Extracellular Vesicle-Derived Bioactive Molecules for Corneal and Ocular Surface Regeneration
Abstract
1. Introduction
2. Extracellular Vesicles
3. Bioactive Molecules in Extracellular Vesicles
3.1. Interaction of Extracellular Vesicles and Recipient Cells
3.2. Bioactive Molecules in Extracellular Vesicles Relevant to Corneal Regeneration
3.2.1. MicroRNAs (miRNAs)
3.2.2. Cytokines and Growth Factors
4. Therapeutic Effect of Extracellular Vesicle-Derived Specific Molecules on the Ocular Surface and Cornea
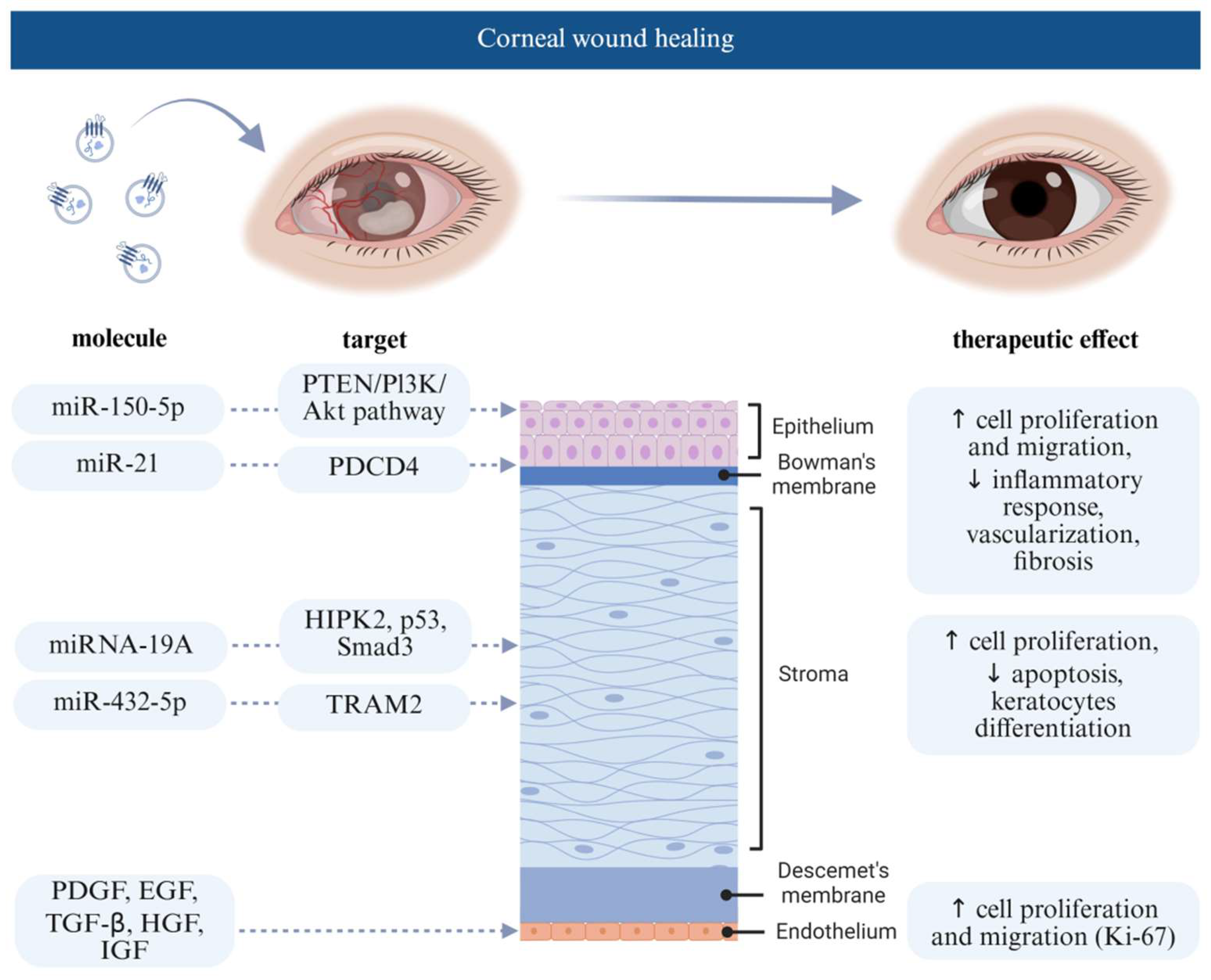
4.1. Extracellular Vesicle Effect on Corneal Wound Healing
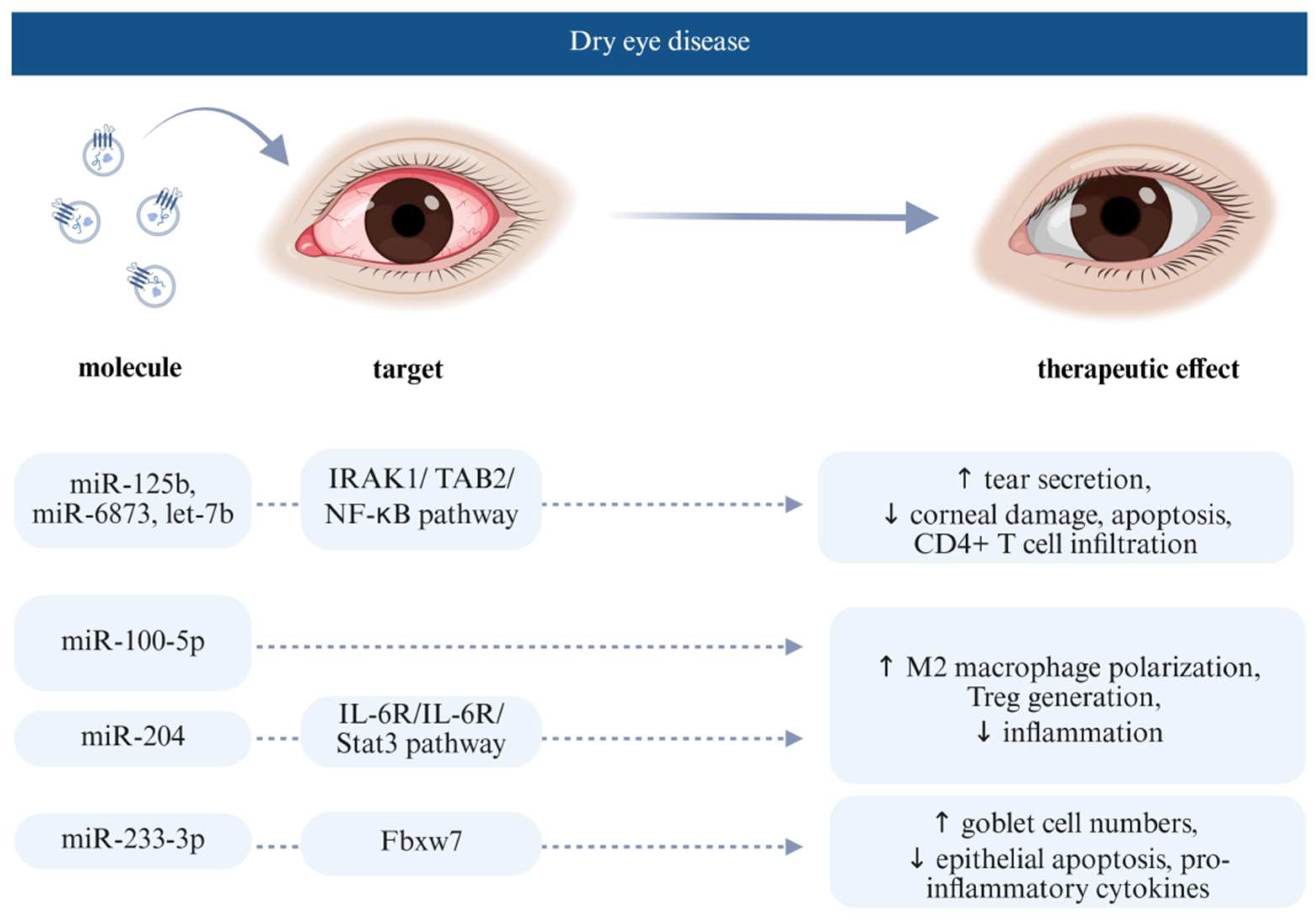
4.2. Extracellular Vesicle Effect on Dry Eye Disease
5. Extracellular Vesicles as Delivery Systems of Therapeutic Molecules
| Therapeutic Molecule | Target | Disease | Producing Cell | Year | Reference |
|---|---|---|---|---|---|
| ocu-microRNA 24-3p (miRNA 24-3p) | rabbit corneal epithelial cells migration and corneal repair | corneal epithelial healing | human adipose-derived MSCs | 2023 | [59] |
| siRNA | / | dry eye disease | hCEC | 2024 | [60] |
| gold nanoparticles (AuNPs) reduced by ascorbic acid | / | dry eye disease | human MSC | 2023 | [61] |
| kaempferol | / | corneal neovascularisation | human platelets | 2025 | [62] |
6. Future Perspectives
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Lužnik, Z.; Hawlina, M.; Ferrari, S.; Ponzin, D.; Schollmayer, P. Ocular surface reconstruction in limbal stem cell deficiency: Current treatment options and perspectives. Expert Rev. Ophthalmol. 2017, 12, 43–56. [Google Scholar] [CrossRef]
- Ueno, H.; Ferrari, G.; Hattori, T.; Saban, D.R.; Katikireddy, K.R.; Chauhan, S.K.; Dana, R. Dependence of corneal stem/progenitor cells on ocular surface innervation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Saccu, G.; Menchise, V.; Gai, C.; Bertolin, M.; Ferrari, S.; Giordano, C.; Manco, M.; Dastrù, W.; Tolosano, E.; Bussolati, B. Bone marrow mesenchymal stromal/stem cell-derived extracellular vesicles promote corneal wound repair by regulating inflammation and angiogenesis. Cells 2022, 11, 3892. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.R.; Bourne, R.R.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Tan, D.T.; Dart, J.K.; Holland, E.J.; Kinoshita, S. Corneal transplantation. Lancet 2012, 379, 1749–1761. [Google Scholar] [CrossRef]
- Li, S.-J.; Cheng, R.-J.; Wei, S.-X.; Xia, Z.-J.; Pu, Y.-Y.; Liu, Y. Advances in mesenchymal stem cell-derived extracellular vesicles therapy for sjogren’s syndrome-related dry eye disease. Exp. Eye Res. 2023, 237, 109716. [Google Scholar] [CrossRef]
- Surico, P.L.; Barone, V.; Singh, R.B.; Coassin, M.; Blanco, T.; Dohlman, T.H.; Basu, S.; Chauhan, S.K.; Dana, R.; Di Zazzo, A. Potential applications of mesenchymal stem cells in ocular surface immune-mediated disorders. Surv. Ophthalmol. 2025, 70, 467–479. [Google Scholar] [CrossRef]
- Kobal, N.; Marzidovšek, M.; Schollmayer, P.; Maličev, E.; Hawlina, M.; Marzidovšek, Z.L. Molecular and cellular mechanisms of the therapeutic effect of mesenchymal stem cells and extracellular vesicles in corneal regeneration. Int. J. Mol. Sci. 2024, 25, 11121. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.; Erdbrügger, U. Minimal information for studies of extracellular vesicles (misev2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, H.; Wu, R.; Guo, Y.; Gong, L.; Fu, K.; Ma, C.; Peng, C.; Li, Y. Mesenchymal stem cell-derived exosomes and non-coding rnas: Regulatory and therapeutic role in liver diseases. Biomed. Pharmacother. 2023, 157, 114040. [Google Scholar] [CrossRef]
- Görgens, A.; Corso, G.; Hagey, D.W.; Wiklander, R.J.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Fan, X.; Hao, R.; Dong, L.; Xue, M.; Tan, L.; Yang, C.; Li, X.; Ren, X. Human embryonic stem cell-derived extracellular vesicles alleviate retinal degeneration by upregulating oct4 to promote retinal müller cell retrodifferentiation via hsp90. Stem Cell Res. Ther. 2021, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Conforti, A.; Scarsella, M.; Starc, N.; Giorda, E.; Biagini, S.; Proia, A.; Carsetti, R.; Locatelli, F.; Bernardo, M.E. Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev. 2014, 23, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Di Trapani, M.; Bassi, G.; Midolo, M.; Gatti, A.; Takam Kamga, P.; Cassaro, A.; Carusone, R.; Adamo, A.; Krampera, M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on t, b and nk cell functions. Sci. Rep. 2016, 6, 24120. [Google Scholar] [CrossRef]
- Wen, C.; Seeger, R.C.; Fabbri, M.; Wang, L.; Wayne, A.S.; Jong, A.Y. Biological roles and potential applications of immune cell-derived extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1400370. [Google Scholar] [CrossRef]
- Barilani, M.; Peli, V.; Manzini, P.; Pistoni, C.; Rusconi, F.; Pinatel, E.M.; Pischiutta, F.; Tace, D.; Iachini, M.C.; Elia, N. Extracellular vesicles from human induced pluripotent stem cells exhibit a unique microrna and circrna signature. Int. J. Biol. Sci. 2024, 20, 6255–6278. [Google Scholar] [CrossRef]
- Maličev, E.; Jazbec, K. An overview of mesenchymal stem cell heterogeneity and concentration. Pharmaceuticals 2024, 17, 350. [Google Scholar] [CrossRef]
- Teck Tan, T.; Kiang Lim, S. Relevance of rna to the therapeutic efficacy of mesenchymal stromal/stem cells extracellular vesicles. RNA Biol. 2025, 22, 1–7. [Google Scholar]
- Marić, I.; Žiberna, K.; Kolenc, A.; Maličev, E. Platelet activation and blood extracellular vesicles: The influence of venepuncture and short blood storage. Blood Cells Mol. Dis. 2024, 106, 102842. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Kimiz-Gebologlu, I.; Oncel, S.S. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control. Release 2022, 347, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to extracellular vesicles: Biogenesis, rna cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Naskou, M.C.; Cochran, A.; Darzenta, N.; Golan, M.E.; Stice, S.L.; Martin, D.R. The characteristics and function of small extracellular vesicles derived from human bone marrow and umbilical cord mesenchymal stromal cells are influenced by cell culture conditions. Stem Cells Dev. 2024, 33, 117–127. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Kolenc, A.; Grundner, M.; Hostnik, I.; Maličev, E. Monitoring cell activation and extracellular vesicle generation in platelet concentrates for transfusion. Int. J. Mol. Sci. 2024, 25, 11577. [Google Scholar] [CrossRef]
- Kolenc, A.; Maličev, E. Current methods for analysing mesenchymal stem cell-derived extracellular vesicles. Int. J. Mol. Sci. 2024, 25, 3439. [Google Scholar] [CrossRef]
- Kwok, Z.H.; Wang, C.; Jin, Y. Extracellular vesicle transportation and uptake by recipient cells: A critical process to regulate human diseases. Processes 2021, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, S.; Zhang, T.; Pan, X.; Ke, Y.; Fan, Y.; Li, J.; Zhang, L.; Chen, C. Glycan-anchored fluorescence labeling of milk-derived extracellular vesicles for investigating their cellular uptake and intracellular fate. Small Methods 2025, 9, 2401996. [Google Scholar] [CrossRef]
- Svensson, K.J.; Christianson, H.C.; Wittrup, A.; Bourseau-Guilmain, E.; Lindqvist, E.; Svensson, L.M.; Mörgelin, M.; Belting, M. Exosome uptake depends on erk1/2-heat shock protein 27 signaling and lipid raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 2013, 288, 17713–17724. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Albero, M.; Navascués, N.; Mendoza, G.; Sebastián, V.; Arruebo, M.; Martín-Duque, P.; Santamaría, J. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnol. 2019, 17, 16. [Google Scholar] [CrossRef]
- Jurgielewicz, B.J.; Yao, Y.; Stice, S.L. Kinetics and specificity of hek293t extracellular vesicle uptake using imaging flow cytometry. Nanoscale Res. Lett. 2020, 15, 170. [Google Scholar] [CrossRef]
- Alisi, L.; Giovannetti, F.; Armentano, M.; Lucchino, L.; Lambiase, A.; Bruscolini, A. Challenging corneal diseases and microrna expression: Focus on rare diseases and new therapeutic frontiers. Surv. Ophthalmol. 2025, 70, 121–131. [Google Scholar] [CrossRef]
- Morán, G.A.G.; Parra-Medina, R.; Cardona, A.G.; Quintero-Ronderos, P.; Rodríguez, É.G. Cytokines, chemokines and growth factors. In Autoimmunity: From Bench to Bedside [Internet]; El Rosario University Press: Bogotá, Colombia, 2013. [Google Scholar]
- Ghafar, N.A.; Jalil, N.A.A.; Kamarudin, T.A. Wound healing of the corneal epithelium: A review. Asian Biomed. (Res. Rev. News) 2021, 15, 199–212. [Google Scholar] [CrossRef]
- Lim, M.; Goldstein, M.H.; Tuli, S.; Schultz, G.S. Growth factor, cytokine and protease interactions during corneal wound healing. Ocul. Surf. 2003, 1, 53–65. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Wu, G.; Qi, P.; Zhang, Y.; Liu, Z.; Li, X.; Yu, Y.; Ye, X.; Li, Y. Umbilical cord mesenchymal stem cell-derived small extracellular vesicles deliver mir-21 to promote corneal epithelial wound healing through pten/pi3k/akt pathway. Stem Cells Int. 2022, 2022, 1252557. [Google Scholar] [CrossRef]
- Shen, T.; Zheng, Q.-Q.; Shen, J.; Li, Q.-S.; Song, X.-H.; Luo, H.-B.; Hong, C.-Y.; Yao, K. Effects of adipose-derived mesenchymal stem cell exosomes on corneal stromal fibroblast viability and extracellular matrix synthesis. Chin. Med. J. 2018, 131, 704–712. [Google Scholar] [CrossRef]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of human corneal mesenchymal stromal cell-derived exosomes on corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Zheng, Q.; Luo, H.; Li, X.; Chen, Z.; Song, Z.; Zhou, G.; Hong, C. Exosomal mir-19a from adipose-derived stem cells suppresses differentiation of corneal keratocytes into myofibroblasts. Aging 2020, 12, 4093–4110. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials 2022, 280, 121320. [Google Scholar] [CrossRef]
- Zhou, T.; He, C.; Lai, P.; Yang, Z.; Liu, Y.; Xu, H.; Lin, X.; Ni, B.; Ju, R.; Yi, W. Mir-204–containing exosomes ameliorate gvhd-associated dry eye disease. Sci. Adv. 2022, 8, eabj9617. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gao, Z.; Zhao, L.; Du, B.; Ma, B.; Nian, H.; Wei, R. Msc-derived small extracellular vesicles attenuate autoimmune dacryoadenitis by promoting m2 macrophage polarization and inducing tregs via mir-100-5p. Front. Immunol. 2022, 13, 888949. [Google Scholar] [CrossRef]
- Widyaningrum, R.; Wu, Y.-W.; Delila, L.; Lee, D.-Y.; Wang, T.-J.; Burnouf, T. In vitro evaluation of platelet extracellular vesicles (pevs) for corneal endothelial regeneration. Platelets 2022, 33, 1237–1250. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, Y.; Zhang, Y.; Zheng, D.; Yan, L.; Guo, M.; Mao, Y.; Yang, L. Exosomes from bone marrow-derived mesenchymal stem cells facilitate corneal wound healing via regulating the p44/42 mapk pathway. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 723–734. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Chen, Q.; Wei, Z.; Xu, X.; Han, D.; Zhang, Y.; Chen, Z.; Liang, Q. Micrornas of extracellular vesicles derived from mesenchymal stromal cells alleviate inflammation in dry eye disease by targeting the irak1/tab2/nf-κb pathway. Ocul. Surf. 2023, 28, 131–140. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, Y.; Liu, Y.; Yang, M.; Zeng, L. Mesenchymal stem cells-derived exosomal mir-223-3p alleviates ocular surface damage and inflammation by downregulating fbxw7 in dry eye models. Investig. Ophthalmol. Vis. Sci. 2024, 65, 1. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, C.; Ma, L.; Zhao, L.; Li, D.; Lin, Y.; Zhou, Q.; Xie, L.; Wang, F. 3d mesenchymal stem cell exosome-functionalized hydrogels for corneal wound healing. J. Control. Release 2025, 380, 630–646. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J. Tfos dews ii epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, W.; Zeng, W.; Feng, K.; Zheng, Y.; Wang, P.; Chen, F.; Zhang, W.; Di, L.; Wang, R. Extracellular vesicles as biomarkers and drug delivery systems for tumor. Acta. Pharm. Sin. B 2025, 15, 3460–3486. [Google Scholar] [CrossRef]
- Fang, L.; Gu, W.; Li, R.; Chen, C.; Cai, S.; Luozhong, S.; Chen, M.; Hsu, A.; Tsai, Y.-C.; Londhe, K. Controlling circular rna encapsulation within extracellular vesicles for gene editing and protein replacement. ACS Nano 2024, 18, 30378–30387. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, D.; Pan, X.; Liang, Y. Targeted therapy using engineered extracellular vesicles: Principles and strategies for membrane modification. J. Nanobiotechnol. 2023, 21, 334. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Du, X.; Chen, S.; Meng, T.; Liu, L.; Li, L.; Xiang, R.; Zhang, H.; Zhu, Y.; Zhang, X.; Lin, S. Extracellular vesicles as precision therapeutic vectors: Charting the future of cell-targeted therapy. Precis. Med. Eng. 2025, 2, 100031. [Google Scholar] [CrossRef]
- Sun, X.; Song, W.; Teng, L.; Huang, Y.; Liu, J.; Peng, Y.; Lu, X.; Yuan, J.; Zhao, X.; Zhao, Q. Mirna 24-3p-rich exosomes functionalized degma-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioact. Mater. 2023, 25, 640–656. [Google Scholar] [CrossRef]
- Xie, M.; Wu, Y.; Zhang, Y.; Lu, R.; Zhai, Z.; Huang, Y.; Wang, F.; Xin, C.; Rong, G.; Zhao, C. Membrane fusion-mediated loading of therapeutic sirna into exosome for tissue-specific application. Adv. Mater. 2024, 36, 2403935. [Google Scholar] [CrossRef]
- Ma, F.; Feng, J.; Liu, X.; Tian, Y.; Wang, W.-J.; Luan, F.-X.; Wang, Y.-J.; Yang, W.-Q.; Bai, J.-Y.; Zhang, Y.-Q. A synergistic therapeutic nano-eyedrop for dry eye disease based on ascorbic acid-coupled exosomes. Nanoscale 2023, 15, 1890–1899. [Google Scholar] [CrossRef]
- Liu, G.-S.; Chen, H.-A.; Chang, C.-Y.; Chen, Y.-J.; Wu, Y.-Y.; Widhibrata, A.; Yang, Y.-H.; Hsieh, E.-H.; Delila, L.; Lin, I.-C. Platelet-derived extracellular vesicle drug delivery system loaded with kaempferol for treating corneal neovascularization. Biomaterials 2025, 319, 123205. [Google Scholar] [CrossRef]
- Azizoglu, M.; Zorba-Yildiz, A.-P.; Yilmaz, H.; Yurtbay, G.; Catli, G.; Yavuz, G.; Yavas, A.-D.; Eren, M.; Arici, M.; Goken, V.; et al. Regenerative medicine in surgery: Stem cells and exosome applications. Cir. Cir. 2025, 93, 86–104. [Google Scholar]
- Xia, Y.; Zhang, J.; Liu, G.; Wolfram, J. Immunogenicity of Extracellular Vesicles. Adv. Mater. 2024, 36, e2403199. [Google Scholar] [CrossRef]
- Phillips, W.; Willms, E.; Hill, A.-F. Understanding extracellular vesicle and nanoparticle heterogeneity: Novel methods and considerations. Proteomics 2021, 21, e2000118. [Google Scholar] [CrossRef]

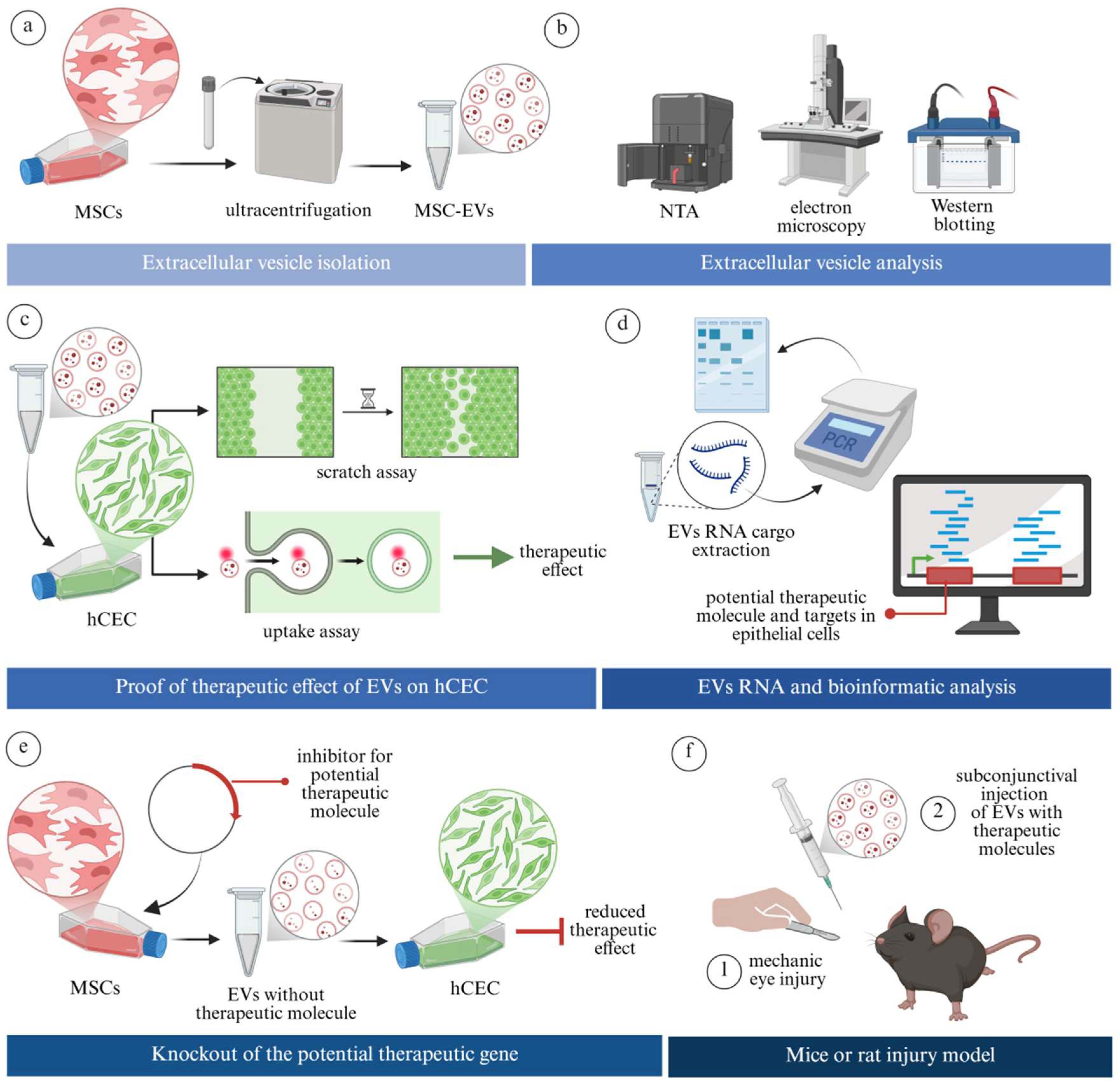
| Molecule | General Function in Relation to Extracellular Vesicles |
| DNA CARGO | |
| dsDNA | Transferred to recipient cell for cell-to-cell communication (influencing gene expression), may act as an immune response trigger (DAMP). |
| mtDNA | Often enriched in EVs, particularly from stressed or cancerous cells. |
| RNA CARGO | |
| mRNA | Transferred to recipient cells for protein synthesis and intercellular communication. |
| miRNA | Gene expression regulation in recipient cells, involved in EV sorting. |
| tRNA | May play roles in stress responses and intercellular signalling. |
| rRNA | Typically minimal in EVs; may be present as fragments. |
| circRNA | Stable RNA molecules that can act as miRNA sponges or regulate transcription. |
| lncRNA | Gene regulation and chromatin remodelling in recipient cells. |
| sncRNA | Various small non-coding RNAs involved in gene regulation. |
| vault RNA | Associated with vault ribonucleoprotein particles; may influence drug resistance and signalling. |
| piRNA | Transposon silencing and genome stability. |
| Y RNA | Degradation of structured and misfolded RNAs in proximity to endosomes. |
| PROTEIN CARGO | |
| Heat shock proteins | |
| HSP60 | Protein folding; may aid in EV cargo stability. |
| HSP70 | Protein folding and protection; involved in EV biogenesis and cargo loading, may assist in membrane fusion. |
| HSP90 | Protein stabilisation and signalling pathways, plays important role in cancer progression. |
| Integrins | Mediation of cell adhesion and signalling; influence on EV targeting (recipient cell specificity) and uptake. |
| Cytoskeletal proteins | |
| Actin | EV motility, possible role in EV biogenesis. |
| Myosin | Motor protein interacting with actin; may facilitate EV movement. |
| Four-transmembrane cross-linked proteins (tetraspanins) | |
| CD9 | Membrane organisation and EV formation (cargo sorting). |
| CD63 | Associated with late endosomes; involved in EV biogenesis. |
| CD81 | Membrane fusion and signalling; role in target cell interaction. |
| CD82 | Cell adhesion and migration. |
| Tspan8 | Cell motility and metastasis (found in tumour-derived EVs; potential in cancer progression). |
| Membrane trafficking proteins | |
| Rab-GTPase | Vesicle trafficking and EV secretion pathways. |
| Annexin | Binds phospholipids; membrane organisation and EV formation (cargo selection). |
| Immuno-regulatory molecules | |
| MHC-I, MHC-II | Presents endogenous antigens; can influence immune recognition. |
| Other proteins | |
| CD106 | Adhesion molecule; EV binding to endothelial cells. |
| ICAM-1 | Adhesion molecule; facilitating EV attachment to target cells. |
| Beta-catenin, P120-catenin | Cell adhesion and Wnt signalling pathway (may influence recipient cell signalling). |
| TGF-β | Cytokine; regulation of cell growth and differentiation; may modulate immune responses. |
| HIF1α | Transcription factor; influences angiogenesis and metabolism in recipient cells. |
| Caveolin-1 | Structural protein of caveolae; involved in endocytosis and signal transduction. |
| ALIX | Endosomal sorting and EV biogenesis; component of the ESCRT pathway in exosomes. |
| TSG101 | ESCRT-I complex; essential for exosome formation (facilitates cargo sorting into EVs). |
| CD86 | Co-stimulatory molecule in immune responses; can modulate T-cell activation. |
| Galectin 9 | Modulates immune responses and apoptosis. |
| LIPID CARGO | |
| Cholesterol | Membrane fluidity and integrity; abundant in EV membranes (essential for structure and function). |
| Flotillin | Membrane formation and endocytosis (may influence cargo sorting). |
| Ceramide | Membrane curvature and EV budding (endosomal membranes). |
| Sphingolipids | Membrane structure and signalling; enriched in EVs. |
| Therapeutic Molecule | Molecular Target | Disease | Producing Cell | Model | Year | Reference | |
|---|---|---|---|---|---|---|---|
| 1 | / | MMP | corneal stromal damage | rabbit ADSC | raCSC | 2018 | [42] |
| 2 | / | / | corneal wound | human cMSC | hCEC | 2018 | [43] |
| 3 | miR-19a | HIPK2, p53 and Smad3 pathways | corneal fibrosis | rabbit ADSC | rabbit cell culture (keratocytes and ADSC) | 2020 | [44] |
| 4 | miR-432-5p | TRAM2 | corneal diseases | human iPSC-MSC | hCEC, rCSSC, rat | 2021 | [45] |
| 5 | miR-204 | IL-6/IL-6R/Stat3 pathway | GVHD-associated dry eye disease | human umbilical cord MSC for clinical patients, mouse bone marrow MSC for in vitro | Fibroblast cell line, macrophage cell line mouse, clinical patients | 2022 | [46] |
| 6 | miR-21 | PTEN/PI3K/ Akt pathway | corneal epithelial wound healing | human umbilical cord MSC | hCEC, rat | 2022 | [41] |
| 7 | miR-100-5p | promoting M2 polarisation and Treg generation | autoimmune dacryoadenitis | human umbilical cord MSC | rabbit | 2022 | [47] |
| 8 | PDGF, EGF, TGF-B, HGF, IGF | / | corneal wound healing | human platelets | hCEC | 2022 | [48] |
| 9 | / | anti-inflammation | corneal damage | human bone marrow MSC | hCEC, mouse | 2022 | [4] |
| 10 | / | p44/42 MAPK pathway | corneal wound | mouse bone marrow MSC | hCEC, mouse | 2023 | [49] |
| 11 | miR-125b-5b, miR-199-3p, let-7b-5p, miR-6873-5p, miR-432-5p, miR-122-5p, miR-4516 | IRAK1/ TAB2/NF-κB | dry eye disease | human umbilical cord MSC | mouse | 2023 | [50] |
| 12 | miR-223-3p | Fbxw7 | dry eye disease | mouse adipose-derived MSC | mCEC, mouse | 2024 | [51] |
| 13 | miR-150-5p | PDCD4 | corneal diseases and injuries | human bone marrow MSC | hCEC, hCSC, rabbit | 2025 | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolenc, A.; Dimnik, Ž.; Marzidovšek, M.; Schollmayer, P.; Hawlina, M.; Maličev, E.; Lužnik Marzidovšek, Z. Extracellular Vesicle-Derived Bioactive Molecules for Corneal and Ocular Surface Regeneration. J. Clin. Med. 2025, 14, 5594. https://doi.org/10.3390/jcm14155594
Kolenc A, Dimnik Ž, Marzidovšek M, Schollmayer P, Hawlina M, Maličev E, Lužnik Marzidovšek Z. Extracellular Vesicle-Derived Bioactive Molecules for Corneal and Ocular Surface Regeneration. Journal of Clinical Medicine. 2025; 14(15):5594. https://doi.org/10.3390/jcm14155594
Chicago/Turabian StyleKolenc, Ana, Živa Dimnik, Miha Marzidovšek, Petra Schollmayer, Marko Hawlina, Elvira Maličev, and Zala Lužnik Marzidovšek. 2025. "Extracellular Vesicle-Derived Bioactive Molecules for Corneal and Ocular Surface Regeneration" Journal of Clinical Medicine 14, no. 15: 5594. https://doi.org/10.3390/jcm14155594
APA StyleKolenc, A., Dimnik, Ž., Marzidovšek, M., Schollmayer, P., Hawlina, M., Maličev, E., & Lužnik Marzidovšek, Z. (2025). Extracellular Vesicle-Derived Bioactive Molecules for Corneal and Ocular Surface Regeneration. Journal of Clinical Medicine, 14(15), 5594. https://doi.org/10.3390/jcm14155594






