Clinical Outcomes of Iron Supplement Therapy in Non-Anemic Female CKD Stage 3 Patients with Low Serum Ferritin Level: A Multi-Institutional TriNetX Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Cohorts
2.3. Data Analysis
3. Results
3.1. Characteristics
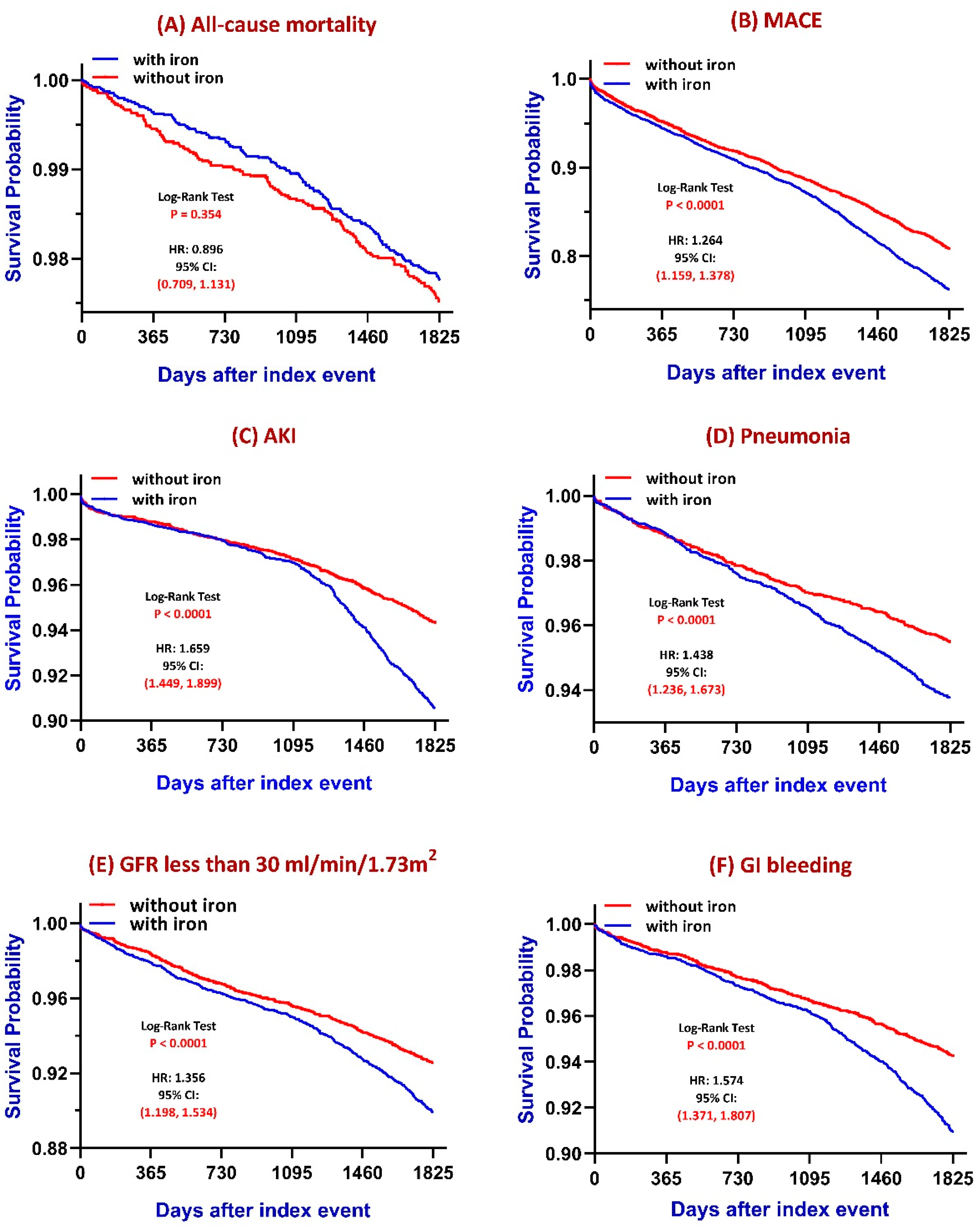
3.2. Five-Year Kaplan–Meier Curves to Evaluate Iron Therapy Primary Outcomes
- All-cause mortality: No statistically significant difference was observed between groups (log-rank p = 0.354), although a subtle trend toward increased mortality was noted in the iron-treated group.
- MACE: Patients receiving iron therapy had a significantly higher cumulative incidence of major adverse cardiovascular events compared to those without iron therapy (log-rank p < 0.0001).
- AKI: The iron group exhibited a significantly greater risk of acute kidney injury (log-rank p < 0.0001). The difference is more prominent after long-term follow-up.
- Pneumonia: Iron supplementation was associated with a significantly increased risk of pneumonia (log-rank p < 0.0001).
- CKD progression: Defined as eGFR declining to ≤30 mL/min/1.73 m2, the iron-treated group showed a significantly faster decline in renal function (log-rank p < 0.0001).
- GI bleeding: The increased risk of GI bleeding is observed in the iron-treated group (p < 0.0001). To specify the relationship between iron supplementation and GI bleeding, we confirmed the consistent outcomes both before and after matching (Table S2). Also, even excluding GI bleeding, these significant differences between the iron-treated group and the group without iron treatment were also unchanged (Table S3).
3.3. Secondary Outcomes: Venous Thromboembolism
3.4. Subgroup Analysis of Iron Therapy
4. Discussion
4.1. Mortality and MACE
4.2. AKI and CKD Progression
4.3. Pneumonia
4.4. Gastrointestinal Bleeding
4.5. Venous Thromboembolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IDWA | Iron deficiency without anemia |
| TSAT | Transferrin saturation |
References
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; DeLoughery, T.G.; Tirnauer, J.S. Iron Deficiency in Adults: A Review. JAMA 2025, 333, 1813–1823. [Google Scholar] [CrossRef]
- Batchelor, E.K.; Kapitsinou, P.; Pergola, P.E.; Kovesdy, C.P.; Jalal, D.I. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. J. Am. Soc. Nephrol. 2020, 31, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; McCulloch, C.E.; Curhan, G.C. Iron status and hemoglobin level in chronic renal insufficiency. J. Am. Soc. Nephrol. 2002, 13, 2783–2786. [Google Scholar] [CrossRef]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Jenča, D.; Melenovský, V.; Mrázková, J.; Šramko, M.; Kotrč, M.; Želízko, M.; Adámková, A.; Piťha, J.; Kautzner, J.; Wohlfahrt, P. Iron deficiency and all-cause mortality after myocardial infarction. Eur. J. Intern. Med. 2024, 126, 102–108. [Google Scholar] [CrossRef]
- Schrage, B.; Rübsamen, N.; Ojeda, F.M.; Thorand, B.; Peters, A.; Koenig, W.; Söderberg, S.; Söderberg, M.; Mathiesen, E.B.; Njølstad, I.; et al. Association of iron deficiency with incident cardiovascular diseases and mortality in the general population. ESC Heart Fail. 2021, 8, 4584–4592. [Google Scholar] [CrossRef]
- Yu, H.; Shao, X.; Guo, Z.; Pang, M.; Chen, S.; She, C.; Cao, L.; Luo, F.; Chen, R.; Zhou, S.; et al. Association of iron deficiency with kidney outcome and all-cause mortality in chronic kidney disease patients without anemia. Nutr. J. 2025, 24, 7. [Google Scholar] [CrossRef]
- Guedes, M.; Muenz, D.G.; Zee, J.; Bieber, B.; Stengel, B.; Massy, Z.A.; Mansencal, N.; Wong, M.M.Y.; Charytan, D.M.; Reichel, H.; et al. Serum Biomarkers of Iron Stores Are Associated with Increased Risk of All-Cause Mortality and Cardiovascular Events in Nondialysis CKD Patients, with or without Anemia. J. Am. Soc. Nephrol. 2021, 32, 2020–2030. [Google Scholar] [CrossRef]
- Hasegawa, T.; Imaizumi, T.; Hamano, T.; Murotani, K.; Fujii, N.; Komaba, H.; Ando, M.; Maruyama, S.; Nangaku, M.; Nitta, K.; et al. Association between serum iron markers, iron supplementation and cardiovascular morbidity in pre-dialysis chronic kidney disease. Nephrol. Dial. Transplant. 2023, 38, 2713–2722. [Google Scholar] [CrossRef]
- Yadav, A.K.; Ghosh, A.; Divyaveer, S.; Mukhopadhyay, B.; Kundu, M.; Kumar, V.; Lele, S.S.; Rajapurkar, M.M.; Jha, V.; Indian Chronic Kidney Disease Study Group. Serum catalytic iron and progression of chronic kidney disease: Findings from the ICKD study. Nephrol. Dial. Transplant. 2021, 37, 1879–1887. [Google Scholar] [CrossRef]
- KDIGO Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Wong, M.M.Y.; Tu, C.; Li, Y.; Perlman, R.L.; Pecoits-Filho, R.; Lopes, A.A.; Narita, I.; Reichel, H.; Port, F.K.; Sukul, N.; et al. Anemia and iron deficiency among chronic kidney disease Stages 3–5ND patients in the Chronic Kidney Disease Outcomes and Practice Patterns Study: Often unmeasured, variably treated. Clin. Kidney J. 2019, 13, 613–624. [Google Scholar] [CrossRef]
- Balendran, S.; Forsyth, C. Non-anaemic iron deficiency. Aust Prescr. 2021, 44, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Cluster, G.N. Guidelines for Iron Supplementation to Treat and Prevent Iron Deficiency Anemia; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Cornelissen, A.; Guo, L.; Sakamoto, A.; Virmani, R.; Finn, A.V. New insights into the role of iron in inflammation and atherosclerosis. eBioMedicine 2019, 47, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Robien, K.; Harnack, L.J.; Park, K.; Jacobs, D.R. Dietary Supplements and Mortality Rate in Older Women: The Iowa Women’s Health Study. Arch. Intern. Med. 2011, 171, 1625–1633. [Google Scholar] [CrossRef]
- Krayenbuehl, P.A.; Battegay, E.; Breymann, C.; Furrer, J.; Schulthess, G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 2011, 118, 3222–3227. [Google Scholar] [CrossRef]
- Holm, C.; Thomsen, L.L.; Langhoff-Roos, J. Intravenous iron isomaltoside treatment of women suffering from severe fatigue after postpartum hemorrhage. J. Matern. Fetal Neonatal Med. 2019, 32, 2797–2804. [Google Scholar] [CrossRef]
- Cho, M.E.; Hansen, J.L.; Peters, C.B.; Cheung, A.K.; Greene, T.; Sauer, B.C. An increased mortality risk is associated with abnormal iron status in diabetic and non-diabetic Veterans with predialysis chronic kidney disease. Kidney Int. 2019, 96, 750–760. [Google Scholar] [CrossRef]
- Mehta, R.C.; Cho, M.E.; Cai, X.; Lee, J.; Chen, J.; He, J.; Flack, J.; Saraf, S.L.; David, V.; Feldman, H.I.; et al. Iron status, fibroblast growth factor 23 and cardiovascular and kidney outcomes in chronic kidney disease. Kidney Int. 2021, 100, 1292–1302. [Google Scholar] [CrossRef]
- Schrage, B.; Rübsamen, N.; Schulz, A.; Münzel, T.; Pfeiffer, N.; Wild, P.S.; Beutel, M.; Schmidtmann, I.; Lott, I.; Blankenberg, S.; et al. Iron deficiency is a common disorder in general population and independently predicts all-cause mortality: Results from the Gutenberg Health Study. Clin. Res. Cardiol. 2020, 109, 1352–1357. [Google Scholar] [CrossRef]
- Eisenga, M.F.; Nolte, I.M.; van der Meer, P.; Bakker, S.J.L.; Gaillard, C.A.J.M. Association of different iron deficiency cutoffs with adverse outcomes in chronic kidney disease. BMC Nephrol. 2018, 19, 225. [Google Scholar] [CrossRef]
- Yang, C.; Hu, T.; Li, C.; Gong, A. Dietary iron intake predicts all-cause and cardiovascular mortality in patients with diabetes. Nutr. Diabetes 2024, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Dewangga, R.; Winston, K. The role of iron supplementation in reducing all-cause mortality of heart failure patients with iron deficiency: A systematic review and meta-analysis. Eur. Heart J. 2021, 42 (Suppl. S1), ehab724.0918. [Google Scholar] [CrossRef]
- Awan, A.A.; Walther, C.P.; Richardson, P.A.; Shah, M.; Winkelmayer, W.C.; Navaneethan, S.D. Prevalence, correlates and outcomes of absolute and functional iron deficiency anemia in nondialysis-dependent chronic kidney disease. Nephrol. Dial. Transplant. 2019, 36, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Gao, Y.; Deng, L.; Liang, Y.; Xia, N.; Pan, L. Association Between Iron Metabolism and Acute Kidney Injury in Critically Ill Patients With Diabetes. Front Endocrinol. 2022, 13, 892811. [Google Scholar] [CrossRef]
- Paul, S.; Shrestha, P.; Sumida, K.; Thomas, F.; Surbhi, S.; Naser, A.M.; Streja, E.; Rhee, C.M.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of oral iron replacement therapy with kidney failure and mortality in CKD patients. Clin. Kidney J. 2023, 16, 2082–2090. [Google Scholar] [CrossRef]
- Ni, L.; Yuan, C.; Wu, X. Targeting ferroptosis in acute kidney injury. Cell Death Dis. 2022, 13, 182. [Google Scholar] [CrossRef]
- Tan, X.; Li, M.; Chen, J.; Liu, G.; Lu, Z. Acute kidney injury and iron metabolism: A narrative review focusing on pathophysiology and therapy. Smart Mol. 2025, e20240026. [Google Scholar] [CrossRef]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef]
- Shah, A.A.; Donovan, K.; Seeley, C.; Dickson, E.A.; Palmer, A.J.R.; Doree, C.; Brunskill, S.; Reid, J.; Acheson, A.G.; Sugavanam, A.; et al. Risk of Infection Associated With Administration of Intravenous Iron: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2133935. [Google Scholar] [CrossRef] [PubMed]
- Nies, I.; Gourde, E.; Newman, W.; Schiele, R. Impact of Iron Supplementation on Hospital Length of Stay for Pneumonia or Skin and Skin Structure Infections: A Retrospective Cohort Study. Hosp. Pharm. 2024, 59, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Moroșan, I.; Fărcaș, A.C.; Kerezsi, A.D.; et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency-A Literature-Based Review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef]
- Lin, Y.; Li, C.; Waters, D.; Kwok, C.S. Gastrointestinal bleeding in chronic kidney disease patients: A systematic review and meta-analysis. Ren Fail. 2023, 45, 2276908. [Google Scholar] [CrossRef]
- Bloor, S.R.; Schutte, R.; Hobson, A.R. Oral Iron Supplementation—Gastrointestinal Side Effects and the Impact on the Gut Microbiota. Microbiol. Res. 2021, 12, 491–502. [Google Scholar] [CrossRef]
- Keung, Y.-K.; Owen, J. Iron deficiency and thrombosis: Literature review. Clin. Appl. Thromb. Hemost. 2004, 10, 387–391. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Barbagallo, A.; Oliveri Conti, G.; Fiore, M.; Cristaldi, A.; Ferrante, M. Oxidative Status, Iron Plasma Levels in Venous Thrombosis Patients. Antioxidants 2024, 13, 689. [Google Scholar] [CrossRef]


| Before Matching | After Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| With Iron (n = 6642) | Without Iron (n = 47,127) | p Value | Std Diff | With Iron (n = 6638) | Without Iron (n = 6638) | p Value | Std Diff | |
| General data | ||||||||
| Age | 59.4 ± 13.8 | 59.1 ± 12.8 | 0.106 | 0.021 | 59.4 ± 13.8 | 59.6 ± 13.2 | 0.297 | 0.018 |
| White | 78.7% | 71.1% | <0.001 | 0.177 | 78.7% | 79.7% | 0.165 | 0.024 |
| Black or African American | 10.6% | 7.5% | <0.001 | 0.107 | 10.6% | 10.8% | 0.633 | 0.008 |
| Asian | 1.2% | 1.2% | 0.928 | 0.001 | 1.2% | 1.2% | 0.690 | 0.007 |
| Comorbidity | ||||||||
| Diabetes mellitus | 10.0% | 6.0% | <0.001 | 0.149 | 9.9% | 9.7% | 0.705 | 0.007 |
| Hypertensive diseases | 21.4% | 21.3% | 0.918 | 0.004 | 20.9% | 20.0% | 0.196 | 0.022 |
| Overweight and obesity | 7.3% | 4.8% | <0.001 | 0.104 | 7.3% | 7.4% | 0.868 | 0.003 |
| Ischemic heart disease | 3.2% | 1.8% | <0.001 | 0.087 | 3.2% | 2.9% | 0.364 | 0.016 |
| Cerebrovascular diseases | 1.6% | 1.0% | <0.001 | 0.051 | 1.6% | 1.4% | 0.518 | 0.011 |
| Medications | ||||||||
| Antilipemic agents | 12.1% | 7.4% | <0.001 | 0.156 | 12.0% | 11.4% | 0.268 | 0.019 |
| Beta blockers | 9.3% | 5.5% | <0.001 | 0.146 | 9.3% | 8.4% | 0.076 | 0.031 |
| Calcium channel blockers | 5.7% | 3.5% | <0.001 | 0.104 | 5.6% | 5.5% | 0.622 | 0.009 |
| Angiotensin II inhibitors | 5.1% | 3.2% | <0.001 | 0.099 | 5.1% | 4.5% | 0.115 | 0.027 |
| NSAIDs | 2.9% | 1.9% | <0.001 | 0.064 | 2.9% | 3.0% | 0.642 | 0.009 |
| Aspirin | 4.8% | 2.5% | <0.001 | 0.127 | 4.7% | 4.0% | 0.045 | 0.035 |
| Rivaroxban | 0.5% | 0.3% | 0.002 | 0.036 | 0.5% | 0.4% | 0.361 | 0.016 |
| Edoxaban | 0.2% | 0.02% | <0.001 | 0.044 | 0.2% | 0% | 0.002 | 0.055 |
| Dabigatran | 0.2% | 0.1% | 0.003 | 0.032 | 0.2% | 0.2% | 1 | <0.001 |
| Laboratory exams | ||||||||
| Creatinine, mg/dL | 0.9 ± 0.3 | 0.9 ± 0.9 | 0.346 | 0.034 | 0.9 ± 0.3 | 1.0 ± 1.3 | 0.153 | 0.054 |
| Calcium, mg/dL | 9.3 ± 0.7 | 9.4 ± 0.6 | <0.001 | 0.106 | 9.3 ± 0.7 | 9.4 ± 0.6 | 0.166 | 0.056 |
| Phosphate, mg/dL | 3.6 ± 0.7 | 3.6 ± 0.7 | 0.990 | 0.001 | 3.6 ± 0.7 | 3.6 ± 0.8 | 0.566 | 0.061 |
| Hemoglobin, g/dL | 13.8 ± 1.1 | 13.8 ± 1.0 | 0.361 | 0.030 | 13.8 ± 1.1 | 13.8 ± 1.1 | 0.478 | 0.032 |
| MCV, fL | 90.6 ± 4.6 | 90.7 ± 4.5 | 0.276 | 0.037 | 90.6 ± 4.6 | 90.6 ± 4.7 | 0.793 | 0.012 |
| Alk phosphatase, U/L | 82.5 ± 31.1 | 82.7 ± 39.8 | 0.907 | 0.005 | 82.5 ± 31.1 | 83.6 ± 32.7 | 0.482 | 0.034 |
| Albumin, g/dL | 4.0 ± 0.4 | 4.1 ± 0.4 | <0.001 | 0.216 | 4.0 ± 0.4 | 4.0 ± 0.5 | 0.088 | 0.081 |
| Total cholesterol, mg/dL | 186.8 ± 47.6 | 193.6 ± 47.2 | 0.001 | 0.144 | 186.9 ± 47.7 | 190.2 ± 53.4 | 0.282 | 0.065 |
| Hemoglobin A1c, % | 6.4 ± 1.3 | 6.4 ± 1.4 | 0.980 | 0.002 | 6.9 ± 1.8 | 6.8 ± 1.7 | 0.457 | 0.048 |
| Intact PTH, pg/mL | 62.3 ± 44.9 | 62.5 ± 43.0 | 0.980 | 0.003 | 62.3 ± 44.9 | 68.4 ± 51.9 | 0.508 | 0.125 |
| Iron, ug/dL | 80.1 ± 38.2 | 83.1 ± 36.2 | 0.330 | 0.082 | 80.1 ± 38.2 | 85.2 ± 42.1 | 0.282 | 0.127 |
| Ferritin, ng/mL | 40.2 ± 23.9 | 49.4 ± 24.5 | <0.001 | 0.380 | 40.2 ± 23.9 | 51.0 ± 25.6 | 0.409 | 0.014 |
| CRP, mg/L | 11.8 ± 18.7 | 8.8 ± 20.3 | 0.097 | 0.153 | 11.9 ± 18.8 | 12.7 ± 30.3 | 0.778 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-C.; Liao, M.-T.; Wang, J.; Tsai, K.-W.; Wu, C.-C.; Lu, K.-C. Clinical Outcomes of Iron Supplement Therapy in Non-Anemic Female CKD Stage 3 Patients with Low Serum Ferritin Level: A Multi-Institutional TriNetX Analysis. J. Clin. Med. 2025, 14, 5575. https://doi.org/10.3390/jcm14155575
Chen H-C, Liao M-T, Wang J, Tsai K-W, Wu C-C, Lu K-C. Clinical Outcomes of Iron Supplement Therapy in Non-Anemic Female CKD Stage 3 Patients with Low Serum Ferritin Level: A Multi-Institutional TriNetX Analysis. Journal of Clinical Medicine. 2025; 14(15):5575. https://doi.org/10.3390/jcm14155575
Chicago/Turabian StyleChen, Hsi-Chih, Min-Tser Liao, Joshua Wang, Kuo-Wang Tsai, Chia-Chao Wu, and Kuo-Cheng Lu. 2025. "Clinical Outcomes of Iron Supplement Therapy in Non-Anemic Female CKD Stage 3 Patients with Low Serum Ferritin Level: A Multi-Institutional TriNetX Analysis" Journal of Clinical Medicine 14, no. 15: 5575. https://doi.org/10.3390/jcm14155575
APA StyleChen, H.-C., Liao, M.-T., Wang, J., Tsai, K.-W., Wu, C.-C., & Lu, K.-C. (2025). Clinical Outcomes of Iron Supplement Therapy in Non-Anemic Female CKD Stage 3 Patients with Low Serum Ferritin Level: A Multi-Institutional TriNetX Analysis. Journal of Clinical Medicine, 14(15), 5575. https://doi.org/10.3390/jcm14155575





