Comparative Effectiveness of Ustekinumab and Vedolizumab as Maintenance Therapy After Tacrolimus-Induced Improvement in Patients with Acute Severe Ulcerative Colitis: A Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Ethical Considerations
2.2. Study Design
2.3. Patients
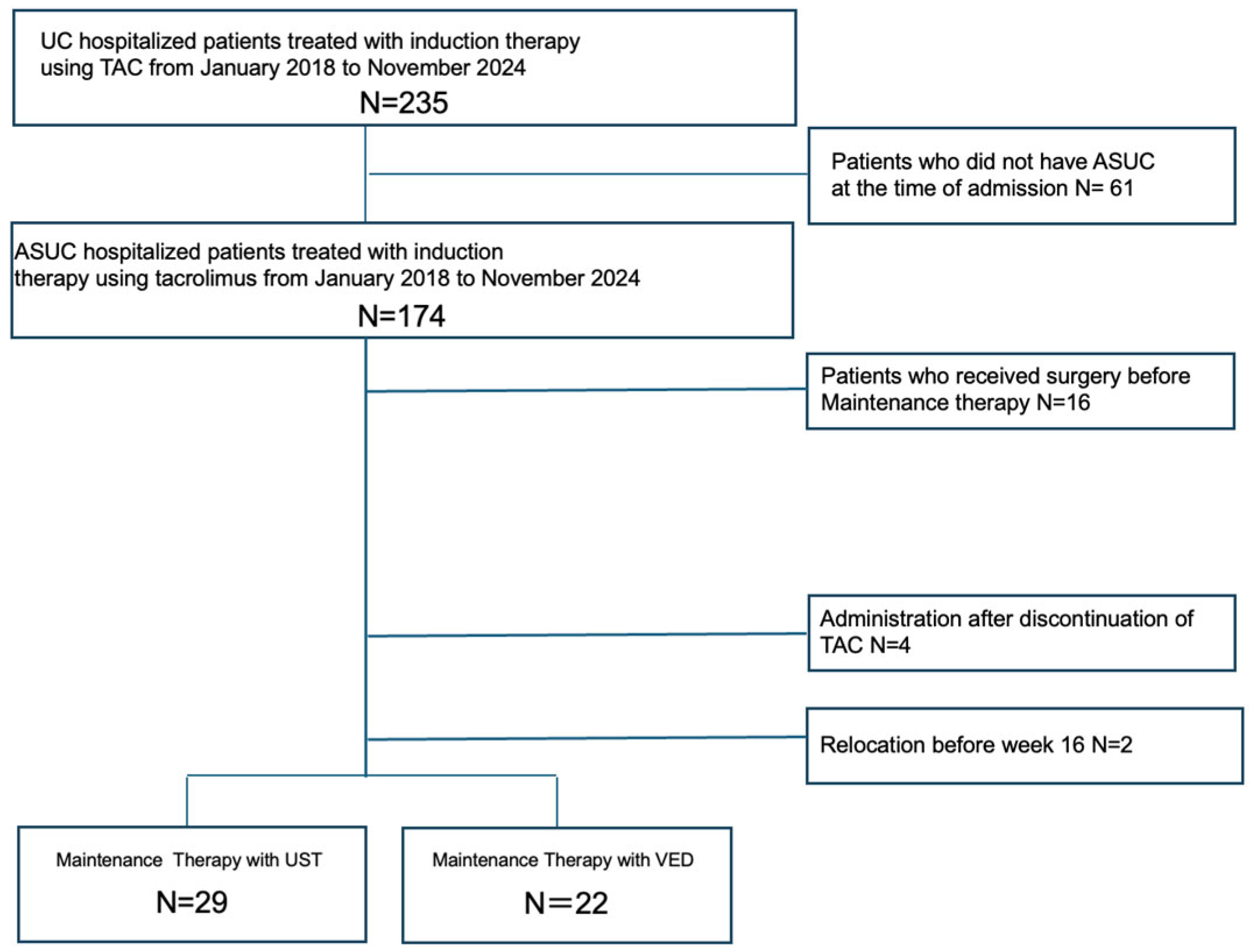
2.4. Inclusion and Exclusion Criteria
2.5. Medications
2.6. Data Collection
2.7. Outcomes
2.8. Definitions
2.9. Statistical Analysis
3. Results
3.1. Patient Demographics and Characteristics
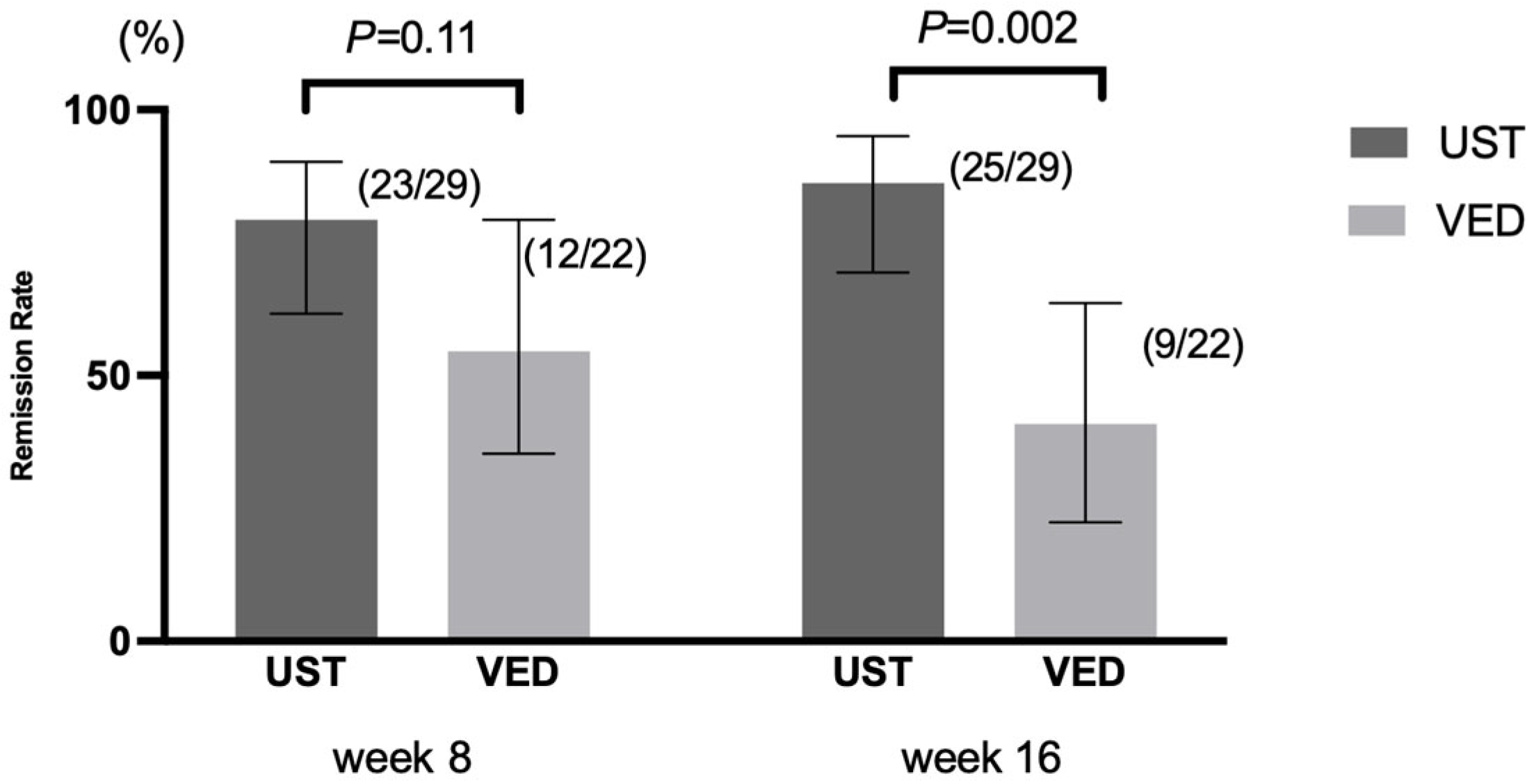
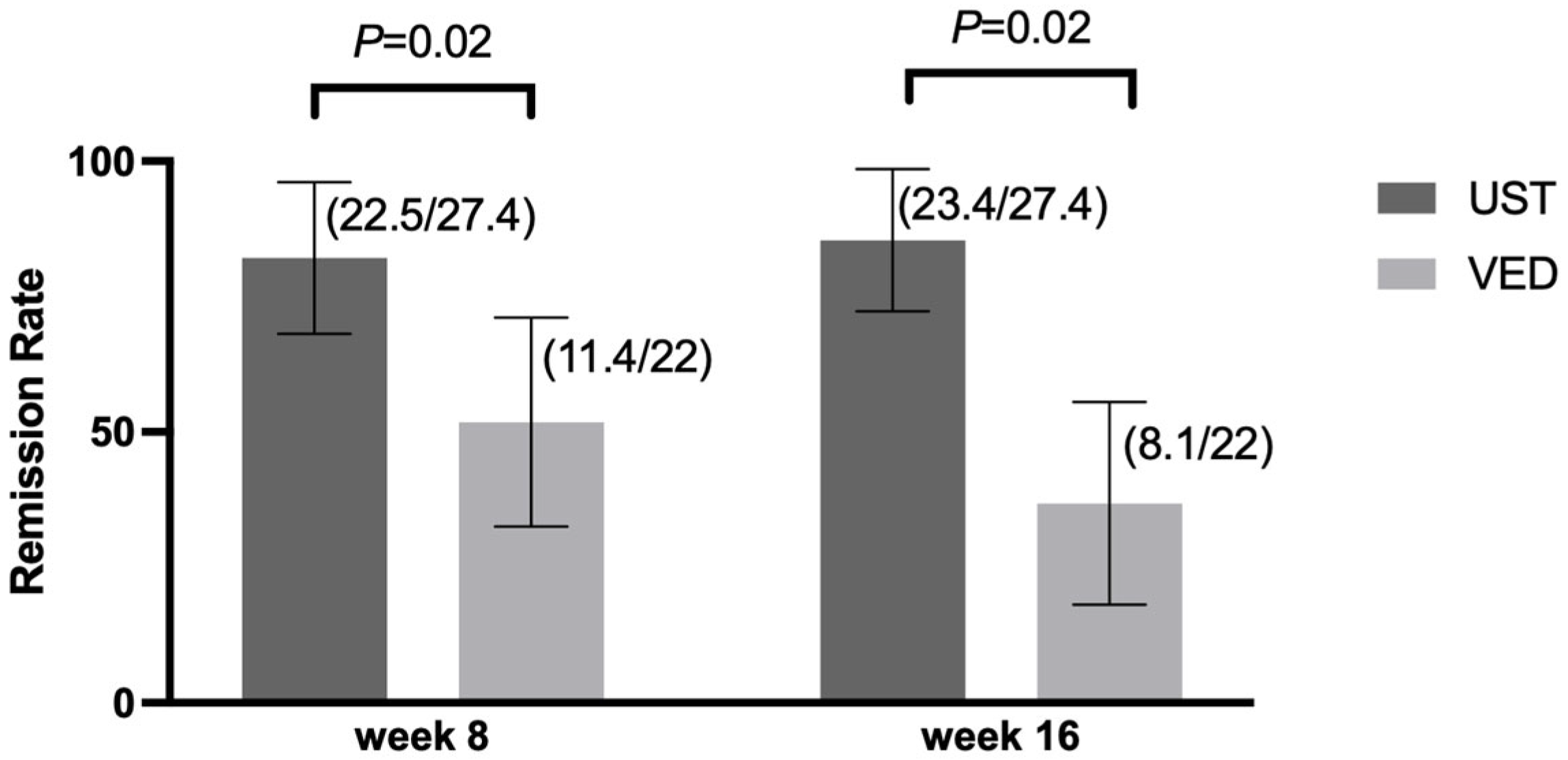
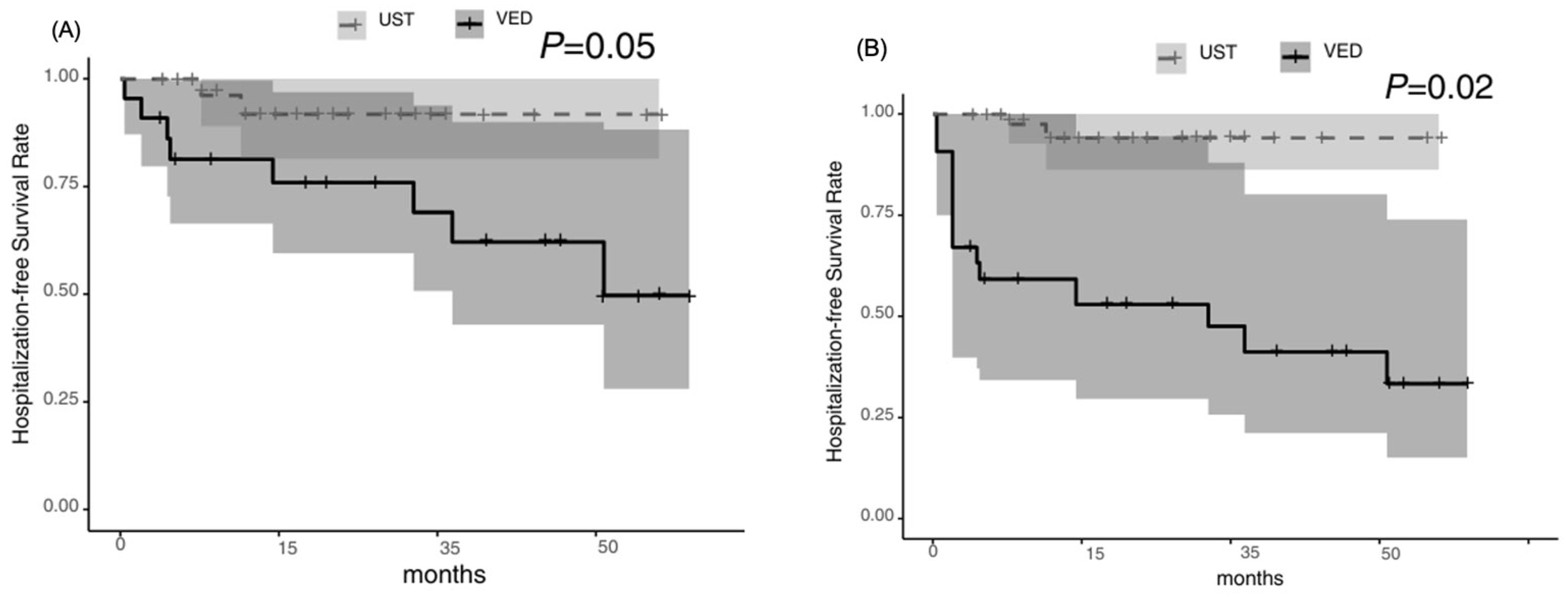
3.2. Primary and Secondary Outcomes
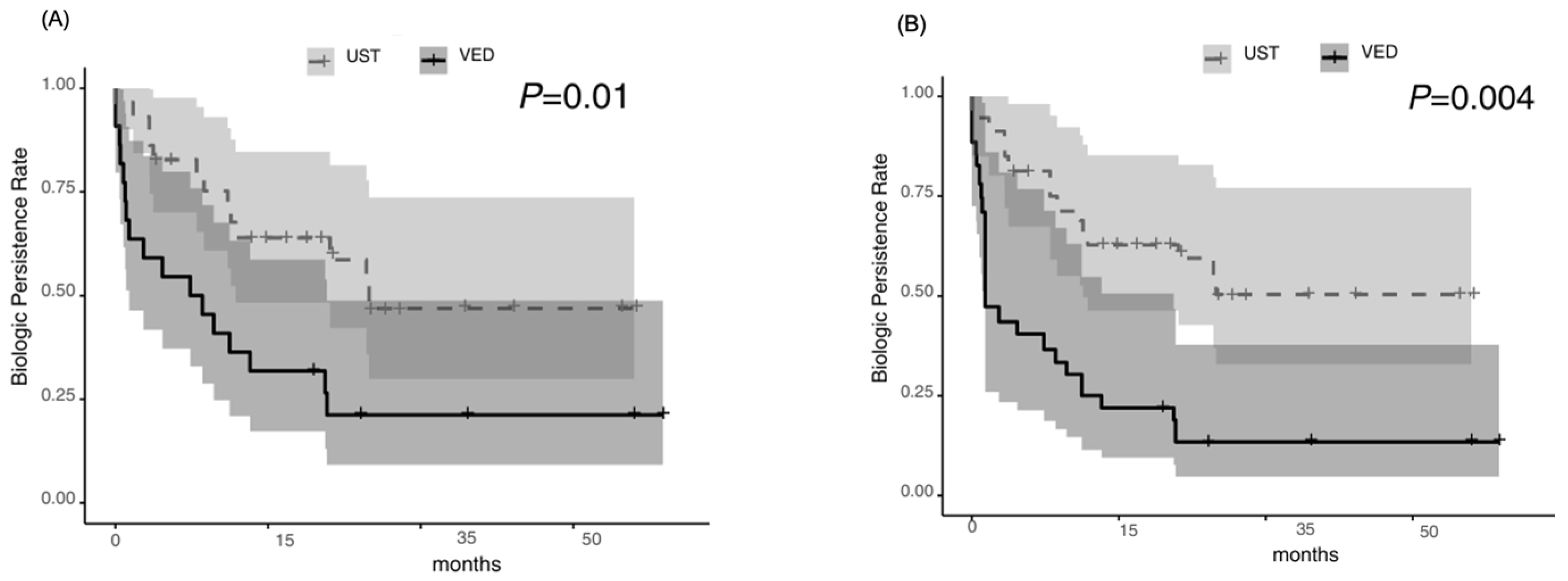
3.3. Exploratory Outcomes
3.4. Subsequent Treatments Following Relapse Within 1 Year of UST or VED Maintenance Therapy
3.5. Risk Factors for Relapse Identified by Univariable and Multivariable Cox Proportional Hazards Models Before and After IPTW Adjustment
3.6. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Feuerstein, J.D.; Moss, A.C.; Farraye, F.A. Ulcerative Colitis. Mayo Clin. Clin. Proc. 2019, 94, 1357–1373. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H. Acute Severe Ulcerative Colitis: Optimal Strategies for Drug Therapy. Gut Liver 2023, 17, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Watanabe, M.; Miwa, H.; Enomoto, N.; Shimosegawa, T.; Koike, K.; Koido, S.; Ishiguro, K.; Hirai, F.; Hibi, T.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef]
- Spinelli, A.; Selvaggi, F.; Verstockt, B.; Doherty, G.; Raine, T.; Panis, Y.; Felice, C.; Rutter, M.; Reinisch, W.; Ghosh, S.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Surgical Treatment. J. Crohns Colitis 2022, 16, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.; Moran, S.; Subramanian, S.; Taylor, S.A.; Tun, G.S.Z.; Verma, A.; Newton, A.C.; Wong, M.; Smith, J.; Patel, R.; et al. British Society of Gastroenterology guidelines on inflammatory bowel disease in adults: 2025. Gut 2025, 74, 1–101. [Google Scholar]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Ogata, H.; Matsui, T.; Nakamura, M.; Iida, M.; Takazoe, M.; Suzuki, Y.; Matsumoto, T.; Hibi, T.; Watanabe, M.; Chiba, T.; et al. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 2006, 55, 1255–1262. [Google Scholar] [CrossRef]
- Landy, J.; Peake, S.T.C.; Hussein, M.; Ng, S.C.; Lindsay, J.O.; Hart, A.L.; Smith, R.; Brown, K.; Wilson, D.; Taylor, P.; et al. Oral tacrolimus as maintenance therapy for refractory ulcerative colitis—an analysis of outcomes in two London tertiary centres. J. Crohns Colitis 2013, 7, e516–e521. [Google Scholar] [CrossRef]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kostakis, A.; Krznarić, Ž.; Lakatos, P.L.; Lomer, M.C.E.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef]
- Gargallo-Puyuelo, C.; Laredo, V.; Gomollón, F. Thiopurines in Inflammatory Bowel Disease. How to Optimize Thiopurines in the Biologic Era? Front. Med. 2021, 8, 681907. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Targan, S.R.; Panés, J.; Present, D.H.; Lashner, B.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Feagan, B.G.; Rutgeerts, P.; Targan, S.R.; Colombel, J.F.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Pellet, G.; Stefanescu, C.; Carbonnel, F.; Peyrin-Biroulet, L.; Roblin, X.; Allimant, C.; Bourreille, A.; Louis, E.; Lamoureux, F.; Lémann, M.; et al. Efficacy and Safety of Induction Therapy with Calcineurin Inhibitors in Combination with Vedolizumab in Patients with Refractory Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 494–501. [Google Scholar] [CrossRef]
- Ollrch, J.E.; Dwadasi, S.; Rai, V.; Peleg, N.; Normatov, I.; Israel, A.; Levine, A.; Kopylov, U.; Shamir, R.; Ben-Horin, S.; et al. Efficacy and safety of induction therapy with calcineurin inhibitors followed by vedolizumab maintenance in 71 patients with severe steroid-refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2020, 51, 637–643. [Google Scholar] [CrossRef]
- Veyrard, P.; Pellet, G.; Laharie, D.; Nachury, M.; Juillerat, P.; Roblin, X. Efficacy of Induction Therapy with Calcineurin Inhibitors in Combination with Ustekinumab for Acute Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2023, 21, 1354–1355. [Google Scholar] [CrossRef]
- Kawakami, K.; Inoue, T.; Murano, M.; Narabayashi, K.; Nouda, S.; Ishida, K.; Yamamoto, T.; Umegaki, E.; Matsumoto, K.; Takeuchi, K.; et al. Effects of oral tacrolimus as a rapid induction therapy in ulcerative colitis. World J. Gastroenterol. 2015, 21, 1880–1886. [Google Scholar] [CrossRef]
- Truelove, S.C.; Witts, L.J. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br. Med. J. 1955, 2, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Dinesen, L.C.; Walsh, A.J.; Protic, M.N.; Heap, G.; Cummings, F.; Warren, B.F.; Travis, S.P.L.; George, B.; Rutter, M.D.; Keshav, S.; et al. The pattern and outcome of acute severe colitis. J. Crohns Colitis 2010, 4, 431–437. [Google Scholar] [CrossRef] [PubMed]
- STELARA® (Ustekinumab) Injection, for Subcutaneous or Intravenous Use. Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125261s161lbl.pdf (accessed on 24 April 2024).
- ENTYVIO® (Vedolizumab) for Injection, for Intravenous Use. Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125476Orig1s046lbl.pdf (accessed on 24 April 2024).
- Sandborn, W.J.; van Assche, G.; Reinisch, W.; Colombel, J.-F.; D’Haens, G.; Wolf, D.C.; Kron, M.; Franchimont, D.; Xu, J.; Kent, J.D.; et al. Adalimumab Induces and Maintains Clinical Remission in Patients with Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2012, 142, 257. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Feagan, B.G.; Schreiber, S.; Wolf, D.C.; Axler, J.L.; Kaviya, A.; James, A.; Ghosh, S.; Panés, J.; Danese, S.; Hanauer, S.B.; et al. Sustained Clinical Remission with Vedolizumab in Patients with Moderate-to-Severe Ulcerative Colitis. Inflamm. Bowel Dis. 2019, 25, 1028–1035. [Google Scholar] [CrossRef]
- Whitehouse, G.; Gray, E.; Mastoridis, S.; Merritt, E.; Kodela, E.; Yang, J.H.M.; Sanchez-Fueyo, A.; Hernandez-Fuentes, M.; Game, D.; Lechler, R.I.; et al. IL-2 therapy restores regulatory T-cell dysfunction induced by calcineurin inhibitors. Proc. Natl. Acad. Sci. USA 2017, 114, 7083–7088. [Google Scholar] [CrossRef] [PubMed]
- Quatresooz, P.; Hermanns-Lê, T.; Piérard, G.E.; Humbert, P.; Delvenne, P.; Piérard-Franchimont, C. Ustekinumab in Psoriasis Immunopathology with Emphasis on the Th17-IL23 Axis: A Primer. J. Biomed. Biotechnol. 2012, 2012, 147413. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Joelson, A.; Green, P.H.R.; Lichtiger, S.; Cadwell, K.; Lebwohl, B.; Smith, M.; Jones, T.; Brown, L.; Davis, R.; et al. Infections Are Common in Patients with Flares of Inflammatory Bowel Disease. Am. J. Gastroenterol. 2018, 113, 1530–1539. [Google Scholar] [CrossRef]
- Limsrivilai, J.; Saleh, Z.M.; Johnson, L.A.; Brown, A.M.; Briggs, E.; Rao, K.; Higgins, P.D.R.; Smith, T.; Patel, A.; Davis, M.; et al. Prevalence and Effect of Intestinal Infections Detected by a PCR-Based Stool Test in Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2020, 65, 3287–3296. [Google Scholar] [CrossRef]
- Shinzaki, S.; Matsuoka, K.; Tanaka, H.; Takeshima, F.; Kato, S.; Torisu, T.; Iijima, H.; Yamada, A.; Yamamoto, T.; Hibi, T.; et al. Leucine-rich alpha-2 glycoprotein is a potential biomarker to monitor disease activity in inflammatory bowel disease receiving adalimumab: PLANET study. J. Gastroenterol. 2021, 56, 560–569. [Google Scholar] [CrossRef]


| UST (n = 29) | VED (n = 22) | p Value | |
|---|---|---|---|
| Partial Mayo score, median [IQR] | 9.0 [9.0–9.0] | 9.0 [9.0–9.0] | 1.00 |
| Number of bloody stools, median [IQR] | 10.0 [8.3–12.0] | 8.00 [6.00–10.00] | 0.01 |
| Heart rate [/min], median [IQR] | 99.5 [88.8–102.0] | 96.0 [87.0–106.0] | 0.65 |
| Body temperature [°C], median [IQR] | 37.1 [36.6–37.7] | 37.1 [36.9–37.8] | 0.31 |
| CRP [mg/L], median [IQR] | 48.8 [24.9–74.7] | 44.5 [24.4–64.6] | 0.89 |
| WBC [/μL], median [IQR] | 9660.0 [7680.0–11,095.0] | 11,220 [6975–12,935] | 0.52 |
| Hb [g/dL], median [IQR] | 11.0 [9.6–12.1] | 11.8 [11.1–13.0] | 0.26 |
| Plt [×104/μL], median [IQR] | 44.0 [32.7–48.7] | 40.9 [33.1–52.5] | 0.72 |
| Alb [g/dL], median [IQR] | 3.1 [2.3–3.6] | 3.2 [2.6–3.7] | 0.46 |
| Concomitant use of prednisolone [%] | 7 [25.9] | 5 [22.7] | 1.00 |
| Dose of prednisolone [mg], median [IQR] | 70.0 [45.0–80.0] | 45.00 [22.5–60.0] | 0.13 |
| IPTW-Unweighted | UST (n = 29) | VED (n = 22) | Overall (n = 51) | p Value | SMD |
|---|---|---|---|---|---|
| Age, years, median [IQR] | 35.0 [27.0, 54.0] | 35.0 [29.3, 50.8] | 35.0 [28.0, 53.0] | 0.83 | 0.10 |
| Males, n [%] | 19 [65.5] | 8 [36.4] | 27 [52.9] | 0.07 | 0.61 |
| Disease type, [pancolitis] [%] | 29 [100.0] | 19 [86.4] | 48 [94.1] | 0.15 | 0.56 |
| Concomitant use of immunomodulatory drug [%] | 11 [37.9] | 10 [45.5] | 21 [41.2] | 0.80 | 0.15 |
| Prior medication of biologics or JAK inhibitor [%] | 21 [72.4] | 12 [54.5] | 33 [64.7] | 0.30 | 0.38 |
| Steroid refractory [%] | 28 [96.6] | 18 [81.8] | 46 [90.2] | 0.23 | 0.49 |
| Duration of TAC therapy, median [IQR] | 185.0 [96.0, 215.0] | 149.5 [147.8, 242.8] | 168.0 [100.0, 239.5] | 0.14 | 0.23 |
| The time from the initiation of TAC to the start of maintenance therapy, median [IQR] | 35.0 [21.0, 45.0] | 46.5 [29.3, 72.8] | 36.0 [21.0, 65.0] | 0.31 | 0.43 |
| Partial Mayo score, median [IQR] | 3.0 [2.0, 6.0] | 3.0 [1.0, 5.0] | 3.0 [1.0, 5.0] | 0.50 | 0.07 |
| CRP [mg/L], median [IQR] | 1.0 [0.4, 2.0] | 2.0 [0.5, 3.0] | 1.0 [0.4, 2.0] | 0.76 | 0.32 |
| WBC [/μL], median [IQR] | 6020.0 [4330.0, 7030.0] | 6670.0 [5475.0, 7852.5] | 6400.0 [4920.0, 7305.0] | 0.20 | 0.39 |
| Hb [g/dL], median [IQR] | 10.2 [9.4, 11.9] | 11.0 [9.8, 12.3] | 10.3 [9.5, 12.1] | 0.34 | 0.22 |
| Plt [×104/μL], median [IQR] | 29.7 [26.7, 36.7] | 32.8 [29.2, 38.8] | 32.1 [26.8, 37.4] | 0.14 | 0.53 |
| Alb [g/dL], median [IQR] | 3.5 [3.0, 4.1] | 4.1 [3.6, 4.2] | 3.8 [3.2, 4.2] | 0.06 | 0.60 |
| IPTW-Weighted | UST (n = 27.4) | VED (n = 22.0) | Overall (n = 49.4) | p Value | SMD | Std-Diff |
|---|---|---|---|---|---|---|
| Age, years, median [IQR] | 37.8 [27.3, 56.9] | 32.7 [29.5, 50.2] | 36.8 [28.2, 55.5] | 0.84 | 0.03 | |
| Males, n [%] | 18.8 [68.8] | 8.1 [36.8] | 26.9 [54.5] | 0.04 | 0.68 | 0.28 |
| Disease type, [pancolitis] [%] | 27.4 [100.0] | 20.7 [94.1] | 48.1 [97.4] | 0.08 | 0.35 | |
| Concomitant use of immunomodulatory drug [%] | 10.3 [37.6] | 8.9 [40.5] | 19.2 [49.5] | 0.82 | 0.07 | |
| Prior medication of biologics or JAK inhibitor [%] | 16.8 [61.3] | 14.2 [64.6] | 31.0 [62.8] | 0.84 | 0.06 | 0.05 |
| Steroid refractory [%] | 24.3 [88.7] | 19.8 [90.0] | 44.1 [89.3] | 0.91 | 0.04 | |
| Duration of TAC therapy, median [IQR] | 139.0 [91.2, 208.7] | 175.5 [149.0, 244.3] | 161.6 [97.2, 237.3] | 0.15 | 0.28 | |
| The time from the initiation of TAC to the start of maintenance therapy, median [IQR] | 24.5 [20.6, 45.0] | 40.1 [18.1, 59.3] | 31.6 [20.1, 53.4] | 0.54 | 0.24 | |
| Partial Mayo score, median [IQR] | 3.0 [1.2, 4.4] | 3.0 [0.4, 5.0] | 3.0 [1.0, 5.0] | 0.73 | 0.15 | 0.12 |
| CRP [mg/L], median [IQR] | 1.1 [0.4, 2.0] | 2.0 [0.5, 4.0] | 1.2 [0.4, 4.0] | 0.65 | 0.50 | 0.13 |
| WBC [/μL], median [IQR] | 6460.0 [4317.8, 7289.7] | 6370.9 [5302.8, 7790.0] | 6395.3 [4929.8, 7723.8] | 0.44 | 0.28 | |
| Hb [g/dL], median [IQR] | 10.8 [9.4, 12.0] | 10.8 [8.8, 12.3] | 10.9 [9.4, 12.3] | 0.90 | 0.01 | |
| Plt [×104/μL], median [IQR] | 29.8 [26.7, 35.5] | 32.2 [29.0, 38.8] | 30.9 [27.1, 37.0] | 0.12 | 0.52 | |
| Alb [g/dL], median [IQR] | 3.4 [3.0, 4.0] | 3.9 [3.5, 4.1] | 3.7 [3.2, 4.1] | 0.10 | 0.51 | 0.01 |
| IPTW-Unweighted Cox Proportional Hazards Model | Univariable | Multivariable | ||||
| Characteristics | HR | 95% CI | p Value | HR | 95% CI | p Value |
| The use of UST as maintenance therapy | 0.39 | 0.19–0.81 | 0.001 | 0.42 | 0.20–0.88 | 0.02 |
| The days between the start of TAC and the administration of the biologics | 1.01 | 1.00–1.01 | 0.05 | 1.00 | 1.00–1.01 | 0.13 |
| IPTW-Weighted Cox Proportional Hazards Model | Univariable | Multivariable | ||||
| Characteristics | HR | 95% CI | p Value | HR | 95% CI | p Value |
| The use of UST as maintenance therapy | 0.30 | 0.14–0.64 | 0.001 | 0.30 | 0.14–0.66 | 0.02 |
| The days between the start of TAC and the administration of the biologics | 1.00 | 1.00–1.01 | 0.42 | 1.00 | 1.00–1.01 | 0.34 |
| UST (n = 29) | VED (n = 22) | |
|---|---|---|
| Arthralgia [%] | 1 (3.4) | 1 (4.5) |
| Upper respiratory tract infection | 1 (3.4) | 2 (9.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaku, K.; Sato, T.; Takeuchi, J.; Yokoyama, K.; Yagi, S.; Takagi, Y.; Ikenouchi, M.; Kawai, M.; Kamikozuru, K.; Yokoyama, Y.; et al. Comparative Effectiveness of Ustekinumab and Vedolizumab as Maintenance Therapy After Tacrolimus-Induced Improvement in Patients with Acute Severe Ulcerative Colitis: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 5588. https://doi.org/10.3390/jcm14155588
Kaku K, Sato T, Takeuchi J, Yokoyama K, Yagi S, Takagi Y, Ikenouchi M, Kawai M, Kamikozuru K, Yokoyama Y, et al. Comparative Effectiveness of Ustekinumab and Vedolizumab as Maintenance Therapy After Tacrolimus-Induced Improvement in Patients with Acute Severe Ulcerative Colitis: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(15):5588. https://doi.org/10.3390/jcm14155588
Chicago/Turabian StyleKaku, Koji, Toshiyuki Sato, Jiro Takeuchi, Keiko Yokoyama, Soichi Yagi, Yasuhiro Takagi, Maiko Ikenouchi, Mikio Kawai, Koji Kamikozuru, Yoko Yokoyama, and et al. 2025. "Comparative Effectiveness of Ustekinumab and Vedolizumab as Maintenance Therapy After Tacrolimus-Induced Improvement in Patients with Acute Severe Ulcerative Colitis: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 15: 5588. https://doi.org/10.3390/jcm14155588
APA StyleKaku, K., Sato, T., Takeuchi, J., Yokoyama, K., Yagi, S., Takagi, Y., Ikenouchi, M., Kawai, M., Kamikozuru, K., Yokoyama, Y., Takagawa, T., Tomita, T., Fukui, H., & Shinzaki, S. (2025). Comparative Effectiveness of Ustekinumab and Vedolizumab as Maintenance Therapy After Tacrolimus-Induced Improvement in Patients with Acute Severe Ulcerative Colitis: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(15), 5588. https://doi.org/10.3390/jcm14155588








