | - | - | |||
| Seasonal | 1938 (57.7) | 248 (45.4) | 1.27 (1.15–1.40), <0.0001 | - | - |
| Drug | 1529 (45.5) | 166 (30.4) | 1.50 (1.31–1.71), <0.0001 | - | - |
| Environmental | 1435 (42.7) | 174 (31.9) | 1.34 (1.18–1.52), 0.0010 | - | - |
| Food | 1169 (34.8) | 142 (26) | 1.34 (1.15–1.55), 0.0285 | - | - |
| Metal | 510 (15.2) | 42 (7.7) | 1.97 (1.46–2.67), 0.0018 | - | - |
| Latex | 477 (14.2) | 40 (7.3) | 1.94 (1.42–2.64), 0.0064 | - | - |
| Anaphylaxis Episodes | 986 (29.3) | 84 (15.4) | 1.91 (1.56–2.34), <0.0001 | - | - |
| Psoriasis | 221 (6.6) | 21 (3.8) | 1.71 (1.10–2.65), ns | 10,780 (3) | 2.16 (1.90–2.46), <0.0001 |
| Lyme disease | 154 (4.6) | 20 (3.7) | 1.25 (0.79–1.98), ns | 3120 (0.9) | 5.21 (4.44–6.10), <0.0001 |
| Cancer | 143 (4.3) | 16 (2.9) | 1.45 (0.87–2.42), ns | 65,980 (18.6) | 0.23 (0.19–0.27), <0.0001 |
| Rheumatoid arthritis (RA) | 137 (4.1) | 19 (3.5) | 1.17 (0.73–1.88), ns | 11,380 (3.2) | 1.27 (1.08–1.50), ns |
| Sjögren syndrome | 127 (3.8) | 14 (2.6) | 1.47 (0.86–2.54), ns | 5600 (1.6) | 2.39 (2.01–2.84), <0.0001 |
| Ankylosing spondylitis | 73 (2.2) | 11 (2) | 1.08 (0.58–2.02), ns | 1360 (0.4) | 5.66 (4.48–7.15), <0.0001 |
| Systemic lupus erythematosus (SLE) | 62 (1.8) | 6 (1.1) | 1.68 (0.73–3.86), ns | 5020 (1.4) | 1.30 (1.02–1.67), ns |
| Scleroderma | 15 (0.4) | 2 (0.4) | - | - | - |
| Other | 168 (5) | 25 (4.6) | 1.09 (0.72–1.65), ns | - | - |
| None of the above | 2608 (77.6) | 452 (82.8) | 0.94 (0.90–0.98), ns | - | - |
| Ocular Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Astigmatism | 2139 (63.7) | 280 (51.3) | 1.24 (1.14–1.35), <0.0001 | 30,440 (8.6) | 7.41 (7.21–7.62), <0.0001 |
| Myopia | 1904 (56.7) | 276 (50.5) | 1.12 (1.03–1.22), ns | 29,380 (8.3) | 6.84 (6.62–7.05), <0.0001 |
| Hyperopia | 669 (19.9) | 75 (13.7) | 1.45 (1.16–1.81), ns | 14,840 (4.2) | 4.75 (4.44–5.10), <0.0001 |
| Macular degeneration | 69 (2.1) | 6 (1.1) | 1.87 (0.82–4.28), ns | 3160 (0.9) | 2.30 (1.82–2.92), <0.0001 |
| Keratoconus | 42 (1.3) | 5 (0.9) | 1.37 (0.54–3.43), ns | 760 (0.2) | 5.83 (4.28–7.94), <0.0001 |
| Retinal detachment | 43 (1.3) | 6 (1.1) | 1.16 (0.50–2.72), ns | 4900 (1.4) | 0.93 (0.69–1.25), ns |
| Blue sclerae | 554 (16.5) | 19 (3.5) | - | - | - |
| Lens subluxation | 21 (0.6) | 1 (0.2) | - | - | - |
| Lens dislocation | 5 (0.1) | 0 (0) | - | - | - |
| None of the above | 600 (17.9) | 149 (27.3) | - | - | - |
| Ocular Symptoms | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Light sensitivity | 2971 (88.4) | 434 (79.5) | 1.11 (1.06–1.16), <0.0001 |
| Visual disturbances | 2802 (83.4) | 400 (73.3) | 1.14 (1.08–1.20), <0.0001 |
| Dry eyes | 2307 (68.7) | 338 (61.9) | 1.11 (1.03–1.19), ns |
| Double vision | 1606 (47.8) | 163 (29.9) | 1.60 (1.40–1.83), <0.0001 |
| Loss of peripheral vision | 709 (21.1) | 59 (10.8) | 1.95 (1.52–2.51), <0.0001 |
| None of the above | 106 (3.2) | 37 (6.8) | 0.47 (0.32–0.67), 0.0208 |
| Dental Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Temporomandibular joint disorder (TMJ disorder) | 1660 (49.4) | 177 (32.4) | 1.52 (1.34–1.73), <0.0001 | 11,120 (3.1) | 15.75 (15.15–16.37), <0.0001 |
| Enamel defects | 661 (19.7) | 68 (12.5) | 1.58 (1.25–1.99), 0.0319 | 300 (0.1) | 232.40 (203.63–265.23), <0.0001 |
| Early onset periodontitis | 580 (17.3) | 64 (11.7) | 1.47 (1.16–1.88), ns | 340 (0.1) | 179.93 (158.08–204.81), <0.0001 |
| None of the above | 1302 (38.8) | 310 (56.8) | 0.68 (0.63–0.74), <0.0001 | - | - |
| Dental Symptoms | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † |
|---|---|---|---|
| Jaw pain | 2753 (81.9) | 375 (68.7) | 1.19 (1.12–1.27), <0.0001 |
| Dental crowding | 2358 (70.2) | 232 (42.5) | 1.65 (1.49–1.83), <0.0001 |
| Subluxation or dislocation of TMJ | 2321 (69.1) | 261 (47.8) | 1.45 (1.32–1.58), <0.0001 |
| Tooth sensitivity without other cause | 2169 (64.6) | 272 (49.8) | 1.30 (1.19–1.41), <0.0001 |
| High or narrow palate | 2000 (59.5) | 152 (27.8) | 2.14 (1.86–2.45), <0.0001 |
| Frequent cavities | 1547 (46) | 172 (31.5) | 1.46 (1.28–1.66), <0.0001 |
| None of the above | 41 (1.2) | 37 (6.8) | 0.18 (0.12–0.28), <0.0001 |
| Mental Health and Sleep Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Anxiety | 2512 (74.8) | 395 (72.3) | 1.03 (0.98–1.09), ns | 112,320 (31.7) | 2.36 (2.31–2.41), <0.0001 |
| Depression | 2285 (68) | 352 (64.5) | 1.05 (0.99–1.13), ns | 112,440 (31.7) | 2.14 (2.09–2.19), <0.0001 |
| Post-traumatic stress disorder (PTSD) | 1388 (41.3) | 150 (27.5) | 1.50 (1.30–1.73), <0.0001 | 21,540 (6.1) | 6.80 (6.51–7.09), <0.0001 |
| Insomnia | 1281 (38.1) | 148 (27.1) | 1.41 (1.22–1.62), 0.0004 | 56,320 (15.9) | 2.40 (2.30–2.51), <0.0001 |
| Panic disorder | 602 (17.9) | 74 (13.6) | 1.32 (1.06–1.65), ns | 14,260 (4) | 4.45 (4.13–4.80), <0.0001 |
| Eating disorder | 592 (17.6) | 77 (14.1) | 1.25 (1.00–1.56), ns | 4820 (1.4) | 12.95 (11.98–14.01), <0.0001 |
| Restless leg syndrome | 553 (16.5) | 56 (10.3) | 1.60 (1.24–2.08), ns | 10,860 (3.1) | 5.37 (4.97–5.81), <0.0001 |
| Obstructive sleep apnea | 427 (12.7) | 51 (9.3) | 1.36 (1.03–1.79), ns | 60,340 (17) | 0.75 (0.68–0.82), <0.0001 |
| Bipolar disorder | 230 (6.8) | 13 (2.4) | 2.88 (1.66–4.99), 0.0386 | 20,540 (5.8) | 1.18 (1.04–1.34), ns |
| Substance use disorder | 96 (2.9) | 15 (2.7) | 1.04 (0.61–1.78), ns | 57,920 (16.3) | 0.17 (0.14–0.21), <0.0001 |
| None of the above | 409 (12.2) | 95 (17.4) | 0.70 (0.57–0.86), ns | - | - |
| Neurodiversity-Related Disorders | hEDS n (%) | HSD n (%) | hEDS vs. HSD RR (95% CI), p-Value † | All of Us n (%) | hEDS vs. All of Us RR (95% CI), p-Value † |
|---|---|---|---|---|---|
| Attention deficit hyperactivity disorder (ADHD/ADD) | 1172 (34.9) | 156 (28.6) | 1.22 (1.06–1.40), ns | 13,740 (3.9) | 9.00 (8.57–9.45), <0.0001 |
| Autism spectrum disorder (ASD) | 466 (13.9) | 58 (10.6) | 1.31 (1.01–1.69), ns | 1060 (0.3) | 46.37 (41.81–51.43), <0.0001 |
| Obsessive–compulsive disorder (OCD) | 466 (13.9) | 45 (8.2) | 1.68 (1.26–2.25), ns | 2860 (0.8) | 17.19 (15.68–18.84), <0.0001 |
| Sensory processing disorder | 306 (9.1) | 34 (6.2) | - | - | - |
| Learning disorder | 299 (8.9) | 37 (6.8) | 1.31 (0.94–1.83), ns | 880 (0.2) | 35.84 (31.57–40.68), <0.0001 |
| Tourette’s syndrome | 38 (1.1) | 4 (0.7) | - | - | - |
| None of the above | 1710 (50.9) | 329 (60.3) | 0.84 (0.78–0.91), 0.0247 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daylor, V.; Griggs, M.; Weintraub, A.; Byrd, R.; Petrucci, T.; Huff, M.; Byerly, K.; Fenner, R.; Severance, S.; Griggs, C.; et al. Defining the Chronic Complexities of hEDS and HSD: A Global Survey of Diagnostic Challenges, Life-Long Comorbidities, and Unmet Needs. J. Clin. Med. 2025, 14, 5636. https://doi.org/10.3390/jcm14165636
Daylor V, Griggs M, Weintraub A, Byrd R, Petrucci T, Huff M, Byerly K, Fenner R, Severance S, Griggs C, et al. Defining the Chronic Complexities of hEDS and HSD: A Global Survey of Diagnostic Challenges, Life-Long Comorbidities, and Unmet Needs. Journal of Clinical Medicine. 2025; 14(16):5636. https://doi.org/10.3390/jcm14165636
Chicago/Turabian StyleDaylor, Victoria, Molly Griggs, Amy Weintraub, Rebecca Byrd, Taylor Petrucci, Matthew Huff, Kathryn Byerly, Roman Fenner, Sydney Severance, Charlotte Griggs, and et al. 2025. "Defining the Chronic Complexities of hEDS and HSD: A Global Survey of Diagnostic Challenges, Life-Long Comorbidities, and Unmet Needs" Journal of Clinical Medicine 14, no. 16: 5636. https://doi.org/10.3390/jcm14165636
APA StyleDaylor, V., Griggs, M., Weintraub, A., Byrd, R., Petrucci, T., Huff, M., Byerly, K., Fenner, R., Severance, S., Griggs, C., Sharma, A., Atwal, P., Kautz, S. A., Shapiro, S., Youkhana, K., Lavallee, M., Wilkerson, A., Nichols, M., Snyder, A., ... Norris, R. A. (2025). Defining the Chronic Complexities of hEDS and HSD: A Global Survey of Diagnostic Challenges, Life-Long Comorbidities, and Unmet Needs. Journal of Clinical Medicine, 14(16), 5636. https://doi.org/10.3390/jcm14165636





