GLP-1 Receptor Agonists and Gastrointestinal Endoscopy: A Narrative Review of Risks, Management Strategies, and the Need for Clinical Consensus
Abstract
1. Clinical and Pharmacological Introduction
2. Material and Methods for the Review of the Existing Evidence
2.1. Review Design and Registration
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Quality and Bias Assessment
2.7. Data Synthesis
2.8. Limitations of the Evidence Base
3. Evidence on Gastric Emptying Delay and Residual Gastric Content
3.1. Physiological Basis
3.2. Experimental and Imaging Data
3.3. Observational Clinical Evidence
3.4. Evidence from Systematic Reviews and Meta-Analyses
3.5. Modifying Factors
Clinical Bottom Line
4. Risk of Aspiration and Endoscopic Complications
5. Colonoscopy and Capsule Endoscopy
5.1. From Gastric Motor Inhibition to Colonic Cleansing Failure
5.2. Clinical Studies of Bowel Preparation Quality
5.3. Downstream Consequences
5.4. Optimizing Preparation Without Universal Drug Suspension
5.5. Capsule Endoscopy
5.6. Practical Recommendations
6. Summary of Clinical Guidelines and Positions
6.1. The Precautionary Era: American Society of Anesthesiologists (ASA)
6.2. Gastroenterology Push-Back: AGA Rapid Update
6.3. Procedural Nuance: American Society for Gastrointestinal Endoscopy (ASGE)
6.4. Multidisciplinary Convergence: Kindel and Colleagues
6.5. European and North American Anesthesia Perspectives
6.6. Key Areas of Agreement and Divergence
Clinical Bottom Line
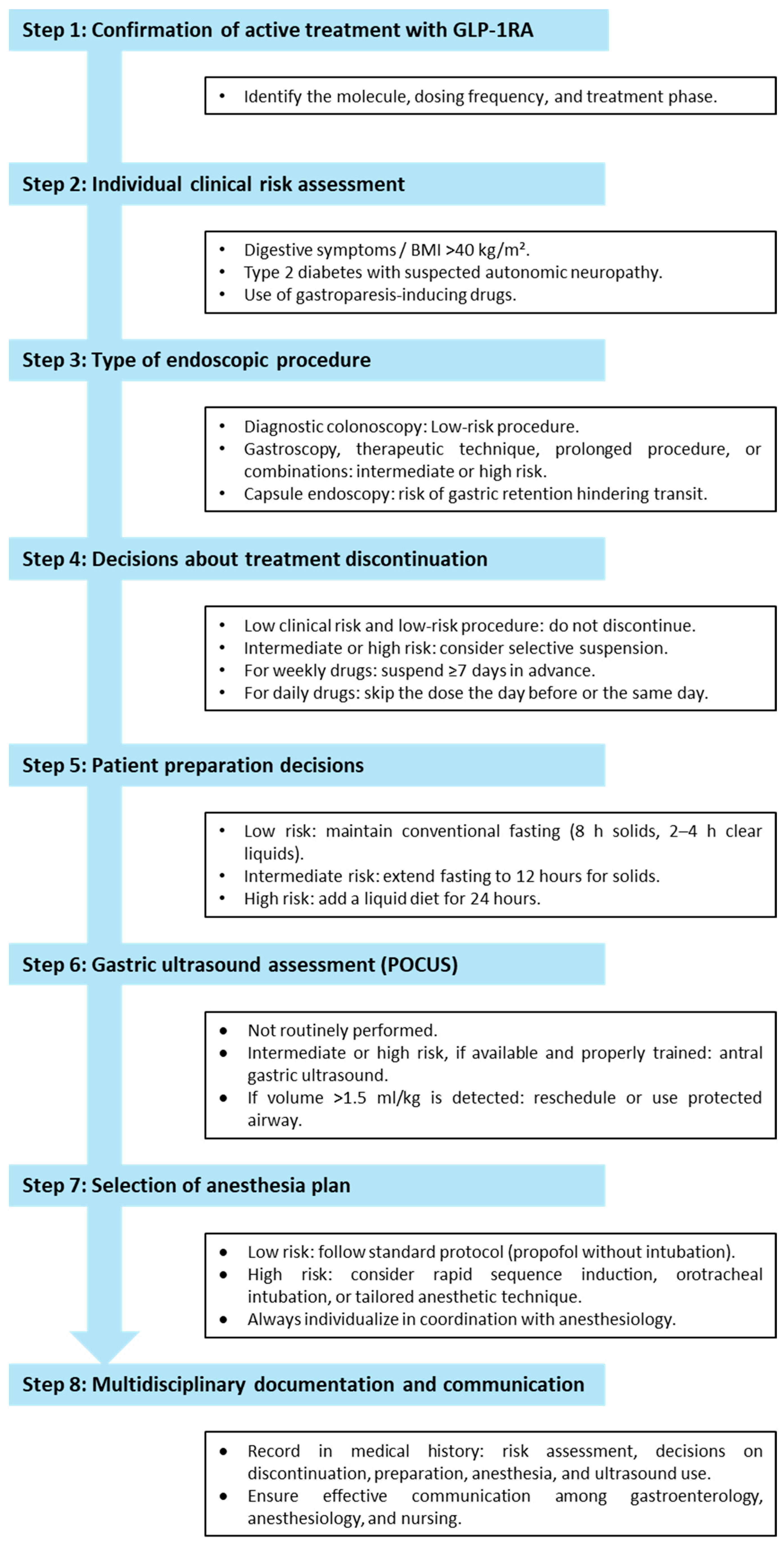
7. Integrated Clinical Decision Algorithm
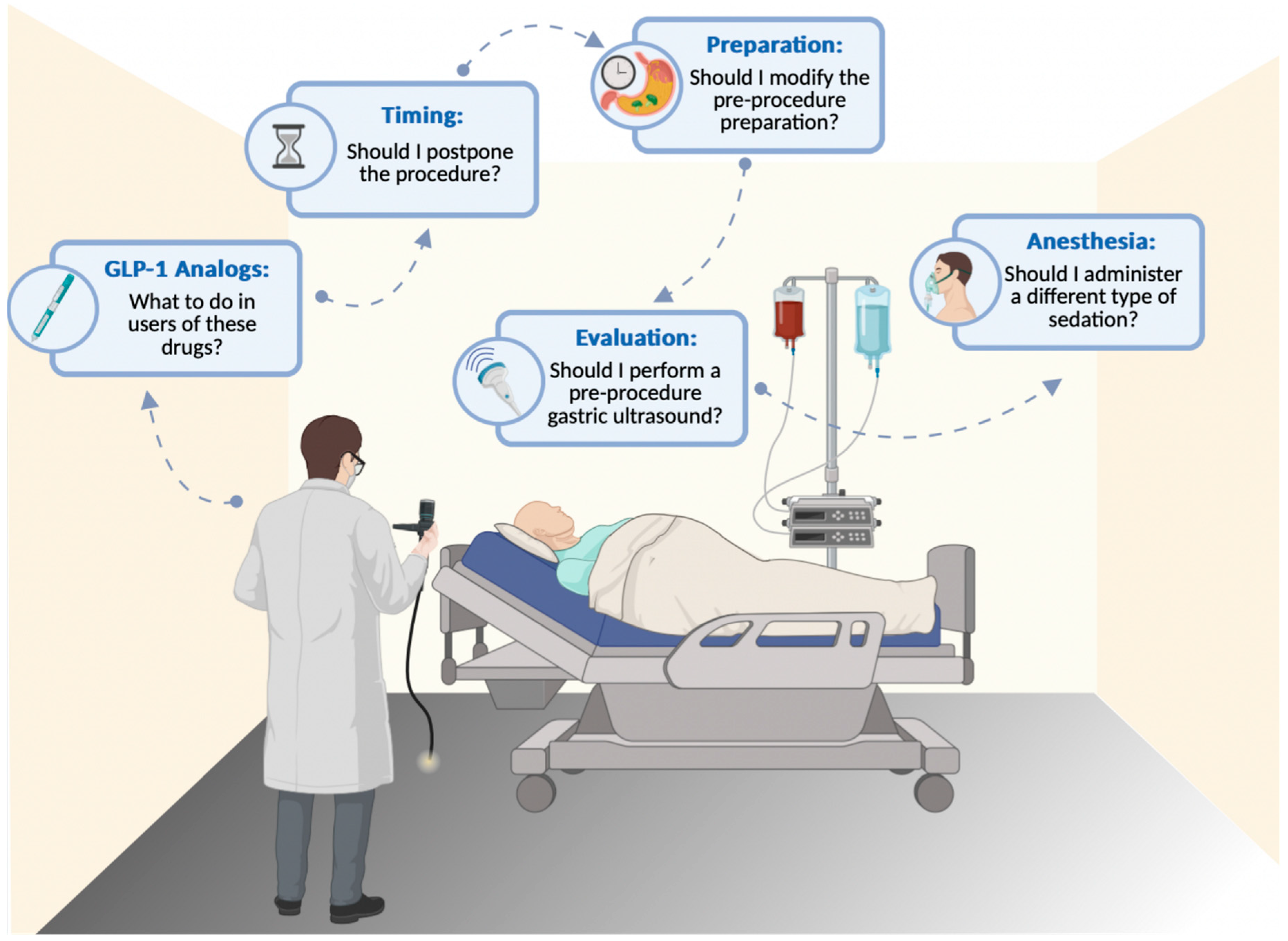
8. Key Clinical Questions and Evidence-Based Recommendations
Clinical Bottom Line
9. Need for Training and Changes in Endoscopic Practice
9.1. Competency-Based Curricula
9.2. Simulation and Team Rehearsal
9.3. Training in Bedside Gastric Ultrasound (POCUS)
9.4. Sedation, Airway Management, and Interdisciplinary Communication
9.5. Patient Education and Digital Engagement Tools
9.6. Workflow and Systems Redesign
9.7. Research and Accreditation Implications
Clinical Bottom Line
10. Conclusions
- (1)
- The adoption of extended clear-liquid fasting (≥24 h), complemented by selective point-of-care gastric ultrasonography in patients reporting upper gastrointestinal symptoms or undergoing high-risk procedures that involve airway sharing. This dual-pronged approach enables the safe continuation of GLP-1 RAs in the vast majority of cases while reserving drug cessation (≥7 days) for a narrowly defined subset, namely, those with recent high-dose exposure, significant residual content on ultrasound, or florid symptoms suggestive of impaired gastric motility.
- (2)
- The prioritization of intensified bowel preparation regimens (particularly split-dose polyethylene glycol solutions in high volume, optionally augmented with stimulant laxatives) over indiscriminate drug withdrawal. Such regimens have been shown to restore colonic cleansing efficacy without compromising metabolic control, thereby safeguarding colonoscopy quality while avoiding glycemic rebound or weight regain [23].
- (3)
- The implementation of targeted training and service reconfiguration. This includes the formal integration of pharmacology-specific fasting algorithms, structured ultrasound training, and rapid-sequence induction competencies into endoscopic curricula, alongside the deployment of digital engagement tools designed to support patient adherence to prolonged fasting protocols. These structural adaptations not only enhance patient safety but also sustain throughput and operational efficiency.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AASLD | American Association for the Study of Liver Diseases |
| AGA | American Gastroenterological Association |
| ASA | American Society of Anesthesiologists |
| ASGE | American Society for Gastrointestinal Endoscopy |
| BMI | Body mass index |
| ESGE | European Society for Gastrointestinal Endoscopy |
| GLP-1 RAs | GLP-1 receptor agonists |
| NNH | Number needed to harm |
| NNT | Number needed to treat |
| POCUS | Point-of-care ultrasound |
| RGC | Residual gastric content |
| T2DM | Type 2 diabetes mellitus |
References
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Madsbad, S.; Kielgast, U.; Asmar, M.; Deacon, C.F.; Torekov, S.S.; Holst, J.J. An overview of once-weekly glucagon-like peptide-1 receptor agonists—Available efficacy and safety data and perspectives for the future. Diabetes Obes. Metab. 2011, 13, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Nauck, M.A. Glucagon-like peptide 1 (GLP-1) in biology and pathology. Diabetes Metab. Res. Rev. 2005, 21, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Glucagon-like peptides 1 and 2 in health and disease: A review. Peptides 2013, 44, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, B.; McCarty, T.R.; Lodhia, N.A.; Jenkins, A.; Elnaiem, A.; Muftah, M.; Flanagan, R.; Chan, W.W. Quantified Metrics of Gastric Emptying Delay by Glucagon-Like Peptide-1 Agonists: A Systematic Review and Meta-Analysis With Insights for Periprocedural Management. Am. J. Gastroenterol. 2024, 119, 1126–1140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jalleh, R.J.; Plummer, M.P.; Marathe, C.S.; Umapathysivam, M.M.; Quast, D.R.; Rayner, C.K.; Jones, K.L.; Wu, T.; Horowitz, M.; Nauck, M.A. Clinical Consequences of Delayed Gastric Emptying with GLP-1 Receptor Agonists and Tirzepatide. J. Clin. Endocrinol. Metab. 2024, 110, 1–15, Erratum in J. Clin. Endocrinol. Metab. 2025, dgaf387. https://doi.org/10.1210/clinem/dgaf387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sherwin, M.; Hamburger, J.; Katz, D.; DeMaria, S., Jr. Influence of semaglutide use on the presence of residual gastric solids on gastric ultrasound: A prospective observational study in volunteers without obesity recently started on semaglutide. Can. J. Anaesth. 2023, 70, 1300–1306. (In English) [Google Scholar] [CrossRef] [PubMed]
- Abdulraheem, A.; Abujaber, B.; Ayers, L.; Al-Zureikat, Q.; Bashiri, K.; Almhanni, G.; Altork, N.; Afzal, U.; Qarkash, D.; Spyros, P.; et al. Impact of GLP-1 receptor agonists on upper gastrointestinal endoscopy: An updated systematic review and meta-analysis. Surg. Endosc. 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.U.; Piazza, A.; Lahooti, A.; Johnson, K.E.; Rangwani, S.; Gouda, Z.; Mahadev, S.; Newberry, C.; Hanscom, M.; Sampath, K.; et al. Glucagon-like peptide-1 receptor agonist use and the risk of residual gastric contents and aspiration in patients undergoing GI endoscopy: A systematic review and a meta-analysis. Gastrointest. Endosc. 2025, 101, 762–771.e13. [Google Scholar] [CrossRef] [PubMed]
- Elkin, J.; Rele, S.; Sumithran, P.; Hii, M.; Thuraisingam, S.; Spelman, T.; Phan, T.; Choong, P.; Dowsey, M.; Shadbolt, C. Association between glucagon-like peptide-1 receptor agonist use and peri-operative pulmonary aspiration: A systematic review and meta-analysis. Anaesthesia 2025, 80, 846–858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santos, L.B.; Mizubuti, G.B.; da Silva, L.M.; Silveira, S.Q.; Nersessian, R.S.F.; Abib, A.C.V.; Bellicieri, F.N.; Lima, H.O.; Ho, A.M.; Dos Anjos, G.S.; et al. Effect of various perioperative semaglutide interruption intervals on residual gastric content assessed by esophagogastroduodenoscopy: A retrospective single center observational study. J. Clin. Anesth. 2024, 99, 111668. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Potnuru, P.P.; Hernandez, N.; Goehl, C.; Praestholm, C.; Sridhar, S.; Nwokolo, O.O. Glucagon-Like Peptide-1 Receptor Agonist Use and Residual Gastric Content Before Anesthesia. JAMA Surg. 2024, 159, 660–667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silveira, S.Q.; da Silva, L.M.; de Campos Vieira Abib, A.; de Moura, D.T.H.; de Moura, E.G.H.; Santos, L.B.; Ho, A.M.; Nersessian, R.S.F.; Lima, F.L.M.; Silva, M.V.; et al. Relationship between perioperative semaglutide use and residual gastric content: A retrospective analysis of patients undergoing elective upper endoscopy. J. Clin. Anesth. 2023, 87, 111091. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, D.; Taye, M.; Still, M.D.; McShea, S.; Satterfield, D.; Dove, J.T.; Wood, G.C.; Addissie, B.D.; Diehl, D.L.; Johal, A.S.; et al. Effects of glucagon-like peptide-1 receptor agonists on upper endoscopy in diabetic and nondiabetic patients. Gastrointest. Endosc. 2024, 100, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Nasser, J.; Hosseini, A.; Barlow, G.; Gianchandani, R.; Rezaie, A.; Pimentel, M.; Mathur, R. Food Retention at Endoscopy Among Adults Using Glucagon-Like Peptide-1 Receptor Agonists. JAMA Netw. Open 2024, 7, e2436783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Facciorusso, A.; Ramai, D.; Dhar, J.; Samanta, J.; Chandan, S.; Gkolfakis, P.; Crinò, S.F.; Maida, M.; Anderloni, A.; Boskoski, I.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists on Upper Gastrointestinal Endoscopy: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2025, 23, 715–725.e3. [Google Scholar] [CrossRef] [PubMed]
- Firkins, S.A.; Yates, J.; Shukla, N.; Garg, R.; Vargo, J.J.; Lembo, A.; Cleveland Clinic Obesity Medicine and Bariatric Endoscopy Working Group. Clinical Outcomes and Safety of Upper Endoscopy While on Glucagon-Like Peptide-1 Receptor Agonists. Clin. Gastroenterol. Hepatol. 2025, 23, 872–873.e3. [Google Scholar] [CrossRef] [PubMed]
- Alkabbani, W.; Suissa, K.; Gu, K.D.; Cromer, S.J.; Paik, J.M.; Bykov, K.; Hobai, I.; Thompson, C.C.; Wexler, D.J.; Patorno, E. Glucagon-like peptide-1 receptor agonists before upper gastrointestinal endoscopy and risk of pulmonary aspiration or discontinuation of procedure: Cohort study. BMJ 2024, 387, e080340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barlowe, T.S.; Anderson, C.; Sandler, R.S.; Subramaniam, D.; Muratore, A.; Buse, J.B.; Gouker, L.N.; Majithia, R.T.; Shaheen, N.J.; Stürmer, T.; et al. Glucagon-Like Peptide-1 Receptor Agonists Do Not Increase Aspiration During Upper Endoscopy in Patients With Diabetes. Clin. Gastroenterol. Hepatol. 2025, 23, 739–747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.H.; Zink, T.; Chen, Y.W.; Nin, D.Z.; Talmo, C.T.; Hollenbeck, B.L.; Grant, A.R.; Niu, R.; Chang, D.C.; Smith, E.L. Postoperative Aspiration Pneumonia Among Adults Using GLP-1 Receptor Agonists. JAMA Netw. Open 2025, 8, e250081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeo, Y.H.; Gaddam, S.; Ng, W.H.; Huang, P.C.; Motility Metabolic Pharmacoepidemiology Group; Ma, K.S.-K.; Rezaie, A. Increased Risk of Aspiration Pneumonia Associated With Endoscopic Procedures Among Patients With Glucagon-like Peptide 1 Receptor Agonist Use. Gastroenterology 2024, 167, 402–404.e3. [Google Scholar] [CrossRef] [PubMed]
- Abu-Freha, N.; Yitzhak, A.; Shirin, H.; Nevo-Shor, A.; Abu-Jaffar, J.; Abu-Rafe, S.; Afianish, Y.; Cohen, D.L.; Bermont, A. Glucagon-like peptide-1 receptor agonists significantly affect the quality of bowel preparation for colonoscopy. Endoscopy 2025, 57, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Beran, A.; Nayfeh, T.; Akhras, A.; Ramai, D.; Mohamed, M.; Guardiola, J.J.; Al-Haddad, M.; Rex, D.K. Effect of Glucagon-Like Peptide-1 Receptor Agonists on Bowel Preparation for Colonoscopy: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2025, 120, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Gala, K.S.; Ghusn, W.; Abboud, D.M.; Wallace, F.K.; Vargas, E.J. Effect of Glucagon-Like Peptide-1 Receptor Agonists on Bowel Preparation for Colonoscopy. Am. J. Gastroenterol. 2024, 119, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Hashash, J.G.; Thompson, C.C.; Wang, A.Y. AGA Rapid Clinical Practice Update on the Management of Patients Taking GLP-1 Receptor Agonists Prior to Endoscopy: Communication. Clin. Gastroenterol. Hepatol. 2024, 22, 705–707. [Google Scholar] [CrossRef] [PubMed]
- El-Boghdadly, K.; Dhesi, J.; Fabb, P.; Levy, N.; Lobo, D.N.; McKechnie, A.; Mustafa, O.; Newland-Jones, P.; Patel, A.; Pournaras, D.J.; et al. Elective peri-operative management of adults taking glucagon-like peptide-1 receptor agonists, glucose-dependent insulinotropic peptide agonists and sodium-glucose cotransporter-2 inhibitors: A multidisciplinary consensus statement: A consensus statement from the Association of Anaesthetists, Association of British Clinical Diabetologists, British Obesity and Metabolic Surgery Society, Centre for Perioperative Care, Joint British Diabetes Societies for Inpatient Care, Royal College of Anaesthetists, Society for Obesity and Bariatric Anaesthesia and UK Clinical Pharmacy Association. Anaesthesia 2025, 80, 412–424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oprea, A.D.; Ostapenko, L.J.; Sweitzer, B.; Selzer, A.; Irizarry-Alvarado, J.M.; Hurtado Andrade, M.D.; Mendez, C.E.; Kelley, K.D.; Stewart, E.; Fernandez Robles, C.R.; et al. Perioperative management of patients taking glucagon-like peptide 1 receptor agonists: Society for Perioperative Assessment and Quality Improvement (SPAQI) multidisciplinary consensus statement. Br. J. Anaesth. 2025, 135, 48–78. [Google Scholar] [CrossRef] [PubMed]
- Tarar, Z.I.; Farooq, U.; Chaudhry, A.; Gandhi, M.; El Alayli, A.; Ayoub, M.; Singh, B.; Daglilar, E.; Thosani, N. Evidence Report on the Safety of Gastrointestinal Endoscopy in Patients on Glucagon-like Peptide-1 Receptor Agonists: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- do Nascimento, T.S.; Pereira, R.O.L.; Maia, E.; Ohnuma, T.; da Costa, M.G.; Slawka, E.; Galhardo, C., Jr.; Krishnamoorthy, V. The impact of glucagon-like peptide-1 receptor agonists in the patients undergoing anesthesia or sedation: Systematic review and meta-analysis. Perioper. Med. 2024, 13, 78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nersessian, R.S.F.; da Silva, L.M.; Carvalho, M.A.S.; Silveira, S.Q.; Abib, A.C.V.; Bellicieri, F.N.; Lima, H.O.; Ho, A.M.; Anjos, G.S.; Mizubuti, G.B. Relationship between residual gastric content and peri-operative semaglutide use assessed by gastric ultrasound: A prospective observational study. Anaesthesia 2024, 79, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Jirapinyo, P.; Siranart, N.; Thompson, C.C. Glucagon-like peptide-1 receptor agonists and upper endoscopy: A real-world experience. Obesity 2025, 33, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Garza, K.; Aminpour, E.; Shah, J.; Mehta, B.; Early, D.; Gyawali, C.P.; Kushnir, V. Glucagon-Like Peptide-1 Receptor Agonists Increase Solid Gastric Residue Rates on Upper Endoscopy Especially in Patients With Complicated Diabetes: A Case-Control Study. Am. J. Gastroenterol. 2024, 119, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Gulak, M.A.; Murphy, P. Regurgitation under anesthesia in a fasted patient prescribed semaglutide for weight loss: A case report. Can. J. Anaesth. 2023, 70, 1397–1400. (In English) [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.B.; Norwood, D.A.; Price, C.; Abdulhadi, B.; Kyanam Kabir Baig, K.; Ahmed, A.M.; Peter, S.; Routman, J.S.; Sánchez-Luna, S.A.; Duggan, E.W.; et al. Effects of glucagon-like peptide-1 receptor agonists on gastric mucosal visibility and retained gastric contents during EGD. Gastrointest. Endosc. 2024, 100, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.R.; Hobai, I.A. Semaglutide, delayed gastric emptying, and intraoperative pulmonary aspiration: A case report. Can. J. Anaesth. 2023, 70, 1394–1396. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kindel, T.L.; Wang, A.Y.; Wadhwa, A.; Schulman, A.R.; Sharaiha, R.Z.; Kroh, M.; Ghanem, O.M.; Levy, S.; Joshi, G.P.; LaMasters, T.; et al. Multi-society clinical practice guidance for the safe use of glucagon-like peptide-1 receptor agonists in the perioperative period. Surg. Endosc. 2025, 39, 180–183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Queiroz, V.N.F.; Falsarella, P.M.; Chaves, R.C.F.; Takaoka, F.; Socolowski, L.R.; Garcia, R.G. Risk of pulmonary aspiration during semaglutide use and anesthesia in a fasting patient: A case report with tomographic evidence. Einstein 2023, 21, eRC0628. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.P.; Abdelmalak, B.B.; Weigel, W.A.; Soriano, S.G.; Harbell, M.W.; Kuo, C.I.; Stricker, P.A.; Domino, K.B.; American Society of Anesthesiologist (ASA) Task Force on Perioperative Fasting. American Society of Anesthesiologists Consensus-Based Guidance on Preoperative Management of Patients (Adults and Children) on Glucagon-like Peptide-1 (GLP-1) Receptor Agonists. June 29, 2023. Available online: https://www.asahq.org/about-asa/newsroom/news-releases/2023/06/american-society-of-anesthesiologists-consensus-based-guidance-on-preoperative (accessed on 20 August 2023).
- Crespo, J.; López-Arias, M.J.; Iruzubieta, P. Variability in the management of GLP-1 agonists and endoscopic procedures: An opportunity for standardization. Rev. Esp. Enferm. Dig. 2025, 117, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Dev, B.; Hadi, Y.; Rizvi, A.; Cao, C.; Horwich, B.; Hoerter, N.A. Low Rates of Aborted Endoscopy Due to Gastric Food Retention in Patients on Glucagon-Like-Peptide-1 Receptor Agonists. Dig. Dis. Sci. 2025, 70, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, K.; Sidhu, R.; Tham, T.; Tziatzios, G.; Guy, C.; Messmann, H.; Arvanitakis, M.; Hassan, C.; Bisschops, R.; Gralnek, I.M. Sedation practices in Gastrointestinal Endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) survey. Endoscopy 2024, 56, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Terán, Á. Endoscopy and sedation: An inseparable binomial for the gastroenterologist. Rev. Esp. Enferm. Dig. 2018, 110, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.J.; Brand, A.; Ngu, W.S.; Stokes, C.; Hoare, Z.; Totton, N.; Bhandari, P.; Sharp, L.; Bastable, A.; Rutter, M.D.; et al. BowelScope: Accuracy of Detection Using Endocuff Optimisation of Mucosal Abnormalities (the B-ADENOMA Study): A multicentre, randomised controlled flexible sigmoidoscopy trial. Gut 2020, 69, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.L.; Stark, J.E. Impact of Glucagon-like Peptide-1 Receptor Agonists on Bowel Preparation for Colonoscopy: A Large, Matched Regional Cohort. Ann. Pharmacother. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Robalino Gonzaga, E.; Farooq, A.; Mohammed, A.; Chandan, S.; Fawwaz, B.; Singh, G.; Malik, A.; Zhang, Y.; Kadkhodayan, K. Real-World Impact of GLP-1 Receptor Agonists on Endoscopic Patient Outcomes in an Ambulatory Setting: A Retrospective Study at a Large Tertiary Center. J. Clin. Med. 2024, 13, 5403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, S.; Chandan, S.; Dahiya, D.S.; Aswath, G.; Ramai, D.; Maida, M.; Anderloni, A.; Muscatiello, N.; Facciorusso, A. Impact of GLP-1 Receptor Agonists in Gastrointestinal Endoscopy: An Updated Review. J. Clin. Med. 2024, 13, 5627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wani, S.; Cote, G.A.; Keswani, R.N.; Yadlapati, R.H.; Hall, M.; O’Hara, J.; Berzin, T.M.; Burbridge, R.A.; Chahal, P.; Cohen, J.; et al. Development of American Society for Gastrointestinal Endoscopy standards for training in advanced endoscopy within dedicated advanced endoscopy fellowship programs. Gastrointest. Endosc. 2025, 101, 12–24. [Google Scholar] [CrossRef] [PubMed]
- American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association Institute; American Society for Gastrointestinal Endoscopy; Society for Gastroenterology Nurses and Associates; Vargo, J.J.; DeLegge, M.H.; Feld, A.D.; Gerstenberger, P.D.; Kwo, P.Y.; et al. Multisociety sedation curriculum for gastrointestinal endoscopy. Gastroenterology 2012, 143, e18–e41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meineri, M.; Arellano, R.; Bryson, G.; Arzola, C.; Chen, R.; Collins, P.; Denault, A.; Desjardins, G.; Fayad, A.; Funk, D.; et al. Canadian recommendations for training and performance in basic perioperative point-of-care ultrasound: Recommendations from a consensus of Canadian anesthesiology academic centres. Can. J. Anaesth. 2021, 68, 376–386. (In English) [Google Scholar] [CrossRef] [PubMed]
- Grillot, N.; Garot, M.; Lasocki, S.; Huet, O.; Bouzat, P.; Le Moal, C.; Oudot, M.; Chatel-Josse, N.; El Amine, Y.; Danguy des Déserts, M.; et al. Assessment of remifentanil for rapid sequence induction and intubation in patients at risk of pulmonary aspiration of gastric contents compared to rapid-onset paralytic agents: Study protocol for a non-inferiority simple blind randomized controlled trial (the REMICRUSH study). Trials 2021, 22, 237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Arguments for SUSPENDING or Delaying | References | Arguments for NOT Suspending or Delaying | References |
|---|---|---|---|
| ↑ Gastric retention (>15%) demonstrated by EGD or ultrasound | [1,3,4,5,6,7,8,9,12,13,14,15,18,21,22,23,24,28,32,33,34] | Absolute risk of aspiration low or not increased in cohorts/meta-analyses | [10,18,19,20,29,35,36] |
| Cases of aspiration/regurgitation despite standard fasting | [7,13,34,36,37] | Liquid fasting 18–24 h + ultrasound allows for continuing | [25,26,38,39] |
| Meta-analysis: ↑ EGD abortions or colonic malpreparation | [5,8,9,16,23,28,39] | Stopping ≥ 7–10 days can uncontrol blood sugar/weight and delay attention | [38,40,41,42] |
| High-risk procedures (EUS, deep sedation without protected airway) | [26,39,42] | Real series show safety with continuous AR | [33,36,40] |
| Physiological basis: gastric emptying ↓ 30–40% | [1,3,4,6] | Consensus and additional research needed | [26,27,43] |
| Study (Reference) | Design/n | Key Finding |
|---|---|---|
| [22] | Multicenter case–control, 9752 colonoscopies | GLP-1 RA doubled inadequate cleansing (Boston Score < 6); risk independent of diabetes and PEG volume. |
| [24] | Single-center cohort, n = 446 | Poor prep 15.5% vs. 6.6% (controls); split-dose 4 L PEG mitigated, but did not abolish, the gap. |
| [44] | Regional database, >12,000 exams | Seven-day semaglutide hold failed to normalize prep (OR 1.6); HbA1c rebounded 0.3%. |
| [23] | Meta-analysis, 8 studies | Pooled RR 1.8 (95% CI 1.4–2.2) for inadequate cleansing; NNT to repeat colonoscopy ≈ 14. |
| Modifier | Evidence | Clinical Implication |
|---|---|---|
| Dose/Potency | Higher weekly doses (≥ 1 mg semaglutide or ≥ 10 mg tirzepatide) show longer gastric half-emptying times [5]. | Consider longer fasting or ultrasound if high doses given within 7 days. |
| Duration of therapy | Gastric adaptation after 12–16 weeks reduces, but does not normalize, RGC rates [6]. | Early-phase users are higher risk. |
| Co-morbid gastroparesis | Diabetic autonomic neuropathy triples RGC odds [32]. | Symptomatic diabetics warrant ultrasound or extended fasting. |
| Procedure type and sedation | EUS or ERCP under general anesthesia carry greater aspiration potential; European survey shows only 38% routinely protect airway [37]. | Prefer endotracheal intubation for high-risk procedures in GLP-1 RA users. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo, J.; Rodríguez-Duque, J.C.; Iruzubieta, P.; Morel Cerda, E.C.; Velarde-Ruiz Velasco, J.A. GLP-1 Receptor Agonists and Gastrointestinal Endoscopy: A Narrative Review of Risks, Management Strategies, and the Need for Clinical Consensus. J. Clin. Med. 2025, 14, 5597. https://doi.org/10.3390/jcm14155597
Crespo J, Rodríguez-Duque JC, Iruzubieta P, Morel Cerda EC, Velarde-Ruiz Velasco JA. GLP-1 Receptor Agonists and Gastrointestinal Endoscopy: A Narrative Review of Risks, Management Strategies, and the Need for Clinical Consensus. Journal of Clinical Medicine. 2025; 14(15):5597. https://doi.org/10.3390/jcm14155597
Chicago/Turabian StyleCrespo, Javier, Juan Carlos Rodríguez-Duque, Paula Iruzubieta, Eliana C. Morel Cerda, and Jose Antonio Velarde-Ruiz Velasco. 2025. "GLP-1 Receptor Agonists and Gastrointestinal Endoscopy: A Narrative Review of Risks, Management Strategies, and the Need for Clinical Consensus" Journal of Clinical Medicine 14, no. 15: 5597. https://doi.org/10.3390/jcm14155597
APA StyleCrespo, J., Rodríguez-Duque, J. C., Iruzubieta, P., Morel Cerda, E. C., & Velarde-Ruiz Velasco, J. A. (2025). GLP-1 Receptor Agonists and Gastrointestinal Endoscopy: A Narrative Review of Risks, Management Strategies, and the Need for Clinical Consensus. Journal of Clinical Medicine, 14(15), 5597. https://doi.org/10.3390/jcm14155597






